Thermodynamics part ii Second Law of Thermodynamics Heat



- Slides: 12

Thermodynamics part ii

Second Law of Thermodynamics Heat flows in Heat can be made to flow the other way, but i. e. air conditioners

Change completely into is ! i. e. rub hands, push object across floor Changing heat Converting some into work is is possible

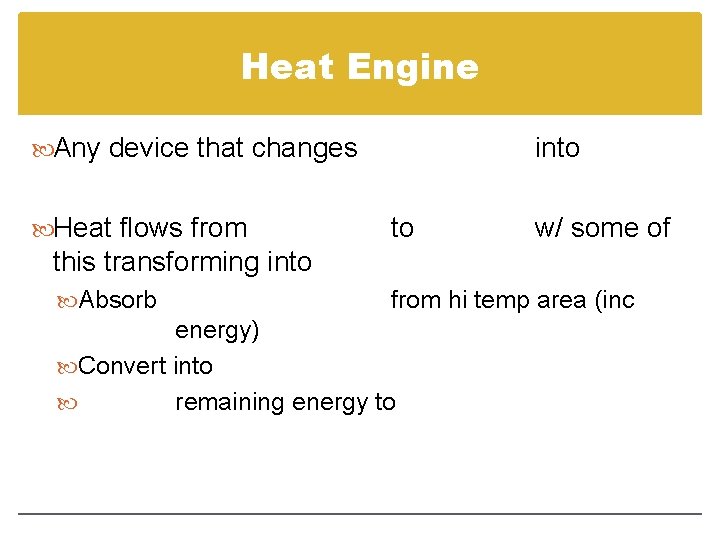
Heat Engine Any device that changes Heat flows from into to w/ some of this transforming into Absorb from hi temp area (inc energy) Convert into remaining energy to

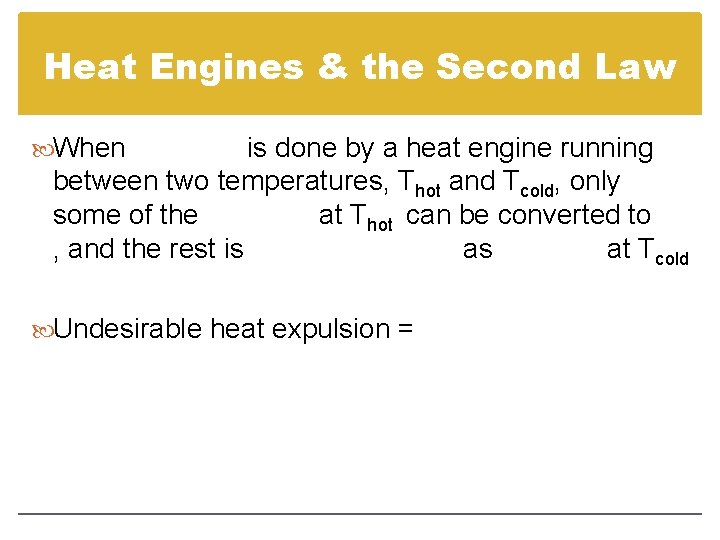
Heat Engines & the Second Law When is done by a heat engine running between two temperatures, Thot and Tcold, only some of the at Thot can be converted to , and the rest is as at Tcold Undesirable heat expulsion =

Carnot Efficiency Upper fraction of heat that can be converted to useful work depends on Ideal efficiency = Depends only on temperature difference between Temperature is expressed in

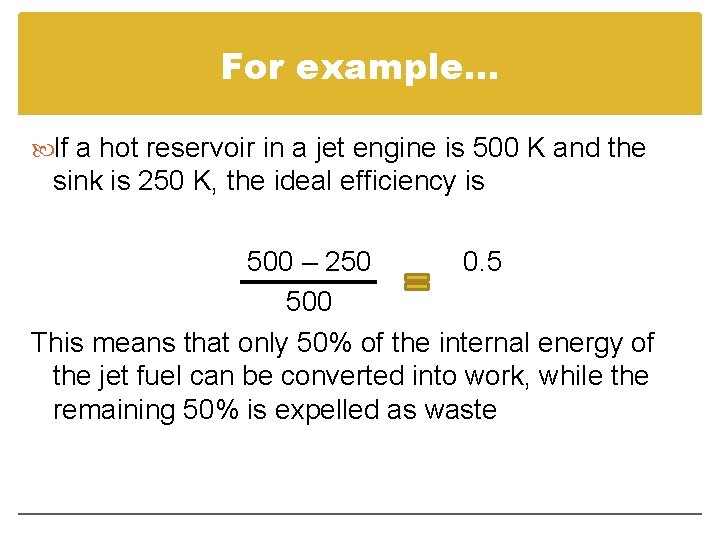
For example… If a hot reservoir in a jet engine is 500 K and the sink is 250 K, the ideal efficiency is 500 – 250 0. 5 500 This means that only 50% of the internal energy of the jet fuel can be converted into work, while the remaining 50% is expelled as waste

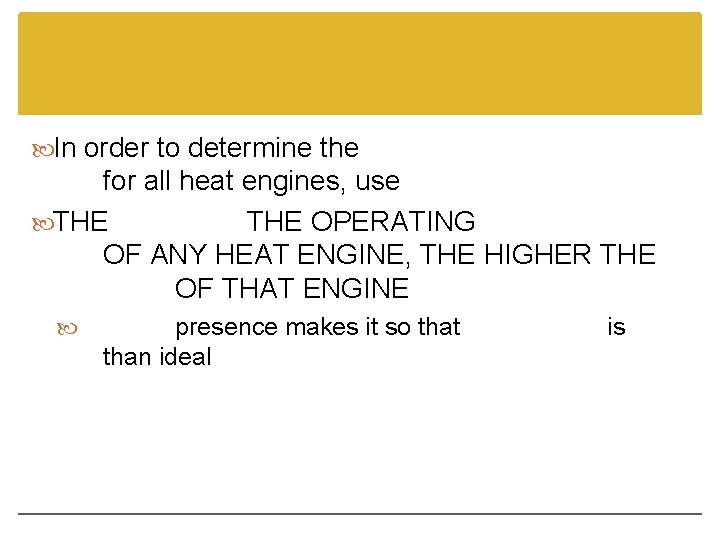
In order to determine the for all heat engines, use THE OPERATING OF ANY HEAT ENGINE, THE HIGHER THE OF THAT ENGINE presence makes it so that than ideal is

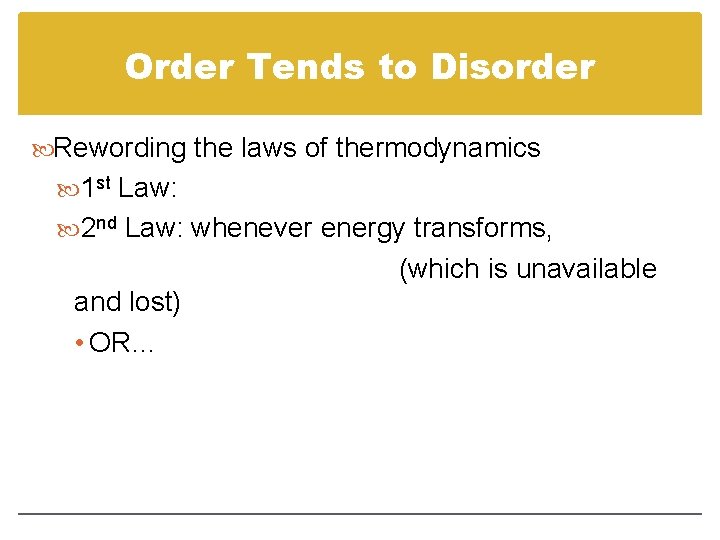
Order Tends to Disorder Rewording the laws of thermodynamics 1 st Law: 2 nd Law: whenever energy transforms, (which is unavailable and lost) • OR…

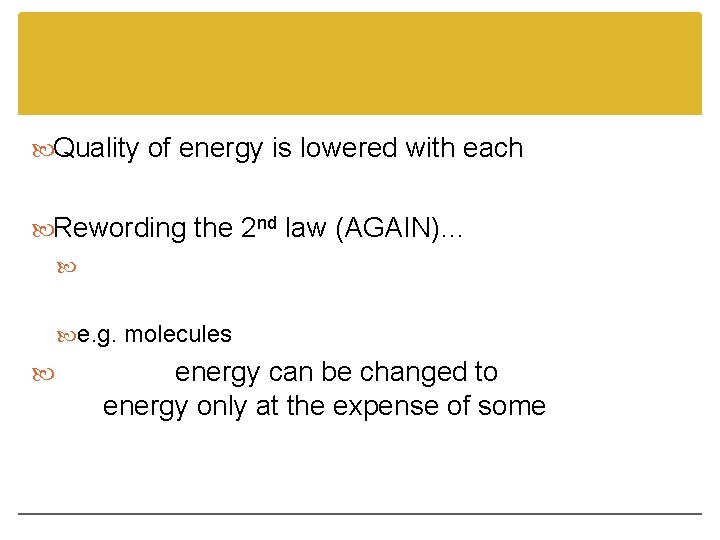
Quality of energy is lowered with each Rewording the 2 nd law (AGAIN)… e. g. molecules energy can be changed to energy only at the expense of some

Entropy The measure of the amount of Entropy w/ in normal physical systems • Humans us/ take from the environment to increase internal organization (decrease of entropy) • Life forms + waste products = net increase in entropy

In Class (finish for homework) RQ#21, 23 -25 P/C#2, 4 T/E#2, 4, 5, 8 -10 CD 23 -1, 23 -2, 24 -1
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Kelvin statement
Kelvin statement State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Equation of second law of thermodynamics
Equation of second law of thermodynamics Quiz 8-3 law of sines and law of cosines
Quiz 8-3 law of sines and law of cosines 186 282 miles per second into meters per second
186 282 miles per second into meters per second Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law Constant in avogadro's law
Constant in avogadro's law