ENTROPHY ERT 206 THERMODYNAMICS OBJECTIVE Apply the second

ENTROPHY ERT 206 THERMODYNAMICS

OBJECTIVE üApply the second law of thermodynamics to processes. üDefine a new property called entropy to quantify the second-law effects. üCalculate the entropy changes that take place during processes for pure substances, incompressible substances, and ideal gases. üExamine a special class of idealized processes, called isentropic processes, and develop the property relations for these processes. üDerive the reversible steady-flow work relations üIntroduce and apply the entropy balance to various systems.
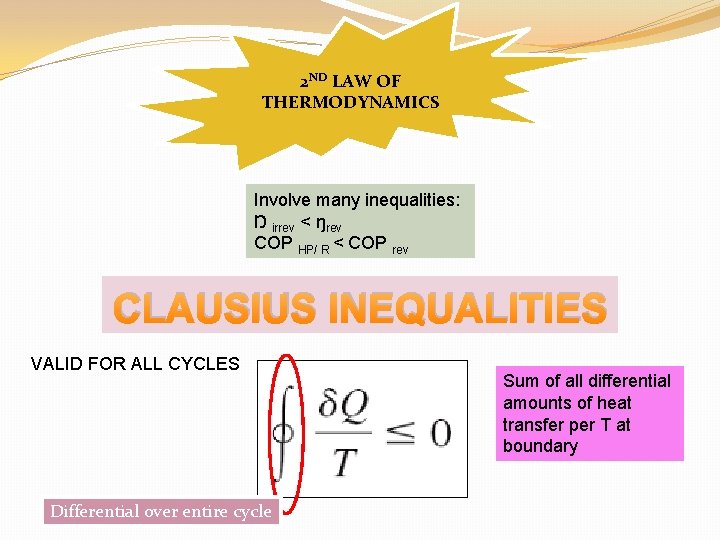
2 ND LAW OF THERMODYNAMICS Involve many inequalities: Ŋ irrev < ŋrev COP HP/ R < COP rev CLAUSIUS INEQUALITIES VALID FOR ALL CYCLES Differential over entire cycle Sum of all differential amounts of heat transfer per T at boundary
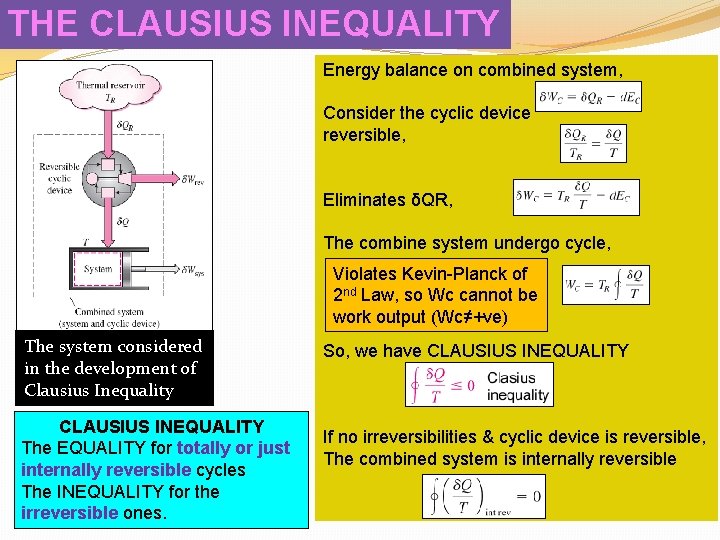
THE CLAUSIUS INEQUALITY Energy balance on combined system, Consider the cyclic device reversible, Eliminates δQR, The combine system undergo cycle, Violates Kevin-Planck of 2 nd Law, so Wc cannot be work output (Wc≠+ve) The system considered in the development of Clausius Inequality CLAUSIUS INEQUALITY The EQUALITY for totally or just internally reversible cycles The INEQUALITY for the irreversible ones. So, we have CLAUSIUS INEQUALITY If no irreversibilities & cyclic device is reversible, The combined system is internally reversible

RELATION TO ENTROPHY? The net change in a property (i. e. volume) during a cycle is always zero ENTROPY PROPERTY Property A quantity which its cyclic integral = 0 Definition of Entrophy SPECIAL CASE: INTERNALLY REVERSIBLE ISOTHERMAL HEAT TRANSFER PROCESSES The entropy change between two specified states is the same whether the process is reversible or irreversible

REMARKS ON ENTROPHY 1. Processes can occur in a certain direction only, not in any direction. A process must proceed in the direction that complies with the increase of entropy principle, that is, S gen ≥ 0. A process that violates this principle is impossible. 2. Entropy is a nonconserved property, and there is no such thing as the conservation of entropy principle. Entropy is conserved during the idealized reversible processes only and increases during all actual processes. (Entropy is generated or created during irreversible process due to the presence of irreversibilities). 3. The performance of engineering systems is degraded by the presence of irreversibilities, and entropy generation is a measure of the magnitudes of the irreversibilities during that process. It is also used to establish criteria for the performance of engineering devices.
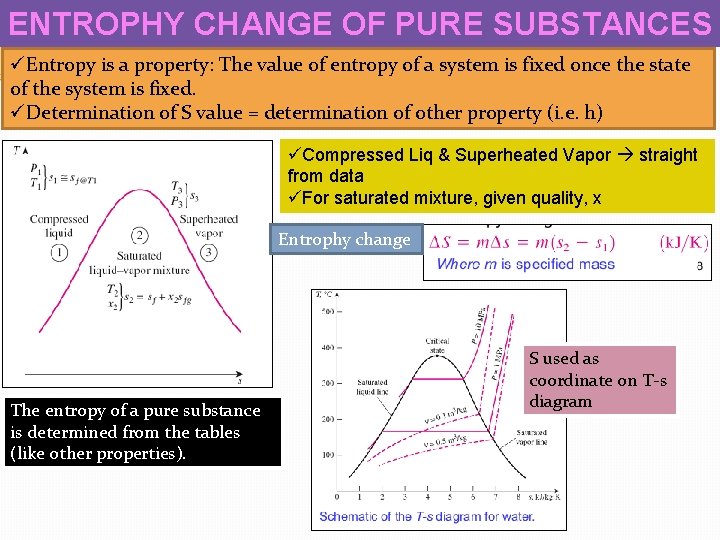
ENTROPHY CHANGE OF PURE SUBSTANCES üEntropy is a property: The value of entropy of a system is fixed once the state of the system is fixed. üDetermination of S value = determination of other property (i. e. h) üCompressed Liq & Superheated Vapor straight from data üFor saturated mixture, given quality, x Entrophy change The entropy of a pure substance is determined from the tables (like other properties). S used as coordinate on T-s diagram
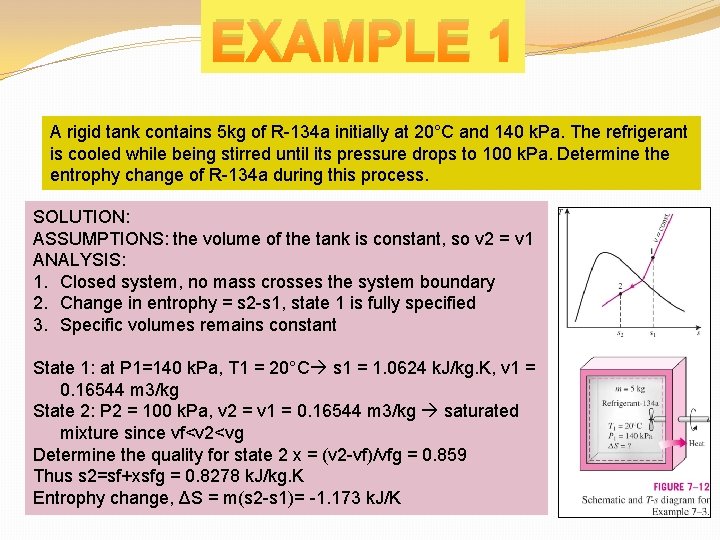
EXAMPLE 1 A rigid tank contains 5 kg of R-134 a initially at 20°C and 140 k. Pa. The refrigerant is cooled while being stirred until its pressure drops to 100 k. Pa. Determine the entrophy change of R-134 a during this process. SOLUTION: ASSUMPTIONS: the volume of the tank is constant, so v 2 = v 1 ANALYSIS: 1. Closed system, no mass crosses the system boundary 2. Change in entrophy = s 2 -s 1, state 1 is fully specified 3. Specific volumes remains constant State 1: at P 1=140 k. Pa, T 1 = 20°C s 1 = 1. 0624 k. J/kg. K, v 1 = 0. 16544 m 3/kg State 2: P 2 = 100 k. Pa, v 2 = v 1 = 0. 16544 m 3/kg saturated mixture since vf<v 2<vg Determine the quality for state 2 x = (v 2 -vf)/vfg = 0. 859 Thus s 2=sf+xsfg = 0. 8278 k. J/kg. K Entrophy change, ΔS = m(s 2 -s 1)= -1. 173 k. J/K
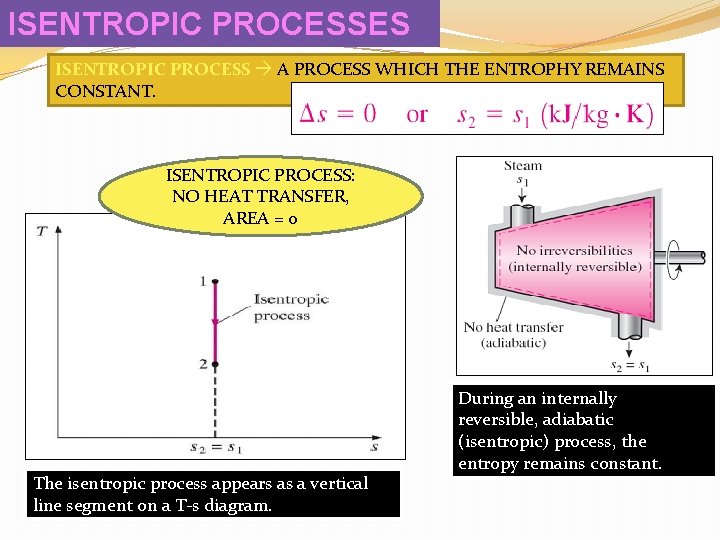
ISENTROPIC PROCESSES ISENTROPIC PROCESS A PROCESS WHICH THE ENTROPHY REMAINS CONSTANT. ISENTROPIC PROCESS: NO HEAT TRANSFER, AREA = 0 The isentropic process appears as a vertical line segment on a T-s diagram. During an internally reversible, adiabatic (isentropic) process, the entropy remains constant.
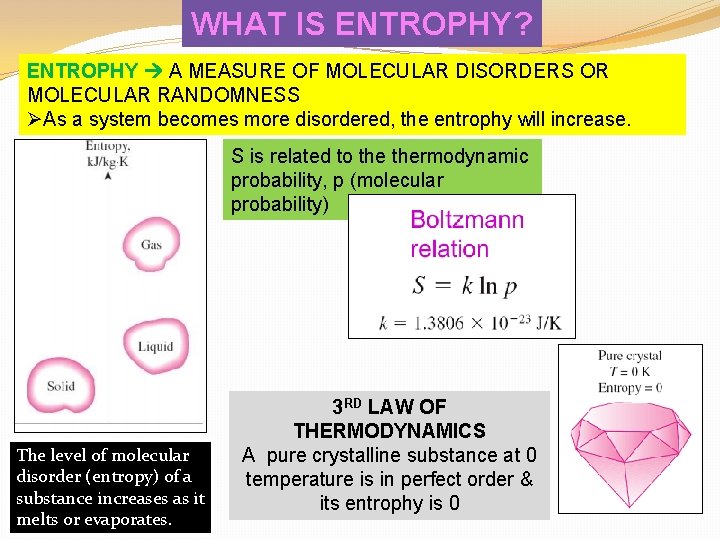
WHAT IS ENTROPHY? ENTROPHY A MEASURE OF MOLECULAR DISORDERS OR MOLECULAR RANDOMNESS ØAs a system becomes more disordered, the entrophy will increase. S is related to thermodynamic probability, p (molecular probability) The level of molecular disorder (entropy) of a substance increases as it melts or evaporates. 3 RD LAW OF THERMODYNAMICS A pure crystalline substance at 0 temperature is in perfect order & its entrophy is 0
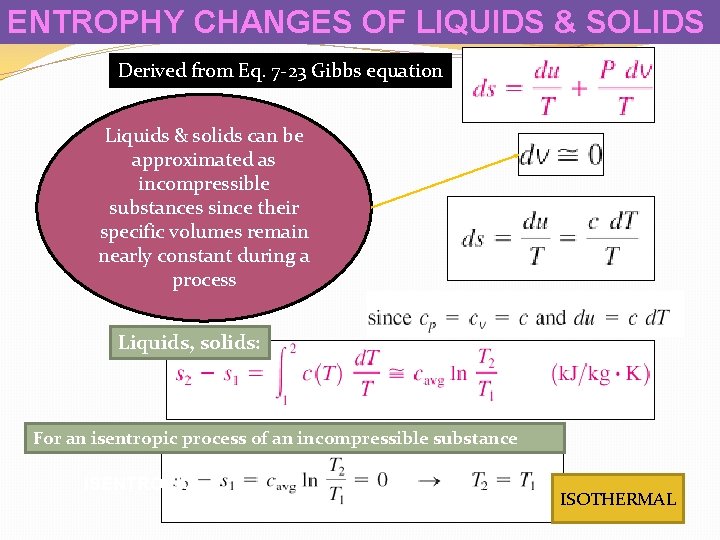
ENTROPHY CHANGES OF LIQUIDS & SOLIDS Derived from Eq. 7 -23 Gibbs equation Liquids & solids can be approximated as incompressible substances since their specific volumes remain nearly constant during a process Liquids, solids: For an isentropic process of an incompressible substance ISENTROPIC ISOTHERMAL
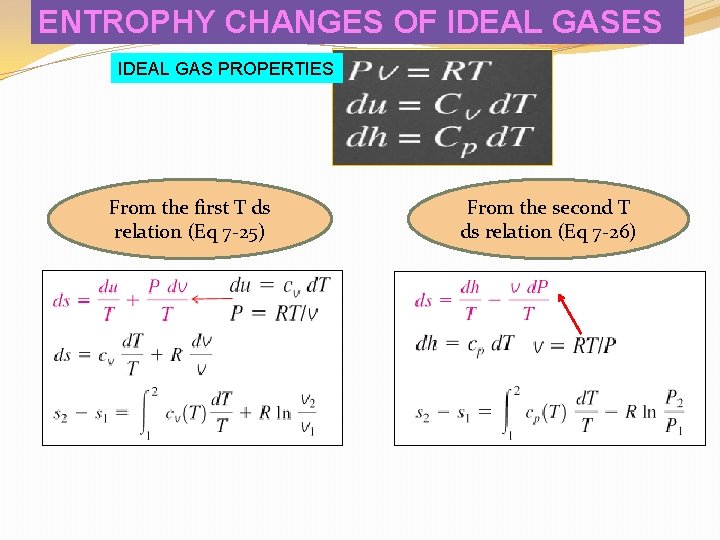
ENTROPHY CHANGES OF IDEAL GASES IDEAL GAS PROPERTIES From the first T ds relation (Eq 7 -25) From the second T ds relation (Eq 7 -26)
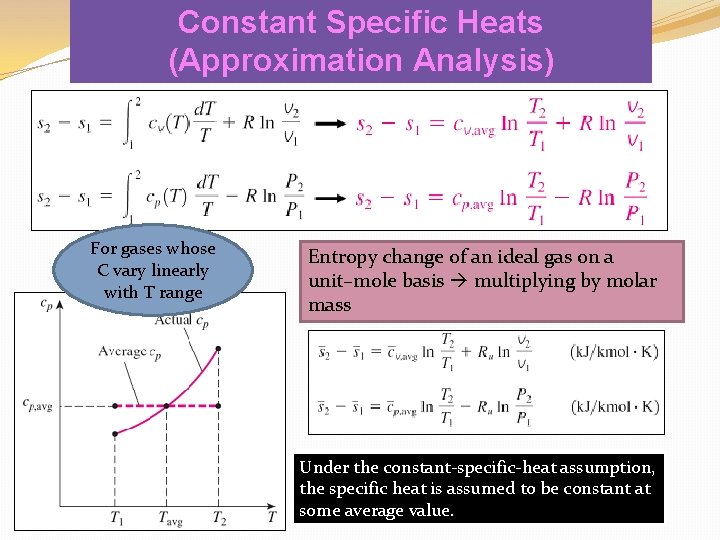
Constant Specific Heats (Approximation Analysis) For gases whose C vary linearly with T range Entropy change of an ideal gas on a unit–mole basis multiplying by molar mass Under the constant-specific-heat assumption, the specific heat is assumed to be constant at some average value.
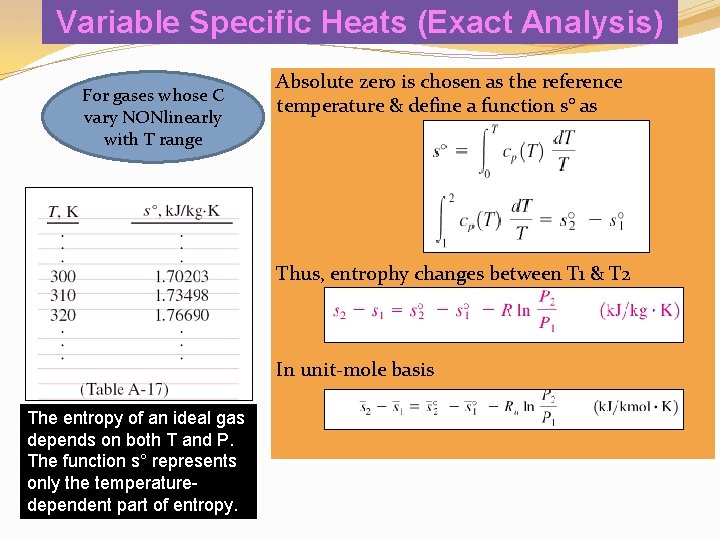
Variable Specific Heats (Exact Analysis) For gases whose C vary NONlinearly with T range Absolute zero is chosen as the reference temperature & define a function s° as Thus, entrophy changes between T 1 & T 2 In unit-mole basis On a unit–mole basis The entropy of an ideal gas depends on both T and P. The function s° represents only the temperaturedependent part of entropy.
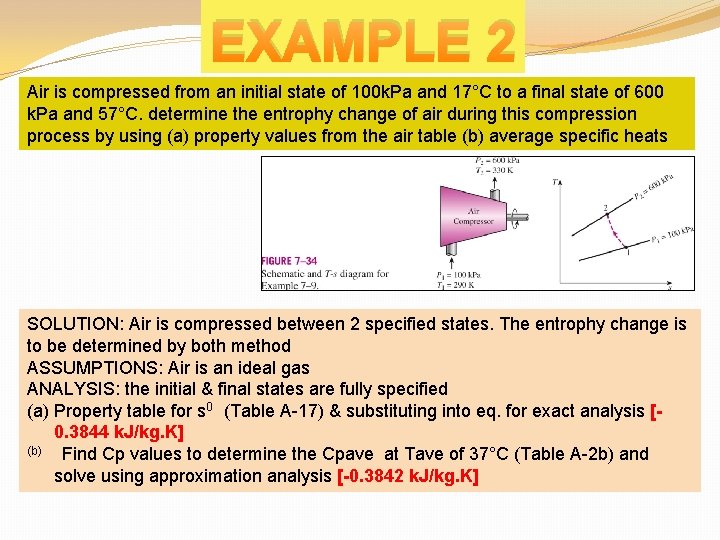
EXAMPLE 2 Air is compressed from an initial state of 100 k. Pa and 17°C to a final state of 600 k. Pa and 57°C. determine the entrophy change of air during this compression process by using (a) property values from the air table (b) average specific heats SOLUTION: Air is compressed between 2 specified states. The entrophy change is to be determined by both method ASSUMPTIONS: Air is an ideal gas ANALYSIS: the initial & final states are fully specified (a) Property table for s 0 (Table A-17) & substituting into eq. for exact analysis [0. 3844 k. J/kg. K] (b) Find Cp values to determine the Cpave at Tave of 37°C (Table A-2 b) and solve using approximation analysis [-0. 3842 k. J/kg. K]
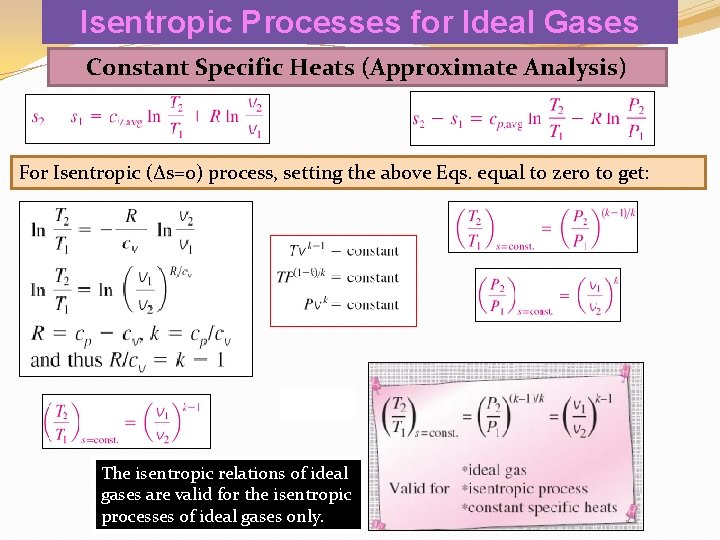
Isentropic Processes for Ideal Gases Constant Specific Heats (Approximate Analysis) For Isentropic (Δs=0) process, setting the above Eqs. equal to zero to get: The isentropic relations of ideal gases are valid for the isentropic processes of ideal gases only.
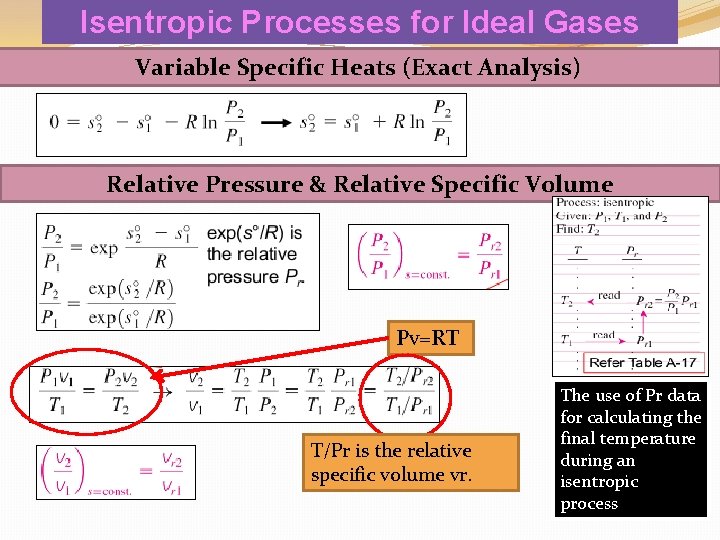
Isentropic Processes for Ideal Gases Variable Specific Heats (Exact Analysis) Relative Pressure & Relative Specific Volume Pv=RT T/Pr is the relative specific volume vr. The use of Pr data for calculating the final temperature during an isentropic process
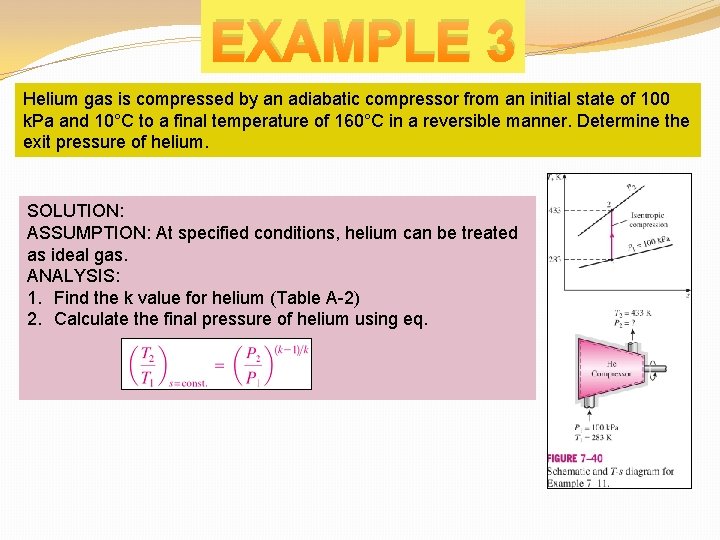
EXAMPLE 3 Helium gas is compressed by an adiabatic compressor from an initial state of 100 k. Pa and 10°C to a final temperature of 160°C in a reversible manner. Determine the exit pressure of helium. SOLUTION: ASSUMPTION: At specified conditions, helium can be treated as ideal gas. ANALYSIS: 1. Find the k value for helium (Table A-2) 2. Calculate the final pressure of helium using eq.
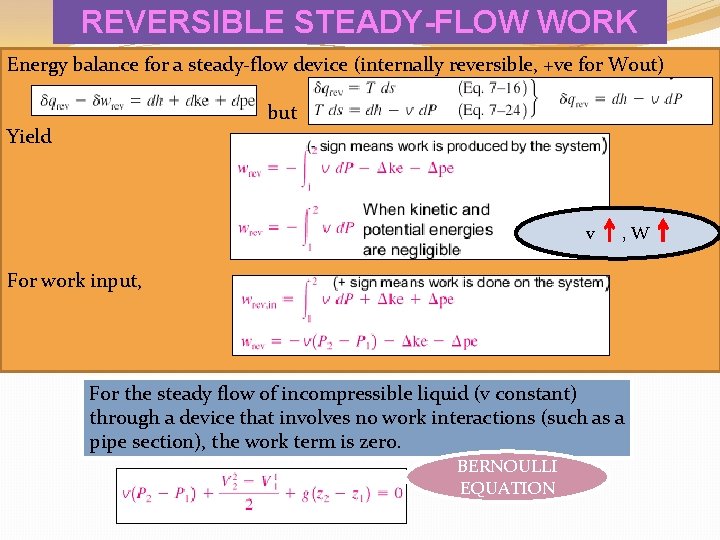
REVERSIBLE STEADY-FLOW WORK Energy balance for a steady-flow device (internally reversible, +ve for Wout) but Yield v , W For work input, For the steady flow of incompressible liquid (v constant) through a device that involves no work interactions (such as a pipe section), the work term is zero. BERNOULLI EQUATION
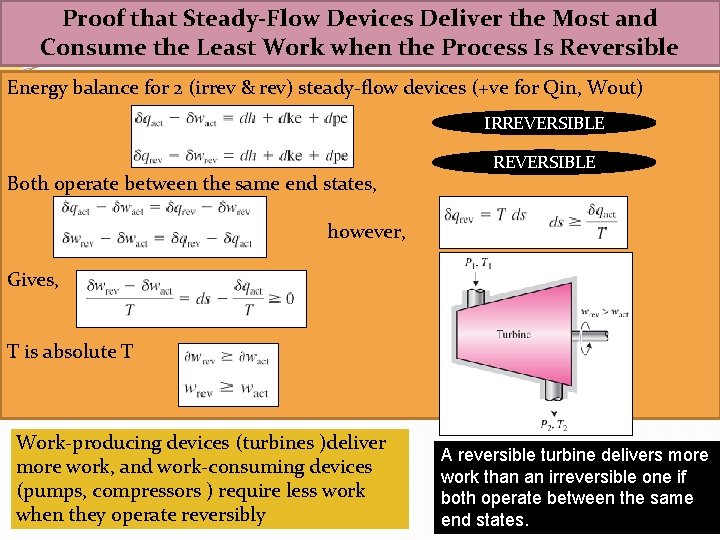
Proof that Steady-Flow Devices Deliver the Most and Consume the Least Work when the Process Is Reversible Energy balance for 2 (irrev & rev) steady-flow devices (+ve for Qin, Wout) IRREVERSIBLE Both operate between the same end states, REVERSIBLE however, Gives, T is absolute T Work-producing devices (turbines )deliver more work, and work-consuming devices (pumps, compressors ) require less work when they operate reversibly A reversible turbine delivers more work than an irreversible one if both operate between the same end states.
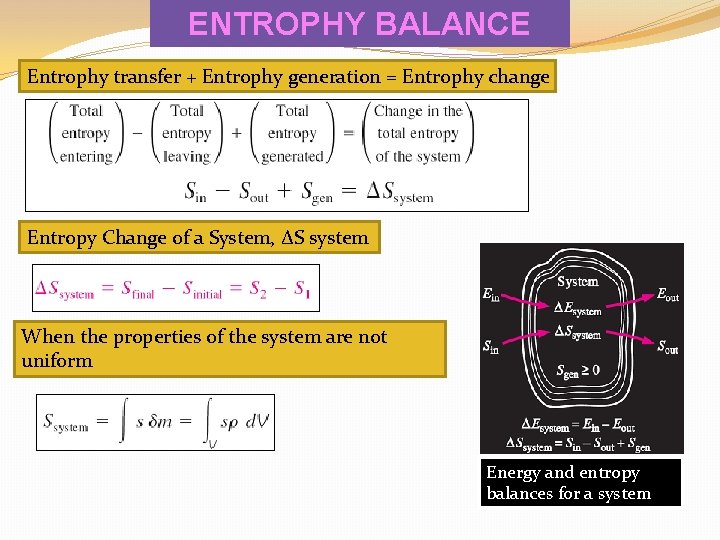
ENTROPHY BALANCE Entrophy transfer + Entrophy generation = Entrophy change Entropy Change of a System, ∆S system When the properties of the system are not uniform Energy and entropy balances for a system
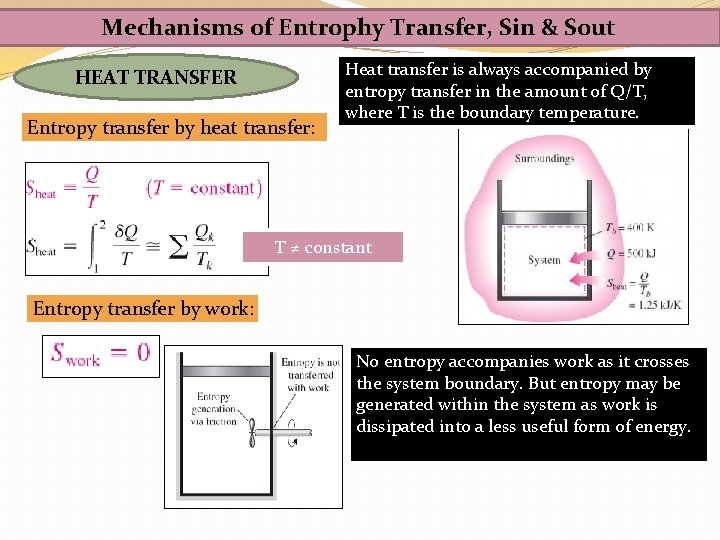
Mechanisms of Entrophy Transfer, Sin & Sout HEAT TRANSFER Entropy transfer by heat transfer: Heat transfer is always accompanied by entropy transfer in the amount of Q/T, where T is the boundary temperature. T ≠ constant Entropy transfer by work: No entropy accompanies work as it crosses the system boundary. But entropy may be generated within the system as work is dissipated into a less useful form of energy.
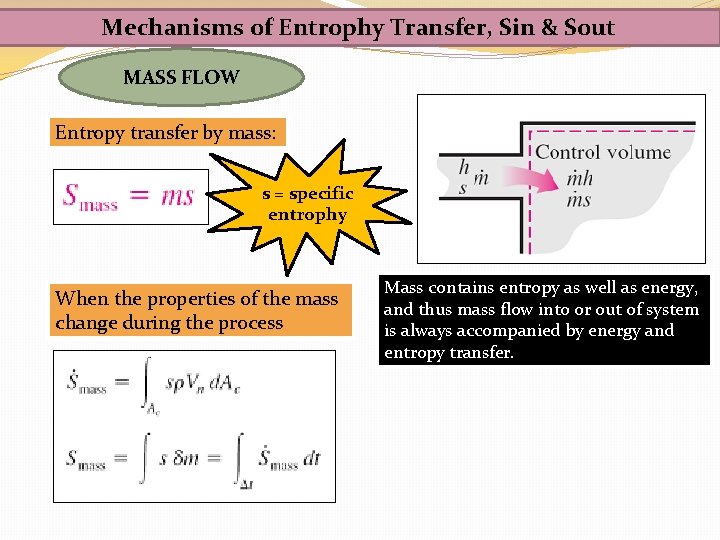
Mechanisms of Entrophy Transfer, Sin & Sout MASS FLOW Entropy transfer by mass: s = specific entrophy When the properties of the mass change during the process Mass contains entropy as well as energy, and thus mass flow into or out of system is always accompanied by energy and entropy transfer.
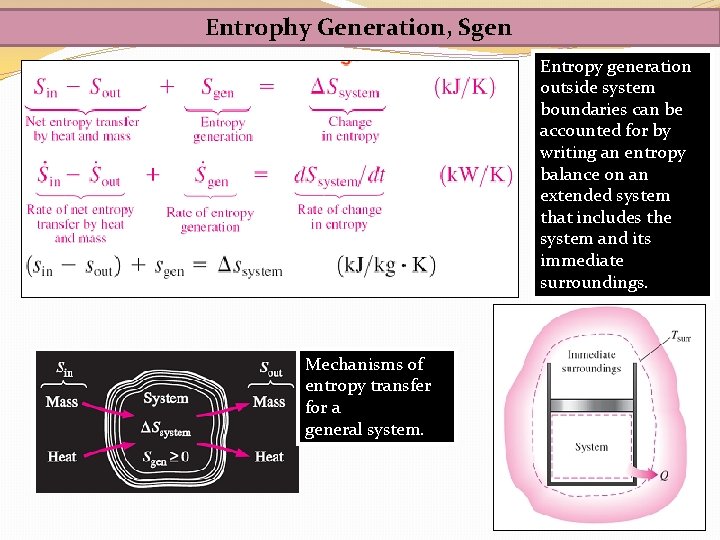
Entrophy Generation, Sgen Entropy generation outside system boundaries can be accounted for by writing an entropy balance on an extended system that includes the system and its immediate surroundings. Mechanisms of entropy transfer for a general system.
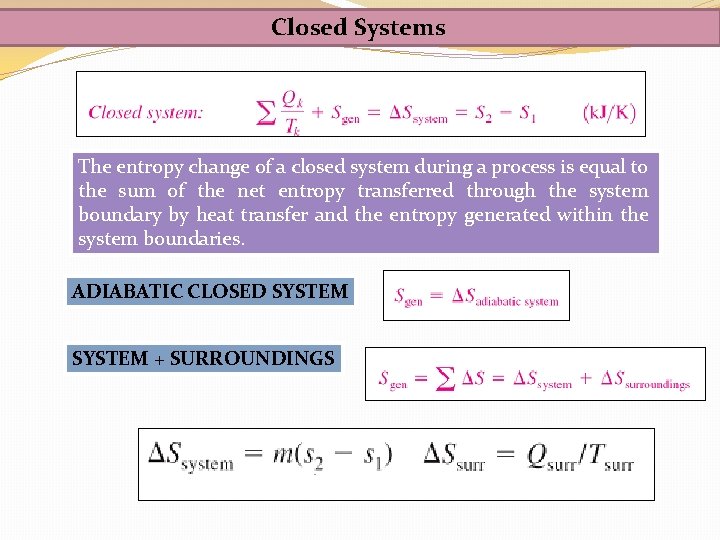
Closed Systems The entropy change of a closed system during a process is equal to the sum of the net entropy transferred through the system boundary by heat transfer and the entropy generated within the system boundaries. ADIABATIC CLOSED SYSTEM + SURROUNDINGS
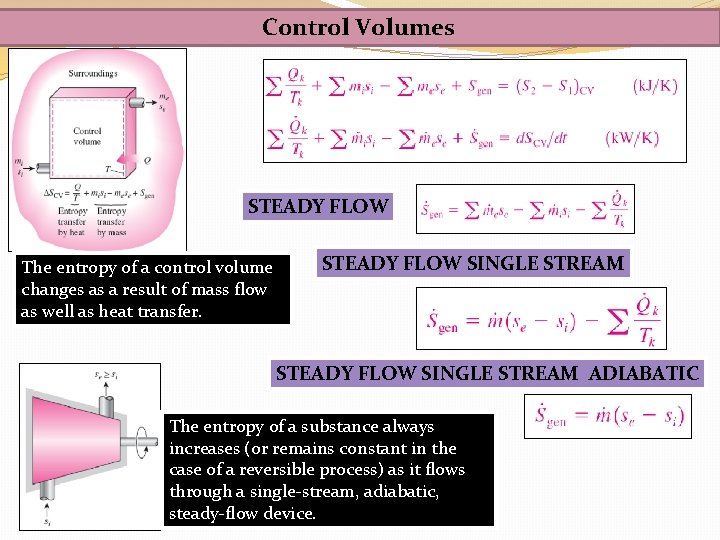
Control Volumes STEADY FLOW The entropy of a control volume changes as a result of mass flow as well as heat transfer. STEADY FLOW SINGLE STREAM ADIABATIC The entropy of a substance always increases (or remains constant in the case of a reversible process) as it flows through a single-stream, adiabatic, steady-flow device.

THANK YOU

TUTORIAL II A horizontal cylinder is separated into two compartments by an adiabatic, frictionless piston. One side contains 0. 2 m 3 of nitrogen and the other side contains 0. 1 kg of helium. . Both initially at 20°C and 95 k. Pa. The sides of the cylinder and the helium end are insulated. Now heat is added to the nitrogen side from a reservoir at 500°C until the pressure of the helium rises to 120 k. Pa. Determine (a) the final temperature of helium, (b) the final volume of the nitrogen, (c) the heat transferred to the nitrogen and (d) the entrophy generation during this process. ANS: (a) 321. 7 K, (b) 0. 2838 m 3, (c) 46. 6287 k. J, (d) 0. 057 k. J/K
- Slides: 28