Emerging Electrical Therapy for Malignant Gliomas Impact of

















- Slides: 38

Emerging Electrical Therapy for Malignant Gliomas: Impact of Tumor Treating Fields and the Role of the Neuroradiologist Aaron Skolnik, MD 1 Harish Poptani, Ph. D 1 Sanjeev Chawla, Ph. D 1 Sumei Wang, MD 1 Guarav Verma, Ph. D 1 Steven Brem, MD 2 Suyash Mohan, MD 1 1 Neuroradiology 2 Neurosurgery Hospital of the University of Pennsylvania ASNR 2016 Abstract: e. Ed. E-78 Submission: 2059

Disclosures Investigator-Sponsored Trial Evaluating Therapeutic Response to Novo-TTF NCT 02441322 Novocure Principal Investigator: Suyash Mohan, MD

Outline: Tumor Treating Fields Scientific Basis Clinical Data Historical Timeline Role of the Neuroradiologist Imaging Assessment Patient & Family Experience Future Projections

Scientific Basis Tumor Treating Fields

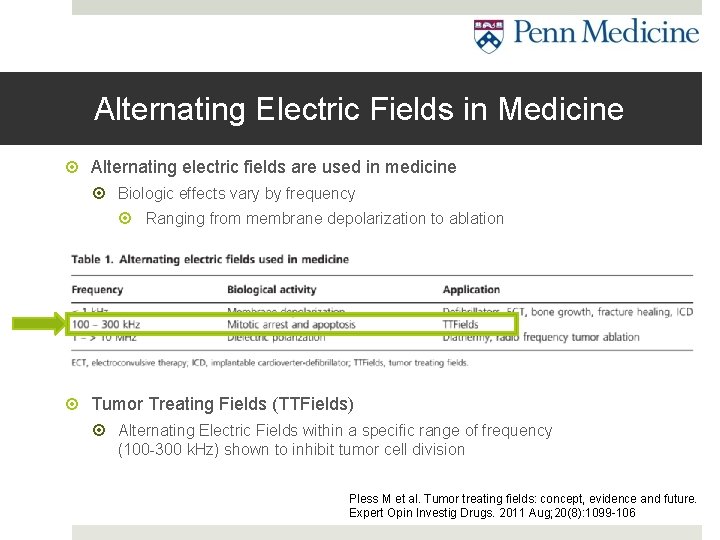
Alternating Electric Fields in Medicine Alternating electric fields are used in medicine Biologic effects vary by frequency Ranging from membrane depolarization to ablation Tumor Treating Fields (TTFields) Alternating Electric Fields within a specific range of frequency (100 -300 k. Hz) shown to inhibit tumor cell division Pless M et al. Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs. 2011 Aug; 20(8): 1099 -106

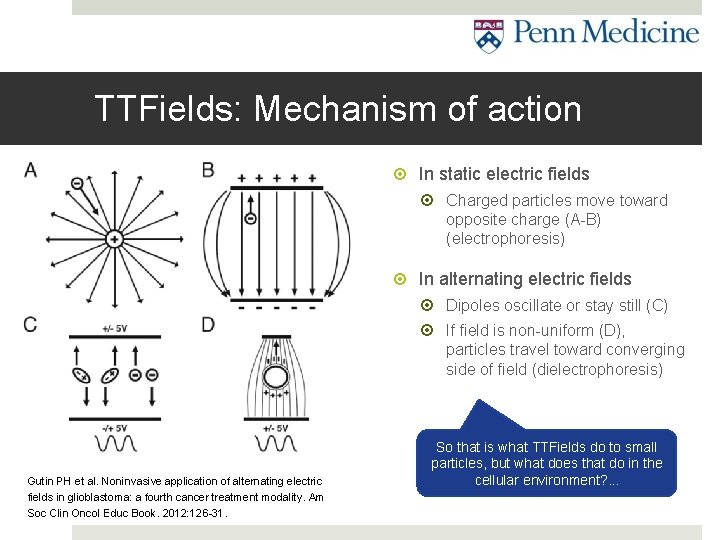
TTFields: Mechanism of action In static electric fields Charged particles move toward opposite charge (A-B) (electrophoresis) In alternating electric fields Dipoles oscillate or stay still (C) If field is non-uniform (D), particles travel toward converging side of field (dielectrophoresis) Gutin PH et al. Noninvasive application of alternating electric fields in glioblastoma: a fourth cancer treatment modality. Am Soc Clin Oncol Educ Book. 2012: 126 -31. So that is what TTFields do to small particles, but what does that do in the cellular environment? . . .

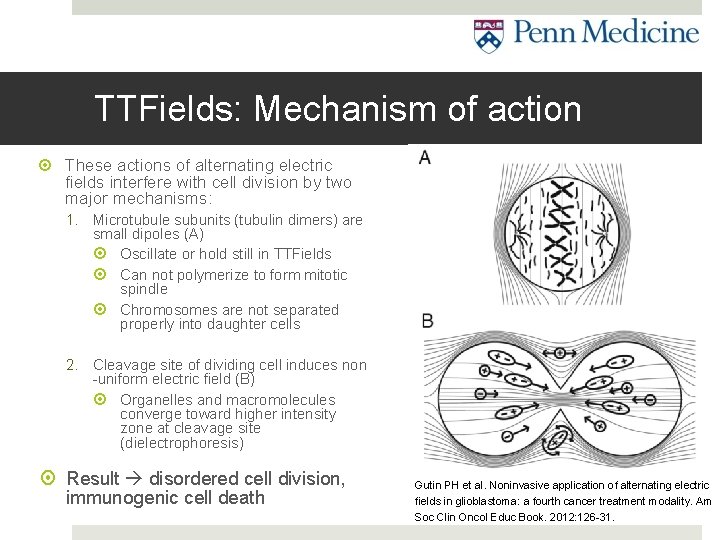
TTFields: Mechanism of action These actions of alternating electric fields interfere with cell division by two major mechanisms: 1. Microtubule subunits (tubulin dimers) are small dipoles (A) Oscillate or hold still in TTFields Can not polymerize to form mitotic spindle Chromosomes are not separated properly into daughter cells 2. Cleavage site of dividing cell induces non -uniform electric field (B) Organelles and macromolecules converge toward higher intensity zone at cleavage site (dielectrophoresis) Result disordered cell division, immunogenic cell death Gutin PH et al. Noninvasive application of alternating electric fields in glioblastoma: a fourth cancer treatment modality. Am Soc Clin Oncol Educ Book. 2012: 126 -31.

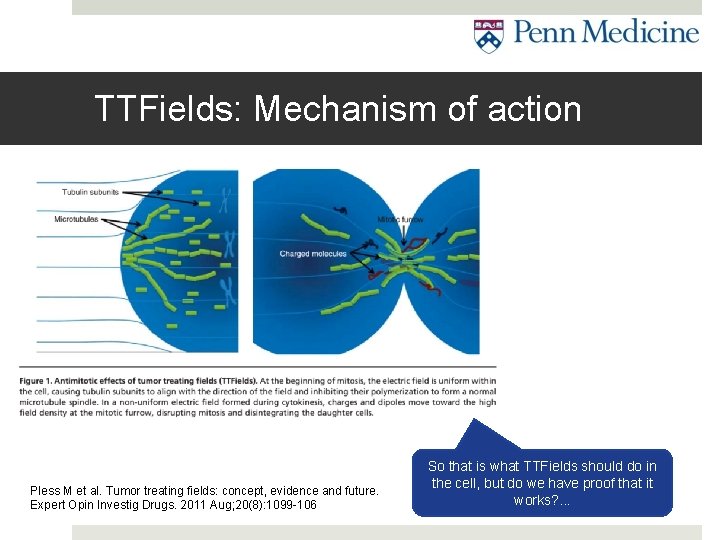
TTFields: Mechanism of action Pless M et al. Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs. 2011 Aug; 20(8): 1099 -106 So that is what TTFields should do in the cell, but do we have proof that it works? . . .

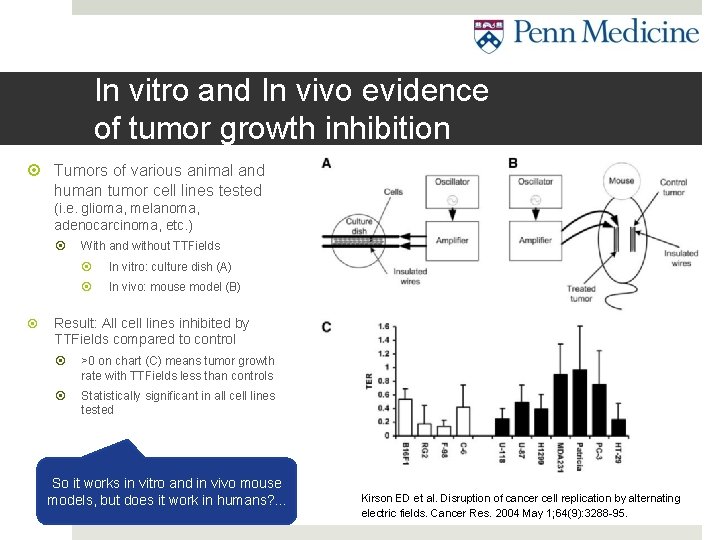
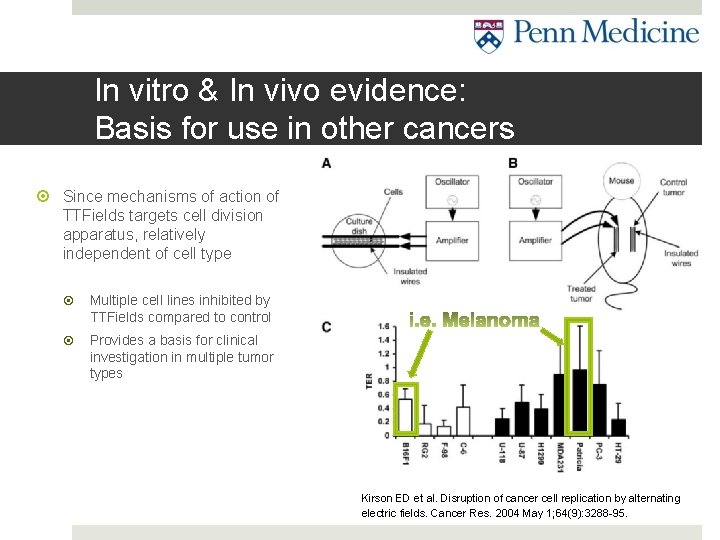
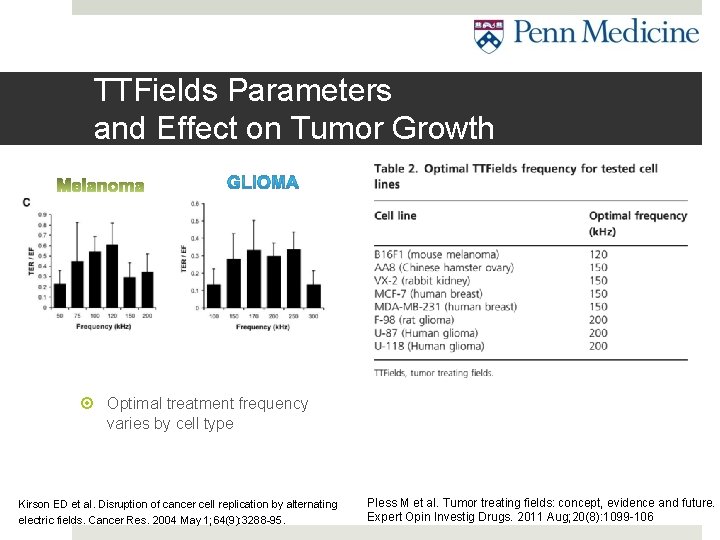
In vitro and In vivo evidence of tumor growth inhibition Tumors of various animal and human tumor cell lines tested (i. e. glioma, melanoma, adenocarcinoma, etc. ) With and without TTFields In vitro: culture dish (A) In vivo: mouse model (B) Result: All cell lines inhibited by TTFields compared to control >0 on chart (C) means tumor growth rate with TTFields less than controls Statistically significant in all cell lines tested So it works in vitro and in vivo mouse models, but does it work in humans? . . . Kirson ED et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004 May 1; 64(9): 3288 -95.

Clinical Data Tumor Treating Fields

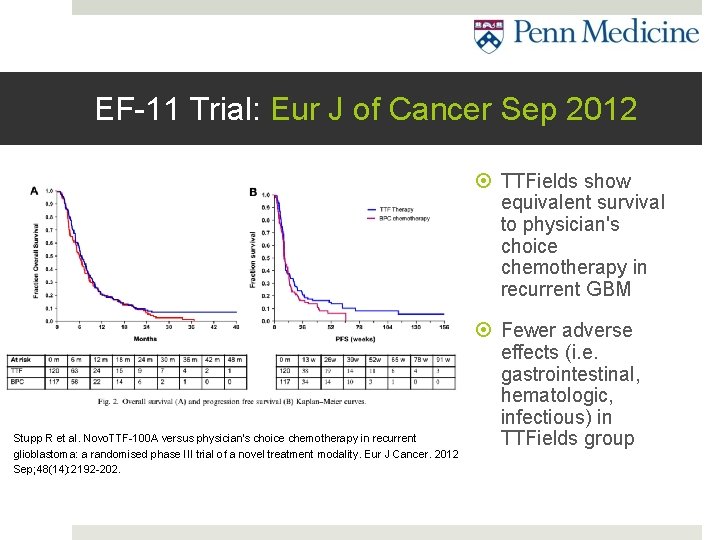
EF-11 Trial: Eur J of Cancer Sep 2012 TTFields show equivalent survival to physician's choice chemotherapy in recurrent GBM Stupp R et al. Novo. TTF-100 A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012 Sep; 48(14): 2192 -202. Fewer adverse effects (i. e. gastrointestinal, hematologic, infectious) in TTFields group

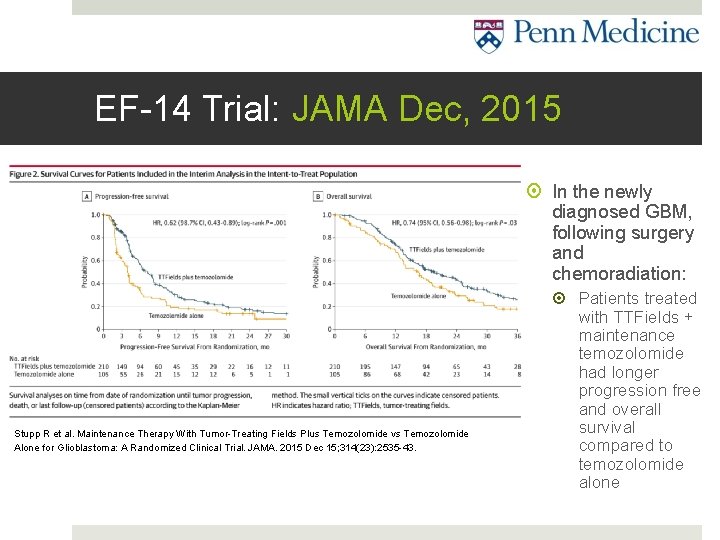
EF-14 Trial: JAMA Dec, 2015 In the newly diagnosed GBM, following surgery and chemoradiation: Stupp R et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015 Dec 15; 314(23): 2535 -43. Patients treated with TTFields + maintenance temozolomide had longer progression free and overall survival compared to temozolomide alone

Major Clinical Trial Summary EF-11 Trial: TTFields show equivalent survival to physician's choice chemotherapy in recurrent GBM, with less toxicity, better quality of life EF-14 Trial: In the newly diagnosed GBM, TTFields extend: Progression free survival by ~3 months (from 4. 0 to 7. 1) Overall survival by ~5 months (from 15. 6 to 20. 5) Relatively low toxicity, most commonly skin irritation Dose dependent treatment efficacy observed with compliance over 18 hours per day of treatment time Stupp R et al. Novo. TTF-100 A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012 Sep; 48(14): 2192 -202. Stupp R et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015 Dec 15; 314(23): 2535 -43.

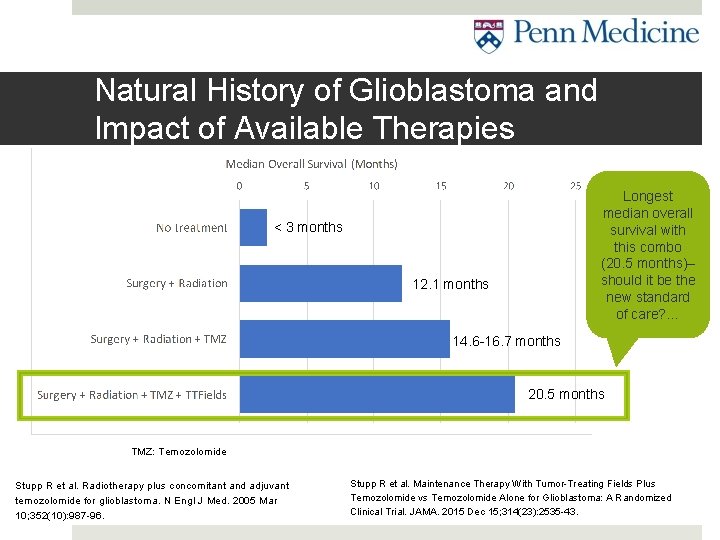
Natural History of Glioblastoma and Impact of Available Therapies Longest median overall survival with this combo (20. 5 months)– should it be the new standard of care? … < 3 months 12. 1 months 14. 6 -16. 7 months 20. 5 months TMZ: Temozolomide Stupp R et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10; 352(10): 987 -96. Stupp R et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015 Dec 15; 314(23): 2535 -43.

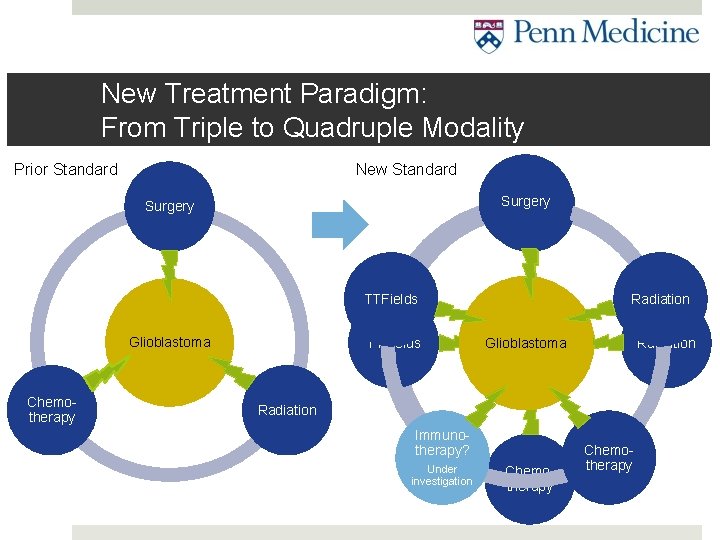
New Treatment Paradigm: From Triple to Quadruple Modality Prior Standard New Standard Surgery TTFields Glioblastoma Chemotherapy TTFields Radiation Glioblastoma Radiation Immunotherapy? Under investigation Chemotherapy

Historical Timeline Tumor Treating Fields

Timeline of FDA - Approved Therapies for Malignant Gliomas June 14, 1996: Carmustine wafer for recurrent glioblastoma January 12, 1999: Temozolomide for anaplastic astrocytoma February 25, 2003: Carmustine wafer for newly diagnosed glioblastoma March 15, 2005: Temozolomide for newly diagnosed glioblastoma May 5, 2009: Bevacizumab for progressive glioblastoma April 15, 2011: TTFields for recurrent glioblastoma October 5, 2015: TTFields for newly diagnosed glioblastoma

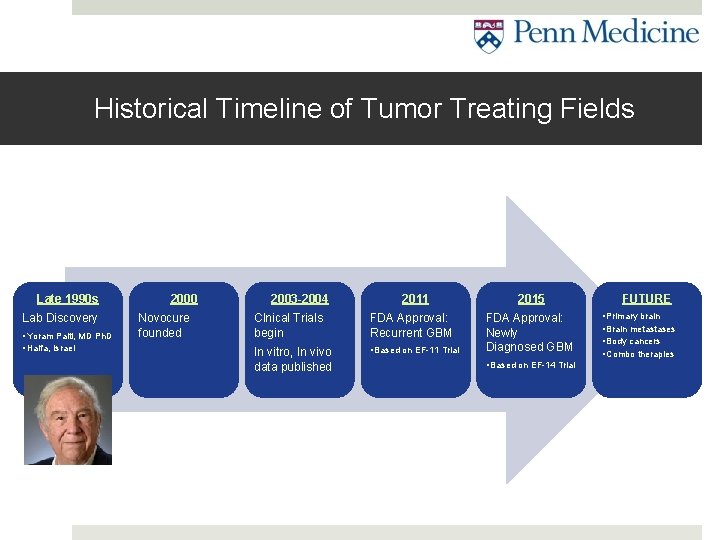
Historical Timeline of Tumor Treating Fields Late 1990 s Lab Discovery • Yoram Palti, MD Ph. D • Haifa, Israel 2000 Novocure founded 2003 -2004 2011 Clnical Trials begin FDA Approval: Recurrent GBM In vitro, In vivo data published • Based on EF-11 Trial 2015 FDA Approval: Newly Diagnosed GBM • Based on EF-14 Trial FUTURE • Primary brain • Brain metastases • Body cancers • Combo therapies

Role of the Neuroradiologist Tumor Treating Fields

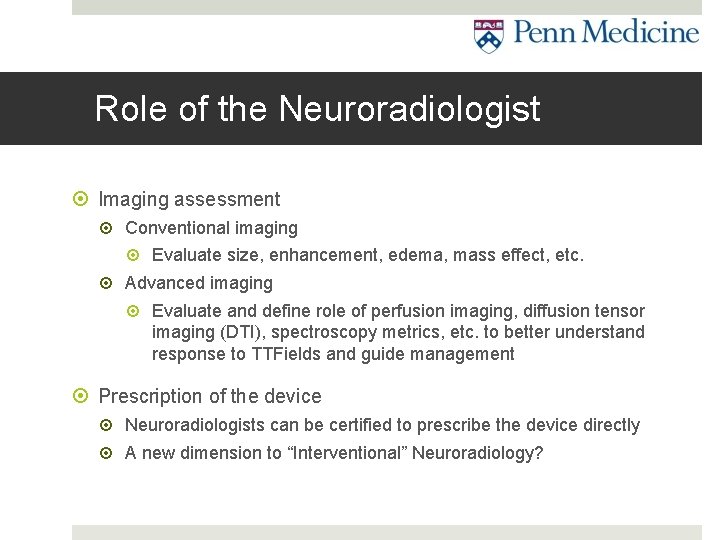
Role of the Neuroradiologist Imaging assessment Conventional imaging Evaluate size, enhancement, edema, mass effect, etc. Advanced imaging Evaluate and define role of perfusion imaging, diffusion tensor imaging (DTI), spectroscopy metrics, etc. to better understand response to TTFields and guide management Prescription of the device Neuroradiologists can be certified to prescribe the device directly A new dimension to “Interventional” Neuroradiology?

Imaging Assessment Tumor Treating Fields

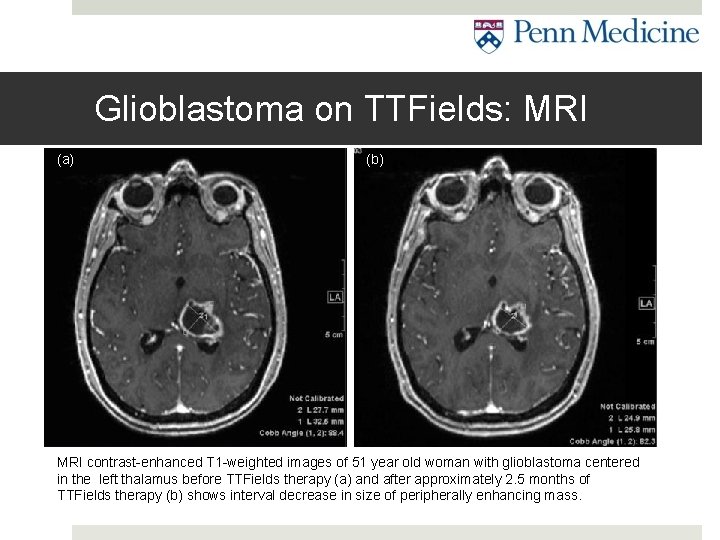
Glioblastoma on TTFields: MRI (a) (b) MRI contrast-enhanced T 1 -weighted images of 51 year old woman with glioblastoma centered in the left thalamus before TTFields therapy (a) and after approximately 2. 5 months of TTFields therapy (b) shows interval decrease in size of peripherally enhancing mass.

Glioblastoma on TTFields: MRI (a) (b) MRI contrast-enhanced T 1 -weighted images of 70 year old man with glioblastoma centered in the right thalamus before TTFields therapy (a) and after approximately 1 month of TTFields therapy (b) shows interval decrease in size and enhancement of mass.

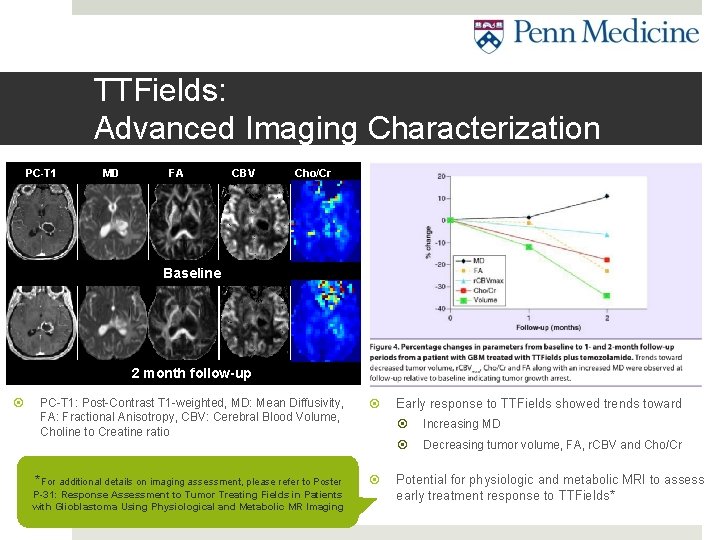
TTFields: Advanced Imaging Characterization PC-T 1 MD FA CBV Cho/Cr Baseline 2 month follow-up PC-T 1: Post-Contrast T 1 -weighted, MD: Mean Diffusivity, FA: Fractional Anisotropy, CBV: Cerebral Blood Volume, Choline to Creatine ratio S Mohan et al. Response Assessment to Tumor Treating *For additional details on imaging assessment, please refer to Poster Fields in newly diagnosed Glioblastoma using Physiologic P-31: Response Assessment to Tumor Treating Fields in Patients and Metabolic MR Imaging: Initial experience. CNS with Glioblastoma Using Physiological and Metabolic MR Imaging Oncology. In Press. Early response to TTFields showed trends toward Increasing MD Decreasing tumor volume, FA, r. CBV and Cho/Cr Potential for physiologic and metabolic MRI to assess early treatment response to TTFields*

Patient and Family Experience Tumor Treating Fields

The Patient & Family Experience TTFields delivered by a portable external device Transducer arrays applied to shaved head by caregiver, wires connected to backpack containing device and battery Arrays removed every 2 -4 days to shave head and apply new arrays Worn as much as possible, goal treatment time >18 hours per day Device interrogated for monthly compliance rates Can be covered by hat, scarf, or wig

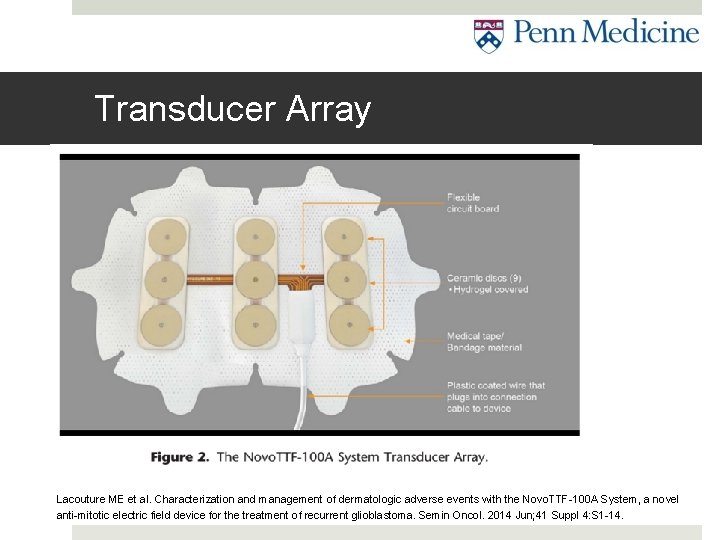
Transducer Array Lacouture ME et al. Characterization and management of dermatologic adverse events with the Novo. TTF-100 A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol. 2014 Jun; 41 Suppl 4: S 1 -14.

TTFields Equipment Lacouture ME et al. Characterization and management of dermatologic adverse events with the Novo. TTF-100 A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol. 2014 Jun; 41 Suppl 4: S 1 -14.

Future Projections Tumor Treating Fields

In vitro & In vivo evidence: Basis for use in other cancers Since mechanisms of action of TTFields targets cell division apparatus, relatively independent of cell type Multiple cell lines inhibited by TTFields compared to control Provides a basis for clinical investigation in multiple tumor types Kirson ED et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004 May 1; 64(9): 3288 -95.

TTFields Parameters and Effect on Tumor Growth Optimal treatment frequency varies by cell type Kirson ED et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004 May 1; 64(9): 3288 -95. Pless M et al. Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs. 2011 Aug; 20(8): 1099 -106

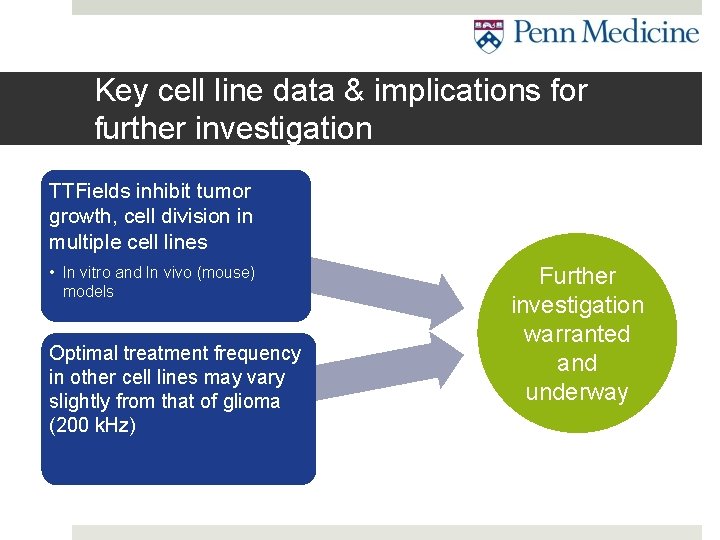
Key cell line data & implications for further investigation TTFields inhibit tumor growth, cell division in multiple cell lines • In vitro and In vivo (mouse) models Optimal treatment frequency in other cell lines may vary slightly from that of glioma (200 k. Hz) Further investigation warranted and underway

Current Clinical Trials of TTFields in Brain Metastases & Body Cancers (as of 4/2016) Brain metastases Small cell lung cancer: NCT 02425072 Non-small cell lung cancer: NCT 01755624 Body cancers Advanced non-small cell lung cancer: NCT 00749346 Mesothelioma: NCT 02397928 Pancreatic adenocarcinoma: NCT 01971281 Recurrent ovarian carcinoma: NCT 02244502 Clinical. Trials. gov

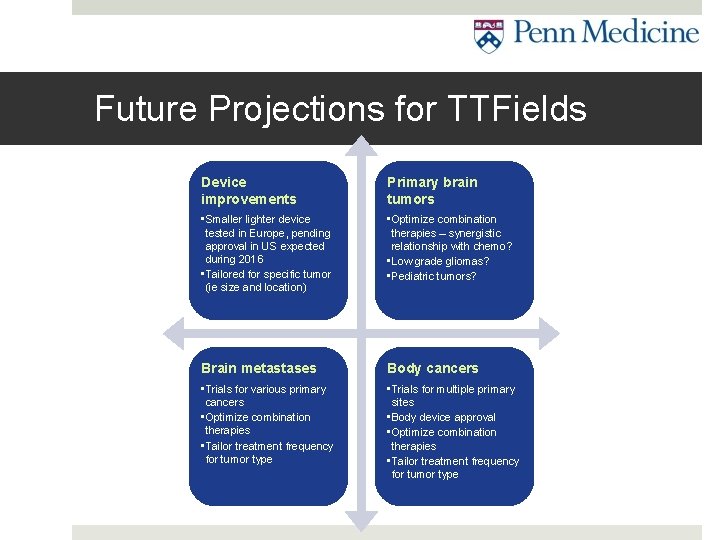
Future Projections for TTFields Device improvements Primary brain tumors • Smaller lighter device tested in Europe, pending approval in US expected during 2016 • Tailored for specific tumor (ie size and location) • Optimize combination therapies – synergistic relationship with chemo? • Low grade gliomas? • Pediatric tumors? Brain metastases Body cancers • Trials for various primary cancers • Optimize combination therapies • Tailor treatment frequency for tumor type • Trials for multiple primary sites • Body device approval • Optimize combination therapies • Tailor treatment frequency for tumor type

Summary: Tumor Treating Fields in Glioblastoma TTFields are a novel mechanism and safe modality to treat cancer TTFields prolong survival in glioblastoma FDA approvals in 2011 and 2015 The Neuroradiologist has the opportunity to add value to TTFields patients by understanding the technology and associated imaging evaluation for response assessment or even prescribing the device Expansion of this treatment modality is expected Laboratory data shows action in multiple tumor types Investigation underway in optimizing combination therapy for glioma treatment, and for use in body cancers and brain metastases

References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987 Aug; 21(2): 201 -6. Chaudhry A, Benson L, Varshaver M, Farber O, Weinberg U, Kirson E, Palti Y. Novo. TTF(™)-100 A System (Tumor Treating Fields) transducer array layout planning for glioblastoma: a Novo. TAL(™) system user study. World J Surg Oncol. 2015 Nov 11; 13(1): 316. Clinical. Trials. gov Gutin PH, Wong ET. Noninvasive application of alternating electric fields in glioblastoma: a fourth cancer treatment modality. Am Soc Clin Oncol Educ Book. 2012: 126 -31. Kirson ED, Dbalý V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S, Gurvich Z, Schneiderman R, Wasserman Y, Salzberg M, Ryffel B, Goldsher D, Dekel E, Palti Y. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007 Jun 12; 104(24): 10152 -7. Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004 May 1; 64(9): 3288 -95. Lacouture ME, Davis ME, Elzinga G, Butowski N, Tran D, Villano JL, Di. Meglio L, Davies AM, Wong ET. Characterization and management of dermatologic adverse events with the Novo. TTF-100 A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol. 2014 Jun; 41 Suppl 4: S 1 -14. Lok E, Hua V, Wong ET. Computed modeling of alternating electric fields therapy for recurrent glioblastoma. Cancer Med. 2015 Aug 26. Mohan S, S Chawla, S Wang, G Verma, A Skolnik, S Brem, K Peters, H Poptani. Response Assessment to Tumor Treating Fields in newly diagnosed Glioblastoma using Physiologic and Metabolic MR Imaging: Initial experience. CNS Oncology. In Press. Novocure. com Optune. com

References 12. 13. 14. 15. 16. 17. Pless M, Weinberg U. Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs. 2011 Aug; 20(8): 1099106. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10; 352(10): 987 -96. Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015 Dec 15; 314(23): 2535 -43. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F, Dbalý V, Ram Z, Villano JL, Rainov N, Weinberg U, Schiff D, Kunschner L, Raizer J, Honnorat J, Sloan A, Malkin M, Landolfi JC, Payer F, Mehdorn M, Weil RJ, Pannullo SC, Westphal M, Smrcka M, Chin L, Kostron H, Hofer S, Bruce J, Cosgrove R, Paleologous N, Palti Y, Gutin PH. Novo. TTF-100 A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012 Sep; 48(14): 2192 -202. Taillibert S, Le Rhun E, Chamberlain MC. Tumor treating fields: a new standard treatment for glioblastoma? Curr Opin Neurol. 2015 Dec; 28(6): 659 -64. Wong ET, Lok E, Swanson KD. An Evidence-Based Review of Alternating Electric Fields Therapy for Malignant Gliomas. Curr Treat Options Oncol. 2015 Aug; 16(8): 40.

Acknowledgments Radiology: Mitchell Schnall MD Ph. D Laurie Loevner, MD Ronald Wolf MD, Ph. D Neurosurgery: Steven Brem MD Donald O’Rourke MD Neuro-oncology/Radiation Oncology: Arati Desai MD Michelle A Basanta MD, Ph. D Robert Lustig MD Neuropathology: Maria Martinez-Lage MD Grants: Novocure EPSI Development Group: Dr. Andrew Maudsley Dr. Albert Thomas Sulaiman Sheriff Dr. Mohammad Sabati Research Personnel Lisa Desiderio Katelyn Reilly Lauren Karpf Krista Huff