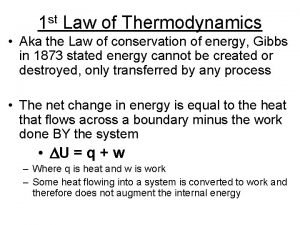
Second Law of thermodynamics The second law of



















- Slides: 25

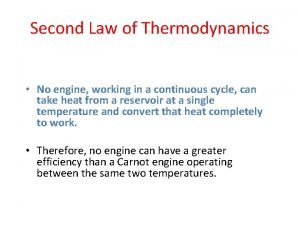
Second Law of thermodynamics

The second law of thermodynamics can be understood through considering these processes: § A rock will fall if you lift it up and then let go § Hot pans cool down when taken out from the stove. § Ice cubes melt in a warm room.

What’s happening in every one of those? Energy of some kind is changing from being localized (concentrated) somehow to becoming more spreed out. i. e in example 1: The potential energy localized in the rock is now totally spread out and dispersed in: § A little air movement. § Little heating of air and ground.

In the previous example § System: rock above ground then rock on ground. § Surroundings: air + ground

§ The second law of thermodynamics states that energy (and matter) tends to become more evenly spread out across the universe. § i. e to concentrate energy (or matter) in one specific place, it is necessary to spread out a greater amount of energy (as heat) across the remainder of the universe ("the surroundings").



What is entropy? Entropy just measures the spontaneous dispersal of energy: or how much energy is spread out in a process as a function of temperature.

Follow the Entropy § Entropy a measure of disorder in the physical system. § the second law of thermodynamics – the universe, or in any isolated system, the degree of disorder (entropy) can only increase. § the movement towards a disordered state is a spontaneous process.

So in a simple equation: Entropy = “ energy dispersed”/ T Entropy couldn't be expressed without the inclusion of absolute temperature. Entropy change ΔS shows us exactly how important to a system is a dispersion of a given amount of energy.


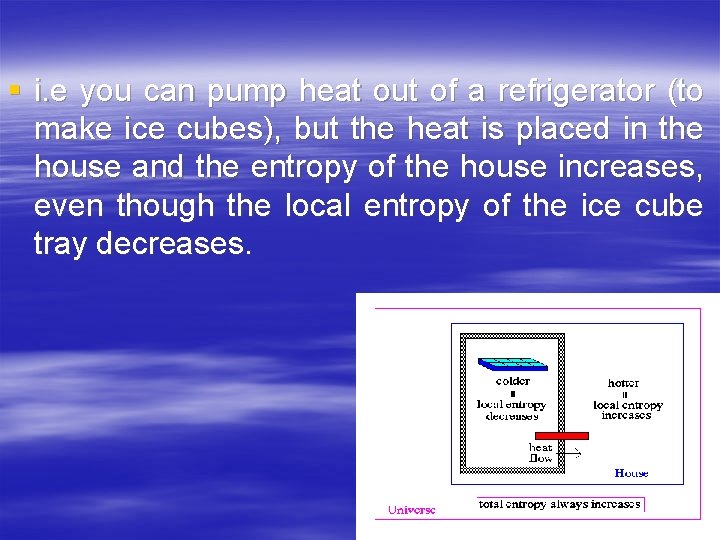
§ i. e you can pump heat out of a refrigerator (to make ice cubes), but the heat is placed in the house and the entropy of the house increases, even though the local entropy of the ice cube tray decreases.

Entropy change Δ S § In chemical terms entropy is related to the random movements of molecules and is measured by T ΔS. § When a system is at equilibrium, no net reaction occurs and the system has no capacity to do work. Q=TΔS entropy. This is a condition of maximum

§ Work can be done by system proceeding to equilibrium and measure of the maximum useful work is given by the following equation W = - ΔH + T ΔS

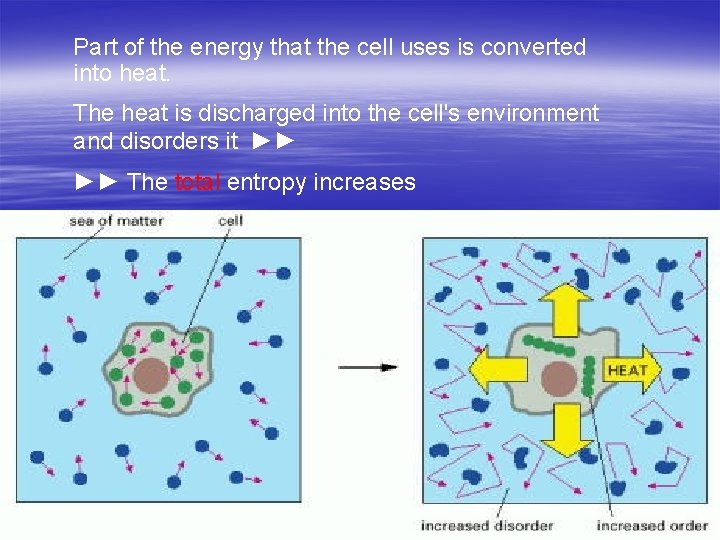
Is the second law of thermodynamics violated in the living cells? NO! § Cell is not an isolated system: it takes energy from its environment to generate order within itself. § Part of the energy that the cell uses is converted into heat. § The heat is discharged into the cell's environment and disorders it. The total entropy increases

Part of the energy that the cell uses is converted into heat. The heat is discharged into the cell's environment and disorders it ►► ►► The total entropy increases

Entropy and Life § For example, living things are highly ordered, low entropy, structures, but they grow and are sustained because their metabolism generates excess entropy in their surroundings. § For living systems, approaching chemical equilibrium means decay and death.

Entropy and Life For living systems, approaching equilibrium means decay and death.

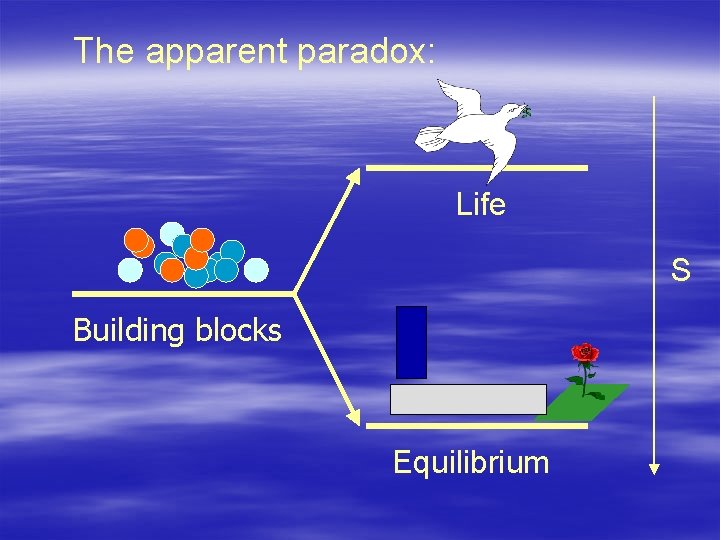
The apparent paradox: Life S Building blocks Equilibrium

Gibbs Free energy § Gibbs introduced the concept of free energy as an another measure of the capacity to do useful work. Free energy G is defined as Δ G = ΔH- T ΔS & W = - ΔH + T ΔS Note that ΔG= -W So that when the measure of W is positive (i. e the system is doing useful work), the measure of ΔG is negative and vice versa.

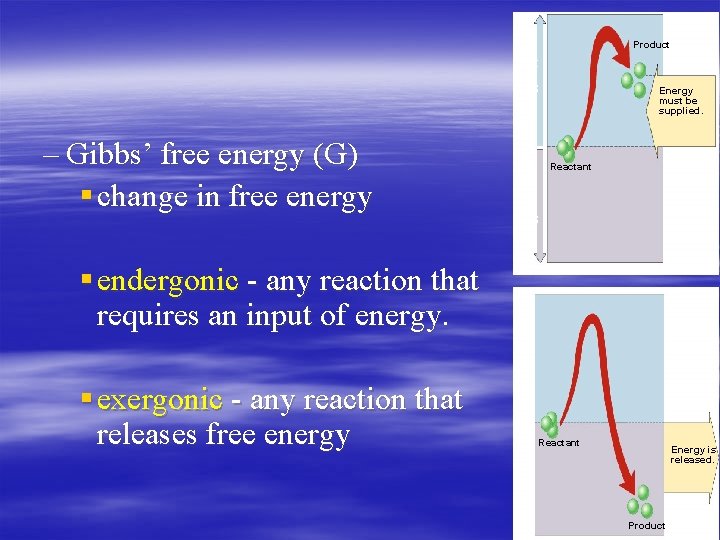
Energy supplied Energy released – Gibbs’ free energy (G) § change in free energy Product Energy must be supplied. Reactant § endergonic - any reaction that requires an input of energy. § exergonic - any reaction that releases free energy Reactant Energy is released. Product

§ Glucose-1 -p Glucose-6 -p Since changes in free energy and enthalpy are related only to the difference between the free energies and enthalpies of reactants and products, so we can characterize the above reaction as: Δ G = G g-6 -p - G g-1 -p or Δ H= H g-6 -p - H g-1 -p

§ If the algebraic sign is: 1 - negative, the reaction is exergonic (i. e it will proceeds spontaneously from left to right as written). 2 -Positive, the reaction is endergonic, (i. e it will not proceeds spontenously. 3 - Zero, the reaction is at equilibrium.

§ When Δ H is: negative, the reaction is exothermic (i. e it gives off heat to its surroundings). Positive, the reaction is endothermic (i. e it take heat from its surroundings). 3 - Zero, the reaction is isothermic ( no net exchange of heat occurs with the surroundings).

Standard free energy “Δ G°” § “Δ G°” of a chemical reaction are calculated at 25 C° and at 1 atmospheric pressure. The biological standard free energy Δ G°−” is more useful in biochemistry, here the standard conditions are: p. H =7 Temp = 37 C° 1 M concentrations of reactants and products.
 Isentropic process
Isentropic process Zeroth law of thermodynamics
Zeroth law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Energy balance equation thermodynamics open system
Energy balance equation thermodynamics open system Zeroth law of thermodynamics definition
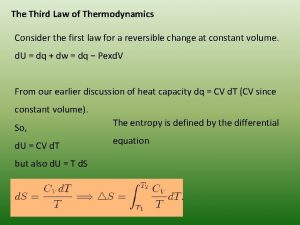
Zeroth law of thermodynamics definition Third law of thermodynamics derivation
Third law of thermodynamics derivation Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement First law of thermodynamics
First law of thermodynamics 1th law of thermodynamics
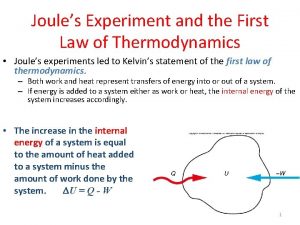
1th law of thermodynamics Joule's experiment first law of thermodynamics
Joule's experiment first law of thermodynamics Isothermal process in thermodynamics
Isothermal process in thermodynamics First law of thermodynamics compressor
First law of thermodynamics compressor Laws of thermodynamics
Laws of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt First law of thermodynamics sign convention
First law of thermodynamics sign convention 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics control mass
First law of thermodynamics control mass