Chapter 6 The Second Law of Thermodynamics 6





- Slides: 14

Chapter 6 The Second Law of Thermodynamics

6. 1 Introduction The first law of thermodynamics is simple, general, but does not constitute a complete theory because certain processes it permits do not occur in nature!

The problems arise from: 1. Classical thermodynamics is connected with states of equilibrium and various processes connecting them. 2. The exact process by which a system reaches the final state from its initial state is immaterial. i. e. the transition is independent of the particular path taken 3. The theory emphasizes reversible processes! Yet, real processes are irreversible!

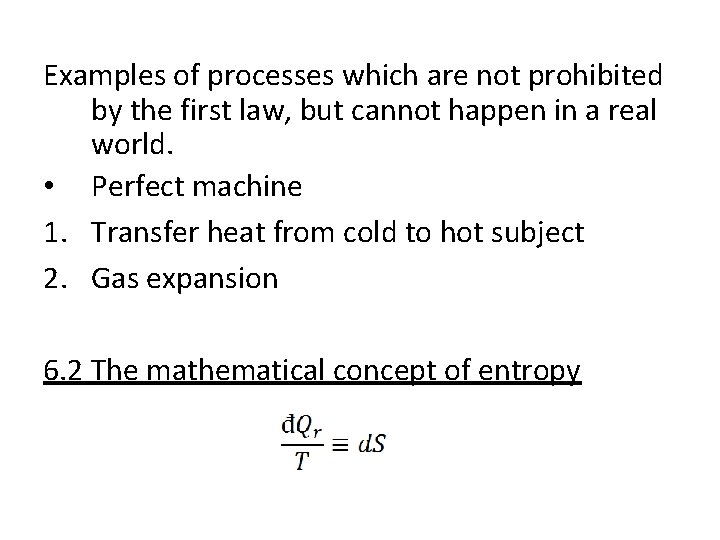
Examples of processes which are not prohibited by the first law, but cannot happen in a real world. • Perfect machine 1. Transfer heat from cold to hot subject 2. Gas expansion 6. 2 The mathematical concept of entropy

The reciprocal of the absolute temperature is an integrating factor that permits the replacement of the inexact differential by the exact differential. The above equation is the Clausius definition of the entropy S. The first law of thermodynamics can be now expressed as for a reversible process

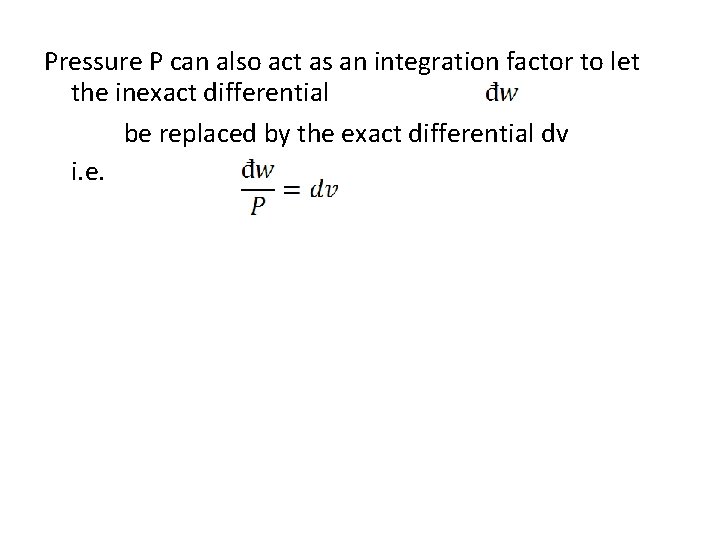
Pressure P can also act as an integration factor to let the inexact differential be replaced by the exact differential dv i. e.

6. 3 Irreversible Processes 1. A battery discharges through a resistor, releasing energy. The reverse process will not occur. 2. Two gases, initially in separated adjoining chambers, will mix uniformly. 3. A free expansion of gas (in Gay-Lussar-Joule experiment) 4. Heat flows from a high temperature body to a low temperature reservoir in the absence of other effect

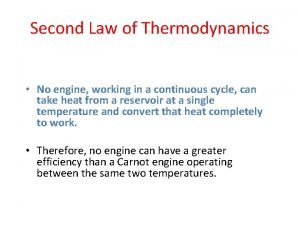
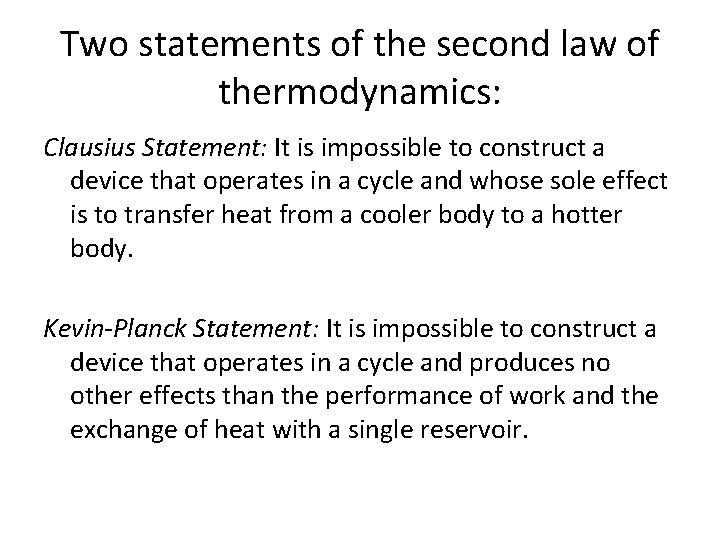
Two statements of the second law of thermodynamics: Clausius Statement: It is impossible to construct a device that operates in a cycle and whose sole effect is to transfer heat from a cooler body to a hotter body. Kevin-Planck Statement: It is impossible to construct a device that operates in a cycle and produces no other effects than the performance of work and the exchange of heat with a single reservoir.

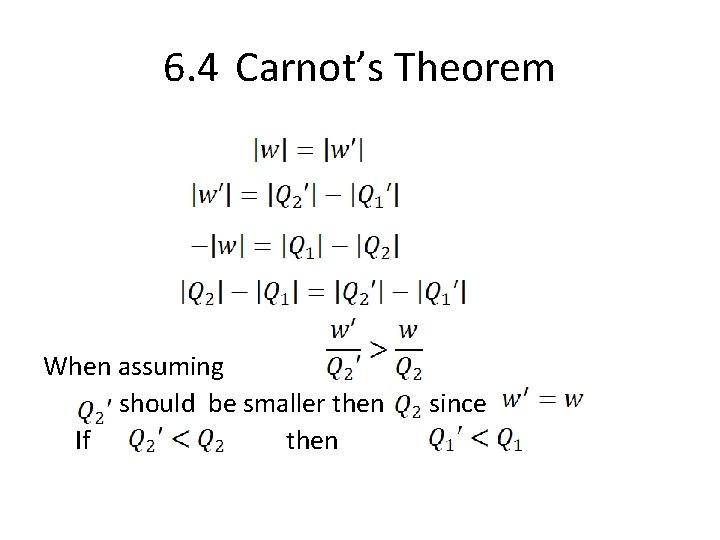
6. 4 Carnot’s Theorem When assuming should be smaller then If then since

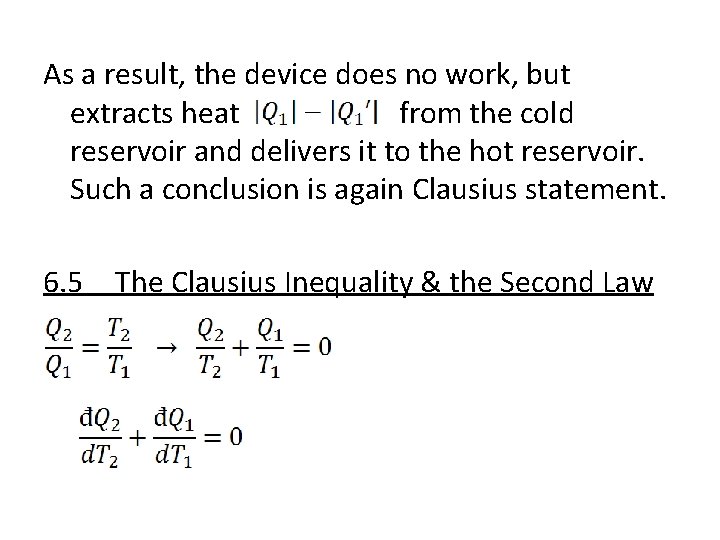
As a result, the device does no work, but extracts heat from the cold reservoir and delivers it to the hot reservoir. Such a conclusion is again Clausius statement. 6. 5 The Clausius Inequality & the Second Law

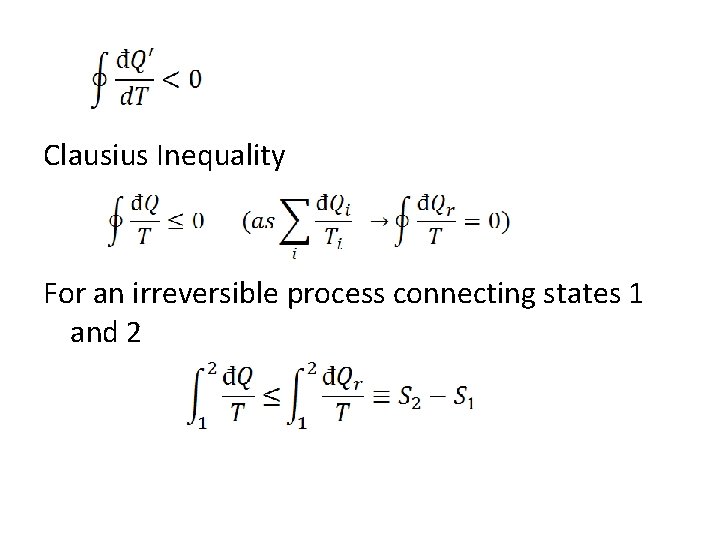
Clausius Inequality For an irreversible process connecting states 1 and 2

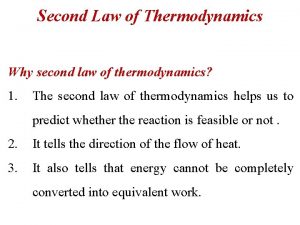
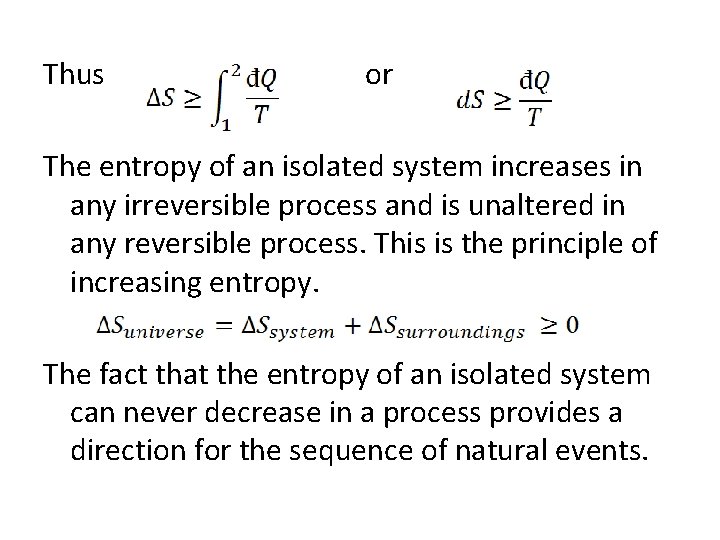
Thus or The entropy of an isolated system increases in any irreversible process and is unaltered in any reversible process. This is the principle of increasing entropy. The fact that the entropy of an isolated system can never decrease in a process provides a direction for the sequence of natural events.

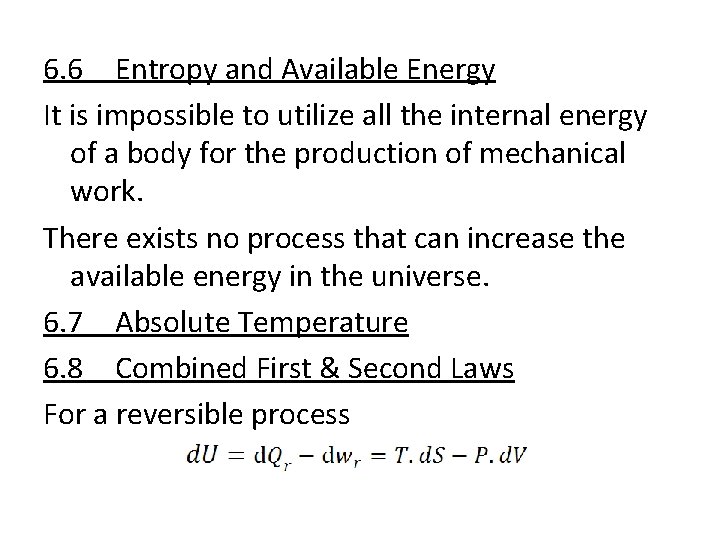
6. 6 Entropy and Available Energy It is impossible to utilize all the internal energy of a body for the production of mechanical work. There exists no process that can increase the available energy in the universe. 6. 7 Absolute Temperature 6. 8 Combined First & Second Laws For a reversible process

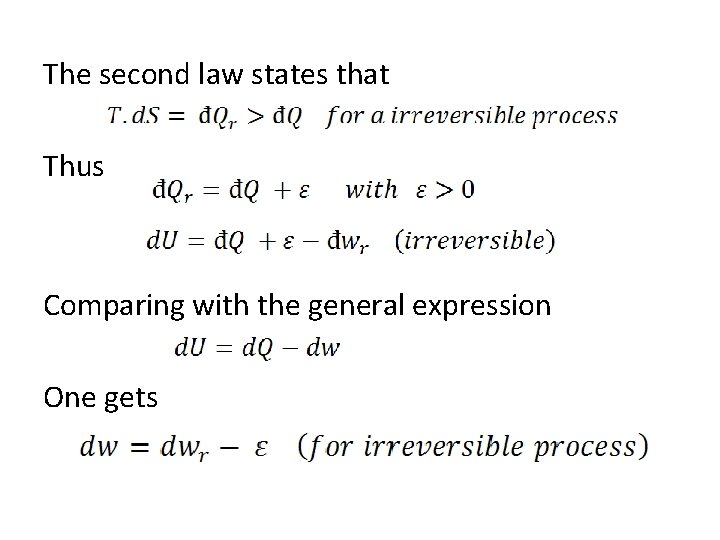
The second law states that Thus Comparing with the general expression One gets
 Kelvin planck statement
Kelvin planck statement Zeroth law of thermodynamics
Zeroth law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law Chapter 2 lesson 3 newton's second law
Chapter 2 lesson 3 newton's second law 27 miles per gallon into kilometers per liter
27 miles per gallon into kilometers per liter First law of thermodynamics equation open system
First law of thermodynamics equation open system Zeroth law of thermodynamics definition
Zeroth law of thermodynamics definition Newton's third law of thermodynamics
Newton's third law of thermodynamics Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement