Epidemiology Gastric cancer was the fourth most common














































- Slides: 65

Epidemiology Gastric cancer was the fourth most common cancer in the world in 2004, and is expected to remain fourth in 2005. Worldwide there are 930, 000 new cases and 700, 000 deaths per year. Sixty percent of new cases occur in developing countries. There is tremendous geographic variation, with the highest death rates in Chile, the former Soviet Union, China, and Japan.

Epidemiology In the United States gastric cancer is the 15 th most common cancer, with 21, 860 new cases expected this year, and 11, 550 deaths. The incidence of gastric cancer has declined significantly worldwide in the last century, with a marked decline in the US since the 1930 s.



Epidemiology In New York State there were an average of 1955 cases annually between 1998 -2002, with 1070 deaths. Male to female ratio of 2: 1 in the US; 3: 2 in New York. Median age at diagnosis is 65 years (40 -70). Incidence increases with age, peaking in the 7 th decade.

Risk Factors Low fat or protein consumption Salted meat or fish High nitrate consumption High complex carbohydrate consumption

Risk Factors Environment Poor food preparation (smoked/salted) Lack of refridgeration Poor drinking water (well water) Smoking

Risk Factors Social Low social class (except in Japan) Medical Prior gastric surgery H. pylori infection Gastric atrophy and gastritis Adenomatous polyps Male gender

Risk Factors Helicobacter pylori Presence of Ig. G to H. pylori in a given population correlates with local incidence and mortality from gastric cancer. Different strains elicit different antibody responses. The cag. A strain causes more mucosal inflammation and thus a higher risk of gastric cancer than cag. A-negative strains.

Risk Factors Adenomatous polyps 10 -20% risk of developing cancer, especially in lesions greater than 2 cm. Multiple lesions increase the risk of developing cancer. Presence of polyps increase the chance of developing cancer in the remainder of mucosa. Endoscopic surveillance is required after removal of polyps.

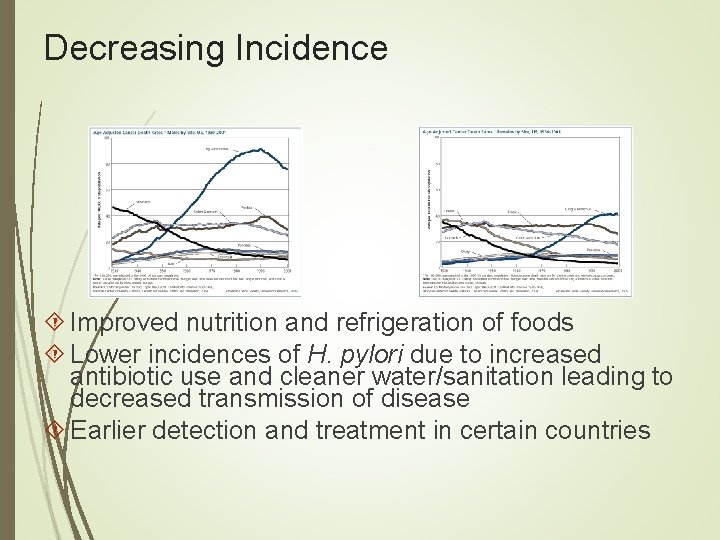
Decreasing Incidence Improved nutrition and refrigeration of foods Lower incidences of H. pylori due to increased antibiotic use and cleaner water/sanitation leading to decreased transmission of disease Earlier detection and treatment in certain countries

Anatomy Most of the blood supply to the stomach is from the celiac artery. Four main arteries: Left and right gastric along the lesser curvature Left and right gastroepiploic along the greater curvature. Blood supply to the proximal stomach also comes from the inferior phrenic and short gastric arteries

Anatomy Occasionally (15 -20%) an aberrant left hepatic artery arises from the left gastric – a concern if the left gastric needs to be divided. The extensive anastomotic connections between these arteries allow, in most cases, three of the four vessels to be ligated as long as the arcades between the curvatures are not disturbed.


Anatomy Venous drainage parallels the arterial supply Left and right gastric veins drain into the portal vein Right gastroepiploic drains into the SMV Left gastroepiploic drains into the splenic vein

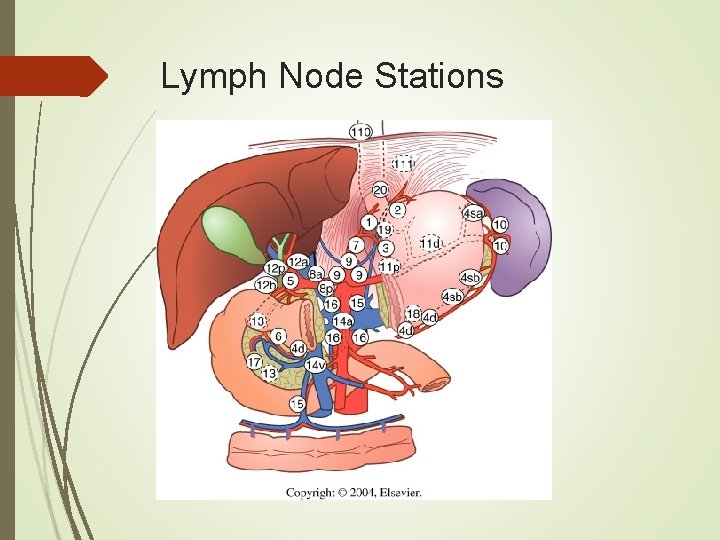
Anatomy Lymphatic drainage is into four zones: Superior gastric Suprapyloric Pancreaticolienal Inferior gastric/subpyloric All four drain into the celiac group of nodes and into the thoracic duct. Gastric cancers drain into any of these groups regardless of location of the tumor.


Anatomy Innervation: Parasympathetic via the vagus. Left anterior and right posterior. Sympathetic via the celiac plexus.


Anatomy Stomach has five layers: Mucosa Epithelium, lamina propria, and muscularis mucosae* Submucosa Smooth muscle layer Subserosa Serosa


Clinical Presentation Symptoms are often absent in early stages, and when present are often ignored, missed, or mistaken for another disease process. Vague discomfort and/or indigestion Epigastric pain that is constant, non-radiating, and unrelieved by food ingestion. Proximal tumors may present with dysphagia. Antral tumors may present with outlet obstruction.

Clinical Presentation Diffuse mural disease may present with early satiety due to decreased distensibility. Up to 15% of patients develop hematemesis and 40% are anemic at presentation.

Clinical Presentation Unfortunately most patients present in later stages of disease, with evidence of metastatic or locally advanced tumor. Palpable abdominal mass, ovarian mass, supraclavicular or periumbilical lymph nodes. Obstruction from tumor invasion into transverse colon. Hepatomegaly, jaundice, ascites, and cachexia.

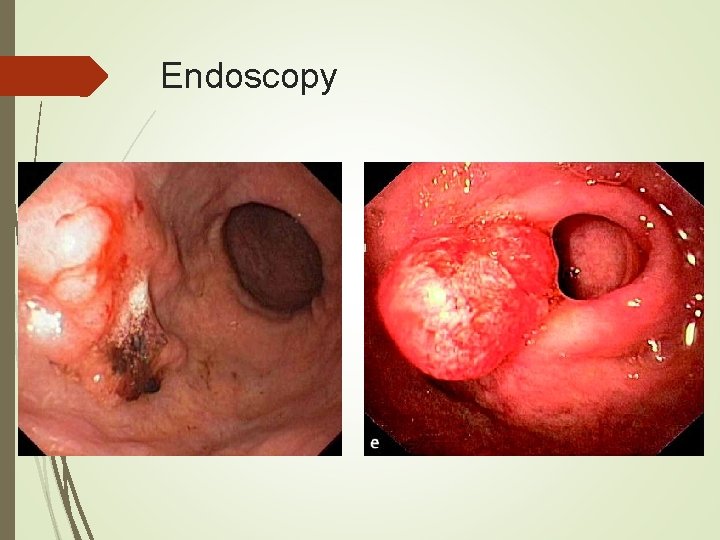
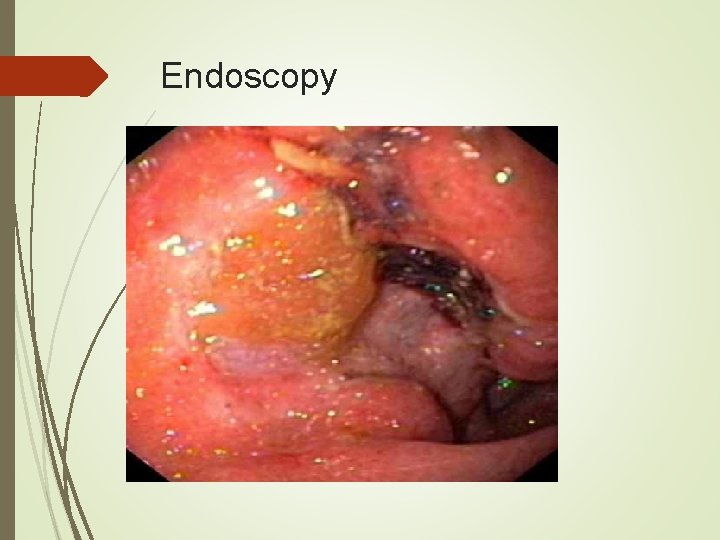
Diagnosis Endoscopy is the diagnostic method of choice. With multiple biopsies (seven or more) the diagnostic accuracy approaches 98%. Cytologic brushings can also be obtained. Size, morphology, and location of tumor can be documented, as well as any other mucosal abnormalities.

Endoscopy

Endoscopy

Diagnosis Double contrast barium swallow has 90% accuracy and is cost effective. No ability to distinguish between malignant and benign ulcers.

Diagnosis Endoscopic Ultrasound (EUS) is a newer modality that is being used in some center to help stage the tumor. Extent of wall invasion and lymph node involvement can be assessed. Overall accuracy is 75%. Poor for T 2 tumors (38%) Better for T 1 (80%) and T 3 (90%) Remains operator dependent.


Preoperative Workup Once diagnosis of gastric cancer has been made, CT scan is useful for evaluation of any distant disease. Limited in detecting early primary and small (<5 mm) metastatic tumors. Accuracy of lymph node staging ranges from 25 to 86%. If CT scan is negative, then laparoscopy is recommended as the next step in evaluation.

Preoperative Workup Laparoscopy detected metastatic disease in 23 to 37% of patients deemed eligible for curative resection by CT scan. Laparoscopy improves palliation in these patients by avoiding unnecessary laparotomy in about one fourth of patients presumed to have local disease on CT scan.






Treatment Surgical resection remains the mainstay of treatment and is the only curative option. More recently pre- and post-chemoradiation therapy has been scrutinized to see if there is any benefit to survival. The issue of extent of resection appears to have been settled. As long as adequate tumor margins are achieved, subtotal gastrectomy has the same survival as total, with decreased morbidity.

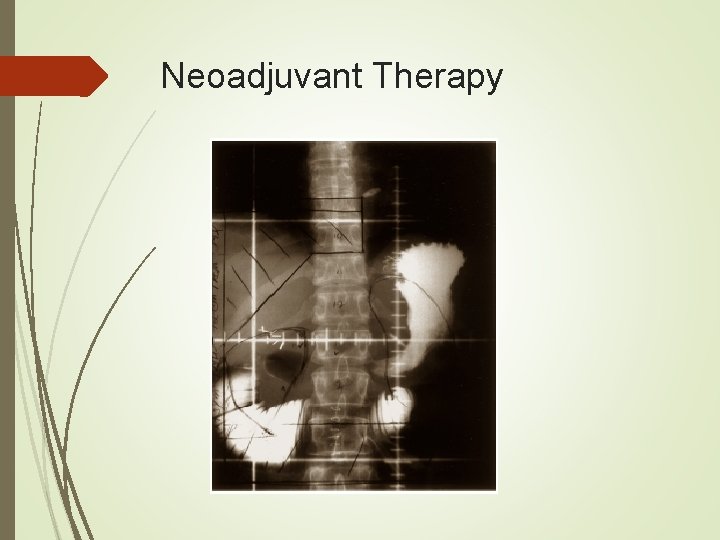
Neoadjuvant Therapy Radiation alone 1970’s in Russia 152 patients were randomly assigned to surgery alone or preop radiation with 20 Gy a week prior to surgery. Five year survival rates were 30% and 39% respectively. In 1998 a Chinese group reported a prospective series of 370 patients who underwent surgery only or had 40 Gy preop radiation. Five year survival was 19. 8% vs 30. 1% with radiation. Resectability and radical resection rates were also improved.

Neoadjuvant Therapy Radiation alone In both studies reported perioperative mortality and anastamotic leak rates were not significantly different. Further studies in radiation alone were largely abandoned in favor of studies including chemotherapy.

Neoadjuvant Therapy

Neoadjuvant Therapy Chemotherapy alone A randomized Netherlands study (DGCT) was unable to show any difference with preop chemotherapy. This may be in part due to the regimen used – FAMTX (FU, doxyrubicin, methotrexate). In the U. K. the MAGIC trial using ECF (epirubicin, cisplatin, FU) has shown promising preliminary results, with 10% more resectable cases and improved disease-free survival.

Neoadjuvant Therapy Combined chemoradiation therapy Has shown a beneficial impact on surgical outcomes in esophageal and rectal cancers, making it an attractive approach for gastric cancer as well. The M. D. Anderson Cancer Center reported several studies, one in 2004 where patients who underwent preop chemoradiotherapy – FU, leucovorin, cisplatin, and 45 Gy in 25 fractions over 5 weeks – achieved pathological complete and partial response in 64% of all operated patients.

Neoadjuvant Therapy Chemoradiation therapy These patients showed a significantly longer median survival of 64 months in comparison to 13 months in patients who did not reach complete or partial response. Further clinical trials are warranted to further show any benefit of neoadjuvant chemoradiation.

Surgical Treatment Aggressive resection of gastric cancer is justified in the absence of distant metastatic spread. The surgery is tailored mainly to the location of the tumor and known pattern of spread. R 0 resection should be achieved, with a minimum of 6 cm margins from gross tumor. R 0 – tumor free margins R 1 – microscopic disease R 2 – gross tumor at margins Minimum of 15 nodes should be removed.

Surgical Treatment Tumors in the cardia and proximal stomach account for 3550% of gastric adenocarcinomas. For these tumors a total gastrectomy should be performed, as opposed to proximal gastric resection which is associated with higher morbidity and mortality rates. Distal tumors may be removed by distal gastrectomy as long as adequate margins are achieved.

Surgical Treatment The extent of lymphadenectomy remains controversial. The JGCA classifies the lymph node basins into 16 basins, and are grouped according to the location of the primary tumor as either D 1, D 2, or D 3 nodes. In general: D 1 – removal of group 1 nodes along the lesser and greater curvature. D 2 – D 1 plus group 2 nodes along the left gastric, common hepatic, celiac, and splenic arteries. D 3 – D 2 plus para-aortic and distal lymph nodes

Lymph Node Stations

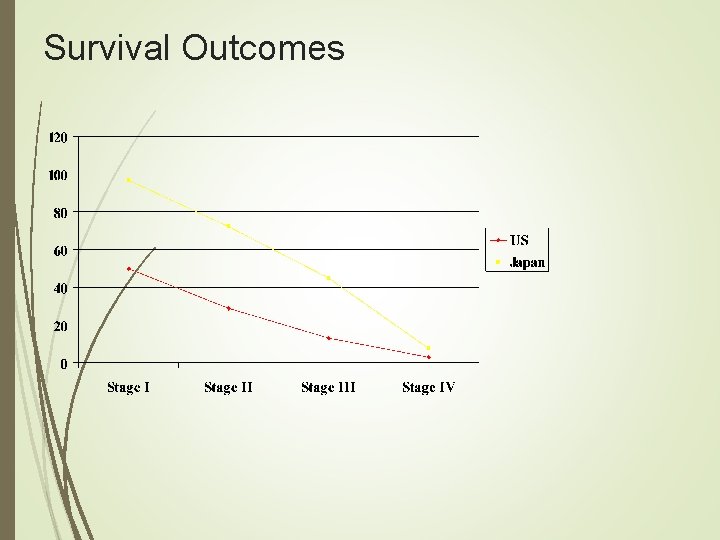
Surgical Treatment A 1993 survey by the ACS showed a 77. 1% resection rate in 18, 365 patients, with a postoperative mortality rate of 7. 2% and 5 -year survival rate of 19%. Of these only 4. 7% were D 2 dissections. In comparison, the Japanese routinely perform D 2 dissections, with 5 -year survival rates above 50%. Although earlier detection accounts for much of the survival benefit, when comparing cancers in the same stage, the Japanese continue to have improved survival.

Survival Outcomes

Surgical Treatment Based on this and other retrospective data, four randomized studies comparing D 1 to D 2 dissections have been conducted. All four trials, including two large ones from the Netherlands and Britain all show the same data; that D 2 dissection significantly increases morbidity and mortality without any significant increase in survival.

Surgical Treatment Splenectomy and pancreatectomy were found to be important risk factors for morbidity and mortality after D 2 dissection. In the DGCT trial a subgroup analysis of patients who underwent D 2 without splenectomy and/or pancreatectomy had a significantly improved survival benefit. A randomized British trial also supported these findings in stage II and III disease.

Surgical Treatment Based on these findings, many groups are recommending “over-D 1” lymphadenectomy for gastric cancers in Western society. The large difference between the Japanese results and Western results remains largely an enigma.

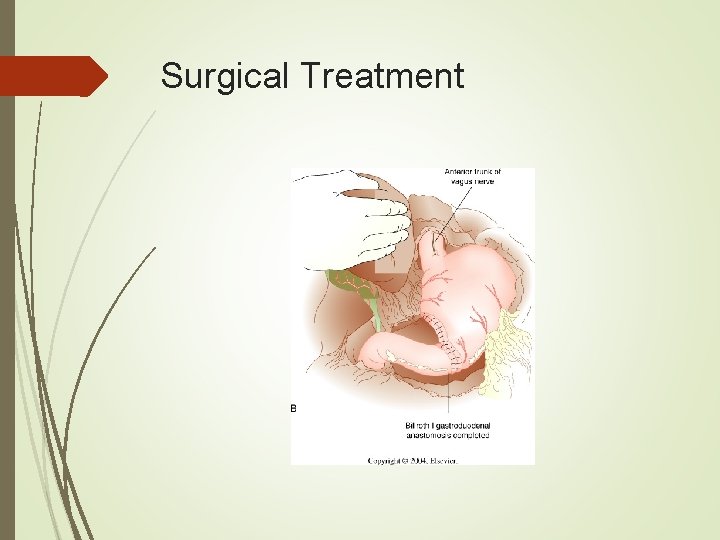
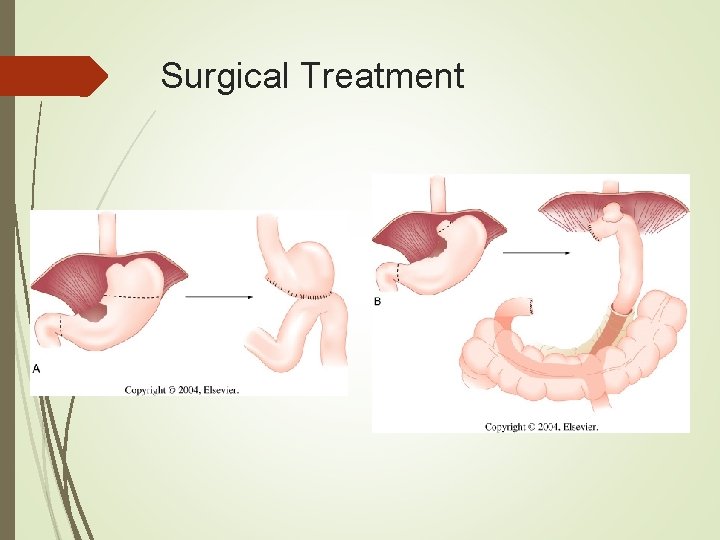
Surgical Treatment Choice of reanastamosis depends on extent of resection. Very distal gastrectomies may be reanastamosed via a Billroth I, II, or Roux-en-Y. Subtotal gastrectomies will require a Billroth II or Roux-en-Y. Total gastrectomies are best served with a Roux-en-Y anastamosis.

Surgical Treatment

Surgical Treatment

Surgical Treatment In the U. S. 20 to 30% of patients present with stage IV disease. Palliative treatment should be geared toward relief of symptoms with minimal morbidity, usually non-operative. Laser recanulization and endoscopic dilatation with or without stent placement has shown success in relieving outlet obstruction.

Adjuvant Therapy A 1999 review of the National Cancer Database reported that only 29% of patients undergoing gastrectomy for cancer had some form of adjuvant therapy. This shows the lack of convincing data up to that point that adjuvant therapy increase survival in gastric cancer.

Adjuvant Therapy In 2001 the Southwest Oncology Group trial was published, showing for the first time in a large prospective randomized trial a survival benefit for patients undergoing gastrectomy for cancer. Median survival was 27 months in the surgery only group, and 36 months after chemoradiotherapy.

Adjuvant Therapy Survival was improved only in the D 0 and D 1 groups. Details on late toxicity have yet to be followed up on and reported. Radiation toxicity had been improved with the use of IMRT (intensity modulated RT), especially renal toxicity.

Adjuvant Therapy

Outcomes What can you expect? Patients who have undergone a potentially curative resection have an average 5 -year survival of 24 to 57%. More useful survival rates are stratified by stage of disease.


Outcomes Recurrence rates remain high, from 40 to 80% depending on the series being quoted. Locoregional failure rate 38 to 45%, with most recurrence in the gastric remnant at the anastamosis, gastric bed, and lymph nodes. Surveillance is important. Patients should be followed every 4 months for the first year, then 6 months for 2 more years. Yearly endoscopy should be performed for subtotal gastrectomies.

Choice of Operation Open gastrectomy with lymph node dissection – at least D 1 – is the current operative standard. Laparoscopic gastrectomy has been shown to be safe with similar survival for patients with distal cancer. Learning curve needs to be overcome, which may be difficult with the decreasing number of gastric cancer cases in the U. S.
 Carcinoid tumor metastatic liver prognosis
Carcinoid tumor metastatic liver prognosis Borrmann classification of gastric cancer
Borrmann classification of gastric cancer How to avoid gastric cancer
How to avoid gastric cancer Ukuran asosiasi
Ukuran asosiasi Nutrition epidemiology definition
Nutrition epidemiology definition Logistic regression epidemiology
Logistic regression epidemiology Point and period prevalence
Point and period prevalence Descriptive vs analytic epidemiology examples
Descriptive vs analytic epidemiology examples Attack rate formula
Attack rate formula Difference between descriptive and analytical epidemiology
Difference between descriptive and analytical epidemiology Descriptive epidemiology
Descriptive epidemiology Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology Pros and cons of cross sectional study
Pros and cons of cross sectional study Recall bias example
Recall bias example Attack rate epidemiology formula
Attack rate epidemiology formula Ramboman analysis
Ramboman analysis Epidemiological wheel
Epidemiological wheel Epidemiology defination
Epidemiology defination Defination of epidemiology
Defination of epidemiology Cholera 1817
Cholera 1817 What is descriptive study in epidemiology
What is descriptive study in epidemiology Spurious association in epidemiology
Spurious association in epidemiology Field epidemiology ppt
Field epidemiology ppt Aims of epidemiology
Aims of epidemiology Certification board of infection control and epidemiology
Certification board of infection control and epidemiology Gordon epidemiology
Gordon epidemiology Epidemiology kept simple
Epidemiology kept simple Diabetic ketoacidosis epidemiology
Diabetic ketoacidosis epidemiology Distribution in epidemiology
Distribution in epidemiology Effect modification epidemiology
Effect modification epidemiology Distribution in epidemiology
Distribution in epidemiology Ramboman
Ramboman Define epidemiology
Define epidemiology Period prevalence formula
Period prevalence formula How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor Nutritional epidemiology
Nutritional epidemiology What is ookinite
What is ookinite Secondary attack rate formula
Secondary attack rate formula How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor Epidemiology definition
Epidemiology definition Seven uses of epidemiology
Seven uses of epidemiology Epidemiology triad
Epidemiology triad John snow epidemiology
John snow epidemiology Epidemiology made easy
Epidemiology made easy What is the greatest common factor of 18, 36, and 90?
What is the greatest common factor of 18, 36, and 90? Common anode and common cathode
Common anode and common cathode 56 prime factorization
56 prime factorization What are the factors for 54
What are the factors for 54 What is the least common multiple of 18 and 27
What is the least common multiple of 18 and 27 Highest common factors and lowest common multiples
Highest common factors and lowest common multiples Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết