POISION DECONTAMINATION METHODS 2 GASTRIC LAVAGE Gastric lavage














- Slides: 18

POISION DECONTAMINATION METHODS

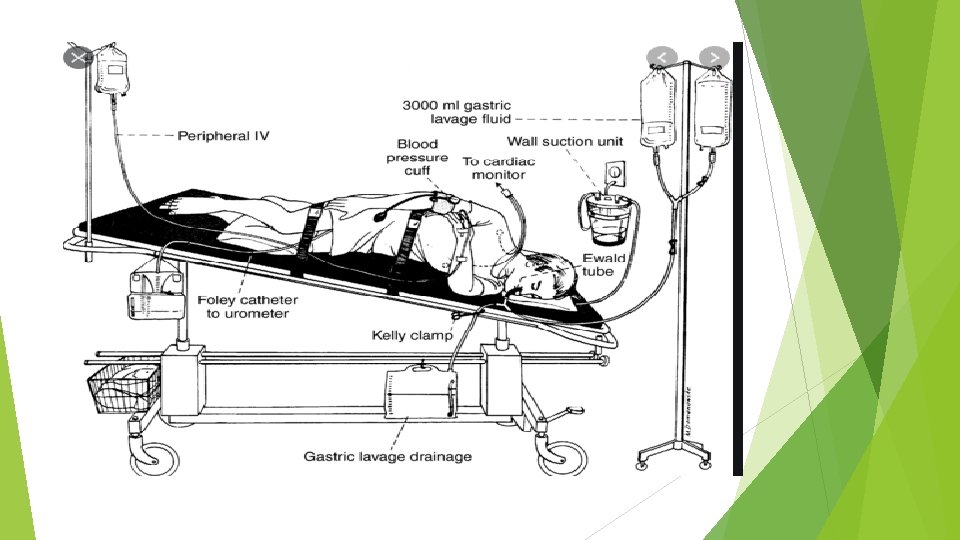
2. GASTRIC LAVAGE Gastric lavage was a time-honored element of the management of poisoned patients, but its routine use is no longer recommended by the American Academy of Clinical Toxicology or the European Association of Poisons Centres and Clinical Toxicologists

TECHNIQUE: Gastric lavage is performed with the patient in the left lateral decubitus position with the head in a 15º Trendelenburg position. Prior endotracheal intubation is not necessary in an awake cooperative patient and the presence of a cuffed endotracheal tube does not preclude aspiration


Although placement of a 36 to 40 French orogastric tube in adults or a 24 to 28 French orogastric tube in children is routinely recommended use of a 16 to 18 French tube placed orally or nasally has not been proven less effective During gastric lavage, drug is usually recovered in dissolved form and not as intact pills because most pills will not fit through the holes of the largest tubes.

Stomach contents should then be removed and gastric lavage performed by gravitational instillation and drainage of multiple sequential aliquots of 200 to 300 m. L of warmed normal saline or tap water. Lavage should be continued until the effluent is relatively clear; 5 liters of fluid are usually sufficient to achieve this goal. The entire procedure is both technically difficult and time-consuming.

EFFICACY: Controlled studies in animals and human volunteers have shown that gastric lavage decreases the absorption of ingested poison by an average of 26 percent when performed 30 minutes after ingestion, and 12 percent when performed at 60 minutes. A different study in which poisoned patients underwent Endoscopy following gastric lavage found that 88 percent of patients had residual solid material in the stomach. A controlled human volunteer study also suggested that the physical force of gastric lavage can propel gastric contents into the small bowel and may thereby promote absorption of the drugs.

Gastric lavage is less effective than activated charcoal in reducing the absorption of simulated toxins but is roughly equivalent in efficacy to ipecac. Gastric lavage in combination with activated charcoal (administered either following lavage or both before and following lavage) is more effective in reducing drug absorption than activated charcoal given alone, but studies have not confirmed a clinical benefit of gastric lavage.

INDICATIONS: The American Academy of Clinical Toxicology and the European Association of Poisons Centers and Clinical Toxicologists state that gastric lavage may be of benefit and is acceptable if a patient has ingested a potentially toxic amount of a substance and the procedure can be performed within one hour of ingestion Its use is not excluded in patients who present greater than one hour following ingestion, particularly when agents that are highly toxic and not bound well by activated charcoal have been ingested.

CONTRAINDICATIONS: Gastric lavage is contraindicated in the following situations: A corrosive agent has been ingested (orogastric tube insertion increases the risk of esophageal perforation) Low viscosity hydrocarbons have been ingested (orogastric tube insertion increases the risk of aspiration) A depressed mental status is present and the patient has not been endotracheally intubated The patient is at risk for hemorrhage or perforation due to esophageal or gastric pathology or recent surgery the patient is unable to cooperate with the procedure

COMPLICATIONS: Gastric lavage is associated with an increased risk of aspiration and ICU admission. Other complications of gastric lavage include laryngospasm, hypoxia and hypercapnia. esophageal and gastric erosions bleeding, perforation, inadvertent tracheal insertion and pulmonary lavage cardiac arrhythmias, cardiac ischemia, pneumothorax, fluid and electrolyte imbalances, and hypothermia.

3. SYRUP OF IPECAC Syrup of ipecac, which contains the alkaloids emetine and cephaline, induces emesis in more than 90 percent of overdose patients with a mean time of onset of 20 minutes. There has been a greater than eight-fold reduction in the use of ipecac for poisoning since 1985; less than 1 percent of all toxic ingestions were treated with ipecac.

Dose The adult dose is 30 m. L by mouth with 240 m. L (8 oz) of water. The recommended dose is 10 m. L for infants’ 6– 12 months of age. 15 m. L for children age 1– 12 years. should not be used in infants younger than 6 month.

EFFICACY: Experimental studies in animals and human volunteers show that recovery of ingested material is highly variable and rapidly diminishes with time. Ipecac is less effective than activated charcoal and approximately equivalent to gastric lavage in reducing poison absorption. The utility of ipecac in conjunction with other decontamination modalities is not well supported by experimental data. Four clinical studies have shown no improvement in patient outcomes from the administration of ipecac before activated charcoal versus activated charcoal alone.

INDICATIONS: Ipecac should not be administered routinely in the management of poisoned patients since there is no evidence from clinical studies that ipecac improves outcome. It may be considered in an alert, conscious patient who has ingested a substantial amount of a toxic substance within 60 minutes of presentation. Thus, its primary utility is in the home or pre-hospital setting immediately following a witnessed ingestion

CONTRAINDICATIONS Ipecac is contraindicated in the following situations; Depressed mental status is present (e. g. coma, seizure). A substance which may compromise airway protective reflexes within one hour has been ingested (e. g. rapidly acting central nervous system depressants) A corrosive agent has been ingested Low viscosity hydrocarbons have been ingested, yielding a high risk of aspiration if vomiting occurs Repeated vomiting poses a health danger (e. g. patients have undergone recent GI surgery, are elderly and debilitated, are in the third trimester of pregnancy, or have severe hypertension)

COMPLICATIONS Ipecac administration may result in protracted vomiting in a substantial number of patients and may result in delayed administration of activated charcoal and oral antidotes such as N-acetylcysteine.

4. WHOLE BOWEL IRRIGATION Whole bowel irrigation (WBI) refers to the enteral administration of a polyethylene glycol balanced electrolyte solution (PEG-ELS) in order to rapidly cleanse the GI tract of its contents and prevent intoxicant absorption. WBI provides an effective means of GI decontamination following ingestion of drug packets, sustained-release or enteric-coated preparations, or agents not well adsorbed by activated charcoal
 Gastric lavage for afb
Gastric lavage for afb Ng tube insertion steps ati
Ng tube insertion steps ati What is this?
What is this? Contamination vs cross contamination
Contamination vs cross contamination Government decontamination service
Government decontamination service Steris pass through window
Steris pass through window Sphair decontamination
Sphair decontamination Ewald tube
Ewald tube Steris decontamination sinks
Steris decontamination sinks Sterile processing decontamination ppe
Sterile processing decontamination ppe Cardioactive drugs
Cardioactive drugs Site:slidetodoc.com
Site:slidetodoc.com No lavage poppy
No lavage poppy Nettoyage en godille
Nettoyage en godille Fonction globale du chariot de lavage
Fonction globale du chariot de lavage Antral lavage
Antral lavage Direct and indirect wax pattern
Direct and indirect wax pattern Gastric pit
Gastric pit Gastric folds
Gastric folds