CONVEGNO INTERREGIONALE SIE PROGRESSI NELLA DIAGNOSI E NELLA



- Slides: 48

CONVEGNO INTERREGIONALE SIE PROGRESSI NELLA DIAGNOSI E NELLA TERAPIA DELLE MALATTIE LINFOPROLIFERATIVE ATTUALITITA‘ NEL TRATTAMENTO DEI LINFOMI AGGRESSIVI S. Cortelazzo Divisione di Ematologia e Centro di Trapianto di Midollo Osseo di Bolzano 17 Novembre 2006 Aula Magna Kolbe, Udine

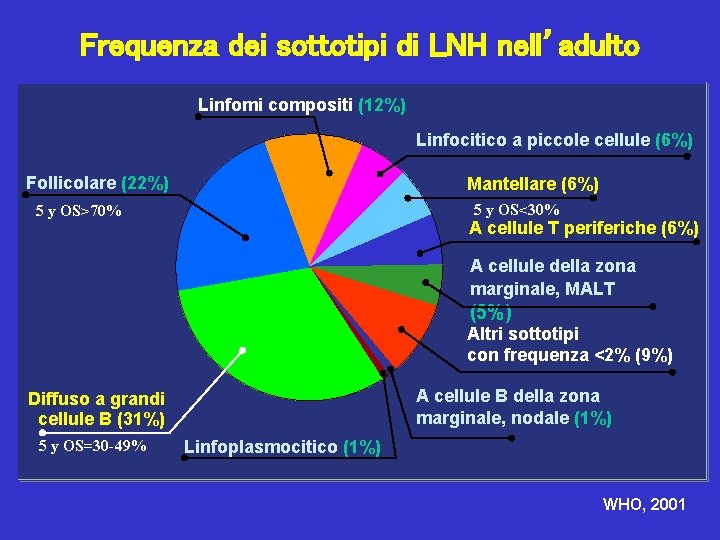
Frequenza dei sottotipi di LNH nell’adulto Linfomi compositi (12%) Linfocitico a piccole cellule (6%) Follicolare (22%) Mantellare (6%) 5 y OS<30% 5 y OS>70% A cellule T periferiche (6%) A cellule della zona marginale, MALT (5%) Altri sottotipi con frequenza <2% (9%) A cellule B della zona marginale, nodale (1%) Diffuso a grandi cellule B (31%) 5 y OS=30 -49% Linfoplasmocitico (1%) WHO, 2001

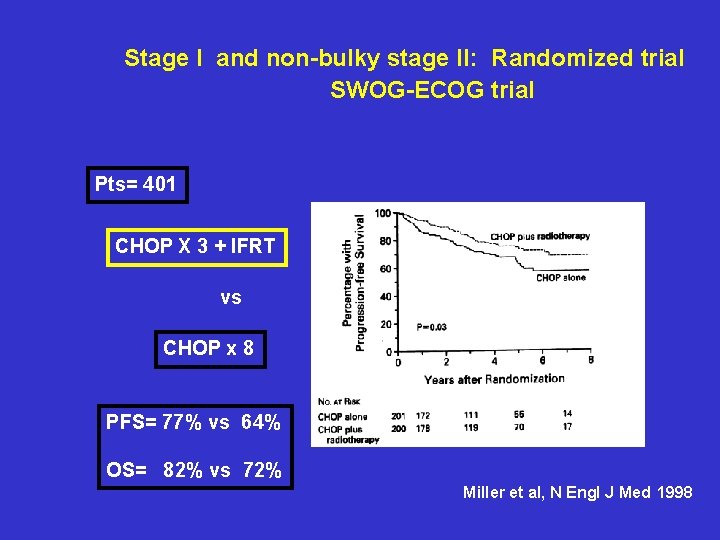
Stage I and non-bulky stage II: Randomized trial SWOG-ECOG trial Pts= 401 CHOP X 3 + IFRT vs CHOP x 8 PFS= 77% vs 64% OS= 82% vs 72% Miller et al, N Engl J Med 1998

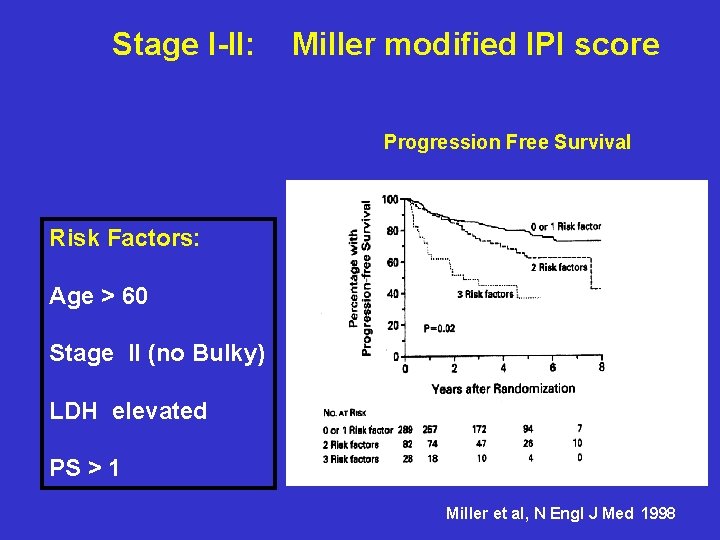
Stage I-II: Miller modified IPI score Progression Free Survival Risk Factors: Age > 60 Stage II (no Bulky) LDH elevated PS > 1 Miller et al, N Engl J Med 1998

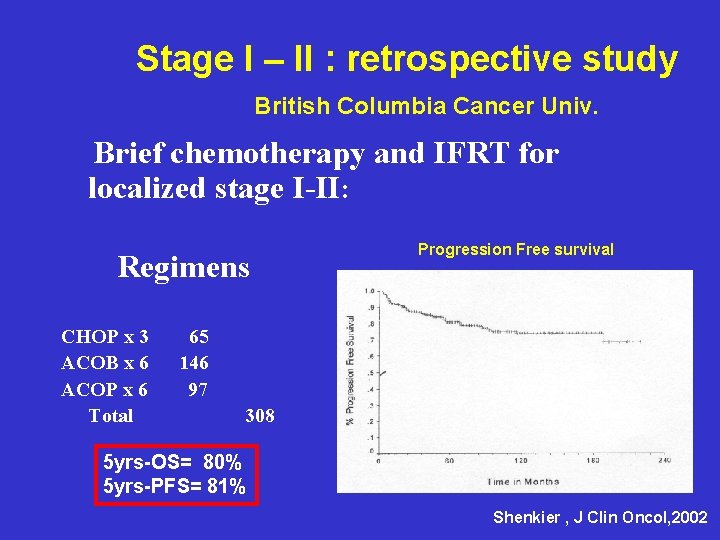
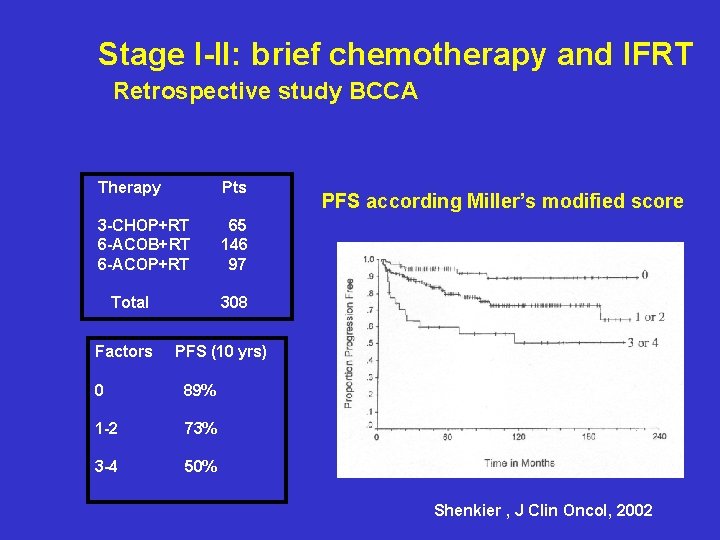
Stage I – II : retrospective study British Columbia Cancer Univ. Brief chemotherapy and IFRT for localized stage I-II: Regimens CHOP x 3 ACOB x 6 ACOP x 6 Total Progression Free survival 65 146 97 308 5 yrs-OS= 80% 5 yrs-PFS= 81% Shenkier , J Clin Oncol, 2002

Stage I-II: brief chemotherapy and IFRT Retrospective study BCCA Therapy Pts 3 -CHOP+RT 6 -ACOB+RT 6 -ACOP+RT 65 146 97 Total Factors PFS according Miller’s modified score 308 PFS (10 yrs) 0 89% 1 -2 73% 3 -4 50% Shenkier , J Clin Oncol, 2002

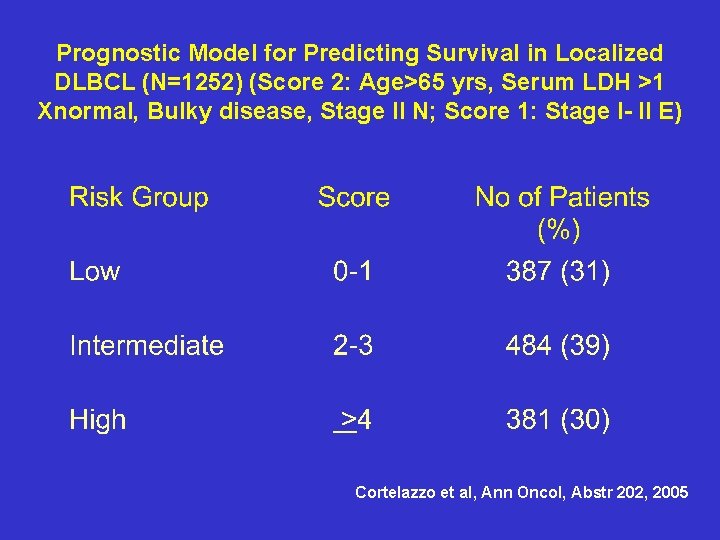
Prognostic Model for Predicting Survival in Localized DLBCL (N=1252) (Score 2: Age>65 yrs, Serum LDH >1 Xnormal, Bulky disease, Stage II N; Score 1: Stage I- II E) Cortelazzo et al, Ann Oncol, Abstr 202, 2005

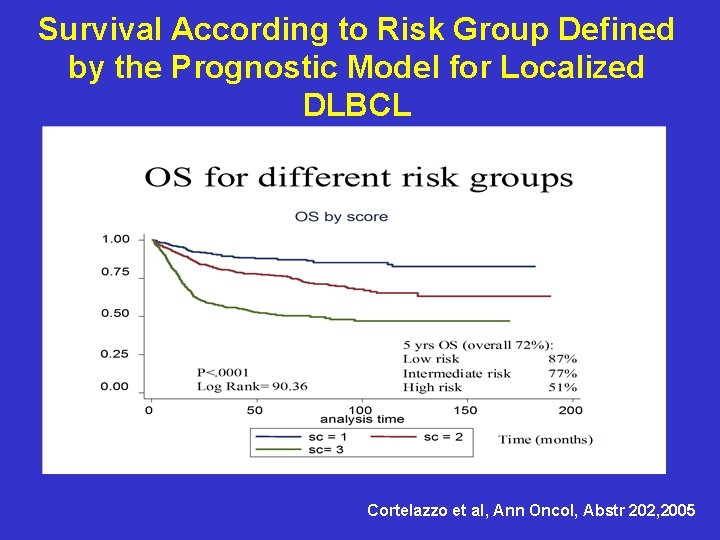
Survival According to Risk Group Defined by the Prognostic Model for Localized DLBCL Cortelazzo et al, Ann Oncol, Abstr 202, 2005

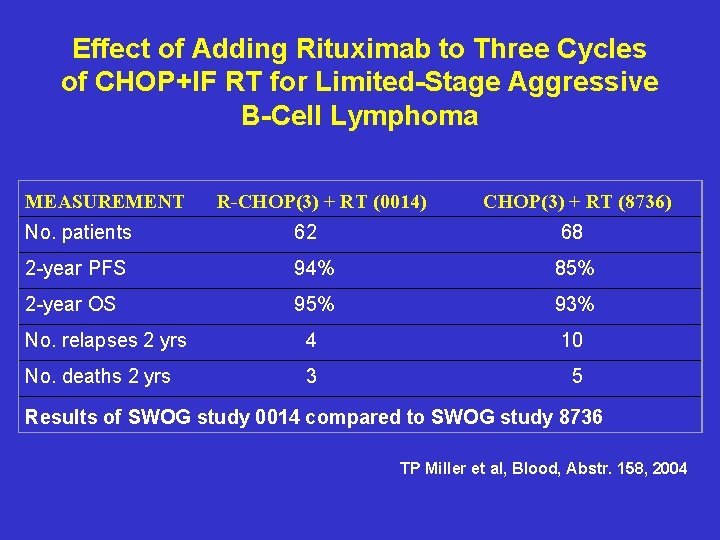
Effect of Adding Rituximab to Three Cycles of CHOP+IF RT for Limited-Stage Aggressive B-Cell Lymphoma MEASUREMENT R-CHOP(3) + RT (0014) CHOP(3) + RT (8736) No. patients 62 68 2 -year PFS 94% 85% 2 -year OS 95% 93% No. relapses 2 yrs 4 10 No. deaths 2 yrs 3 5 Results of SWOG study 0014 compared to SWOG study 8736 TP Miller et al, Blood, Abstr. 158, 2004

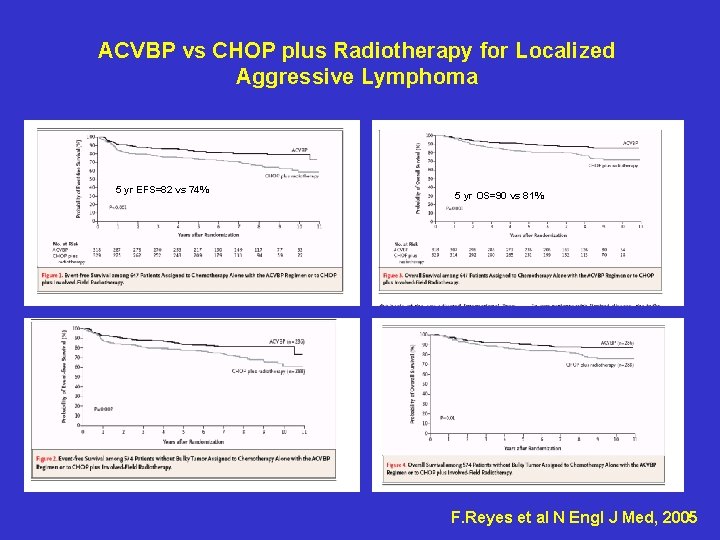
ACVBP vs CHOP plus Radiotherapy for Localized Aggressive Lymphoma 5 yr EFS=82 vs 74% 5 yr OS=90 vs 81% F. Reyes et al N Engl J Med, 2005

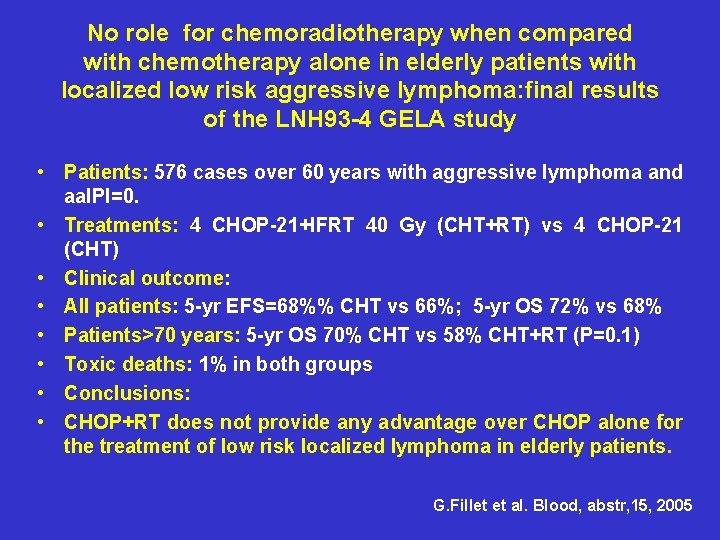
No role for chemoradiotherapy when compared with chemotherapy alone in elderly patients with localized low risk aggressive lymphoma: final results of the LNH 93 -4 GELA study • Patients: 576 cases over 60 years with aggressive lymphoma and aa. IPI=0. • Treatments: 4 CHOP-21+IFRT 40 Gy (CHT+RT) vs 4 CHOP-21 (CHT) • Clinical outcome: • All patients: 5 -yr EFS=68%% CHT vs 66%; 5 -yr OS 72% vs 68% • Patients>70 years: 5 -yr OS 70% CHT vs 58% CHT+RT (P=0. 1) • Toxic deaths: 1% in both groups • Conclusions: • CHOP+RT does not provide any advantage over CHOP alone for the treatment of low risk localized lymphoma in elderly patients. G. Fillet et al. Blood, abstr, 15, 2005

Recommendations (Practice Guidelines from the SIE, SIES, GITMO) Patients of all ages with stage I-II DLBCL and IPI equal to 0 ( normal LDH serum levels, ECOG performance status < 2), should receive abbreviated chemotherapy with an anthracycline-containing regimen plus involved field RT(35 -40 Gy) or a full course of chemotherapy alone [grade C]. Patients with stage I-II disease, and at least one adverse prognostic factor (bulky disease, elevated LDH, performance status ECOG >1, ) should be treated according to the recommendations for stage III-IV [grade D]. Barosi et al, Haematologica, 2006

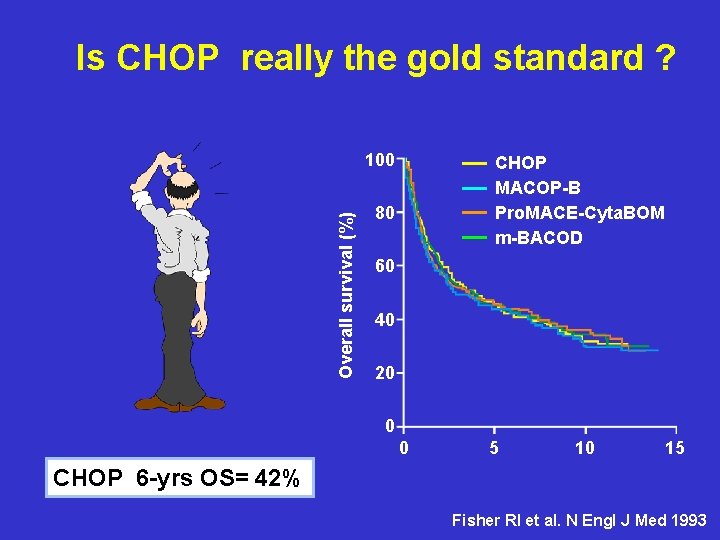
Is CHOP really the gold standard ? Overall survival (%) 100 CHOP MACOP-B Pro. MACE-Cyta. BOM m-BACOD 80 60 40 20 0 0 5 10 15 CHOP 6 -yrs OS= 42% Fisher RI et al. N Engl J Med 1993

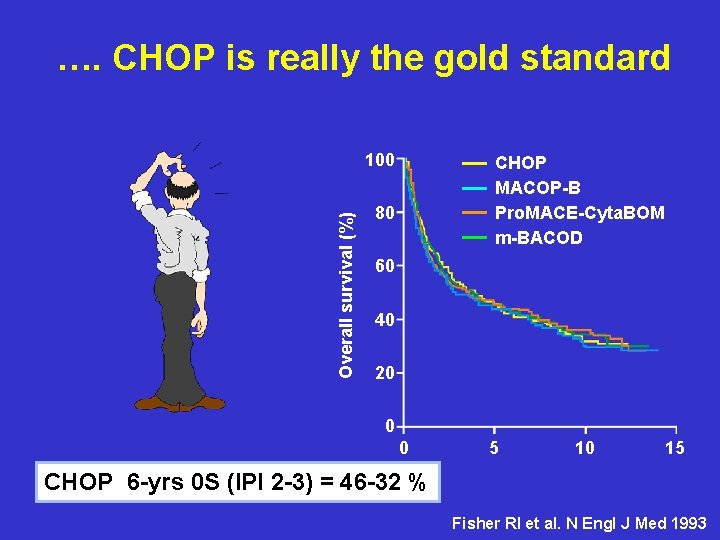
…. CHOP is really the gold standard Overall survival (%) 100 CHOP MACOP-B Pro. MACE-Cyta. BOM m-BACOD 80 60 40 20 0 0 5 10 15 CHOP 6 -yrs 0 S (IPI 2 -3) = 46 -32 % Fisher RI et al. N Engl J Med 1993

Strategie terapeutiche Intensificazione di dose Rituximab Alte dosi + ASCT Chemioterapia dose dense Chemioterapia standard In prima linea terapeutica

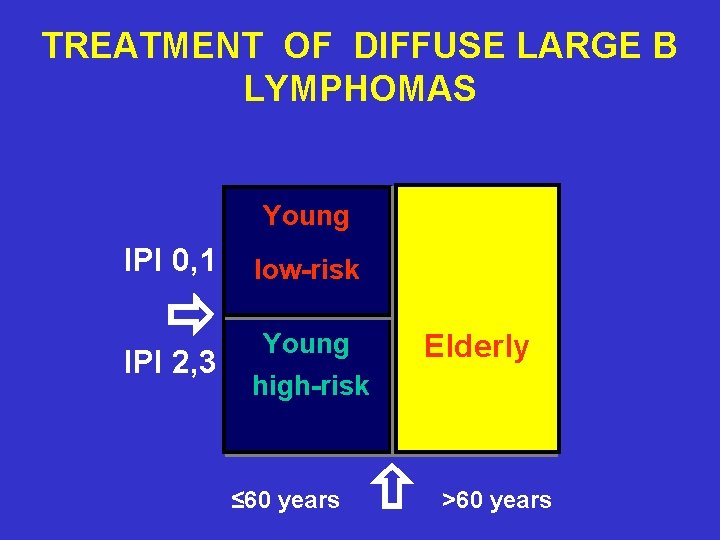
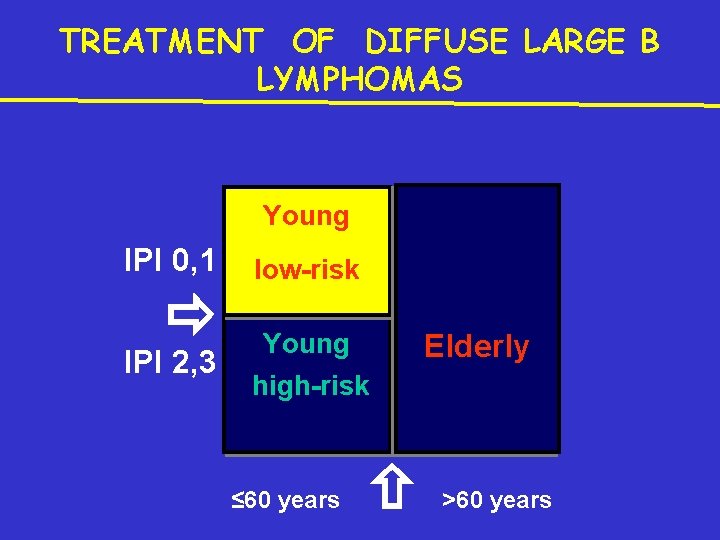
TREATMENT OF DIFFUSE LARGE B LYMPHOMAS Young IPI 0, 1 IPI 2, 3 low-risk Young Elderly high-risk ≤ 60 years >60 years

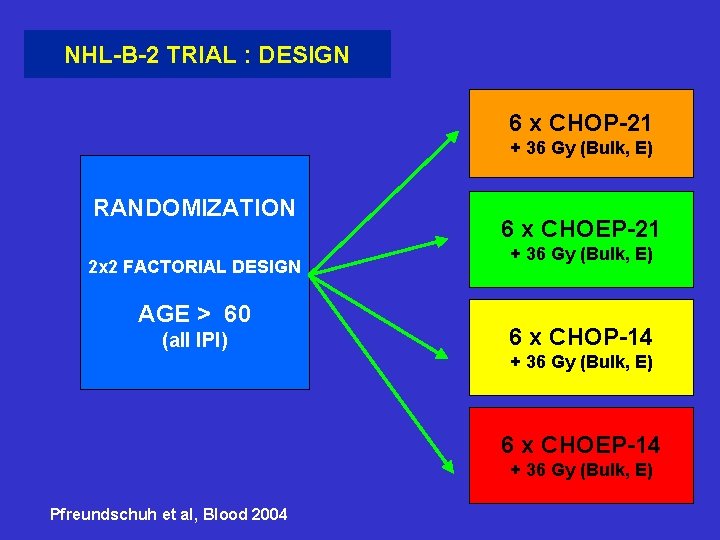
NHL-B-2 TRIAL : DESIGN 6 x CHOP-21 + 36 Gy (Bulk, E) RANDOMIZATION 2 x 2 FACTORIAL DESIGN AGE > 60 (all IPI) 6 x CHOEP-21 + 36 Gy (Bulk, E) 6 x CHOP-14 + 36 Gy (Bulk, E) 6 x CHOEP-14 + 36 Gy (Bulk, E) Pfreundschuh et al, Blood 2004

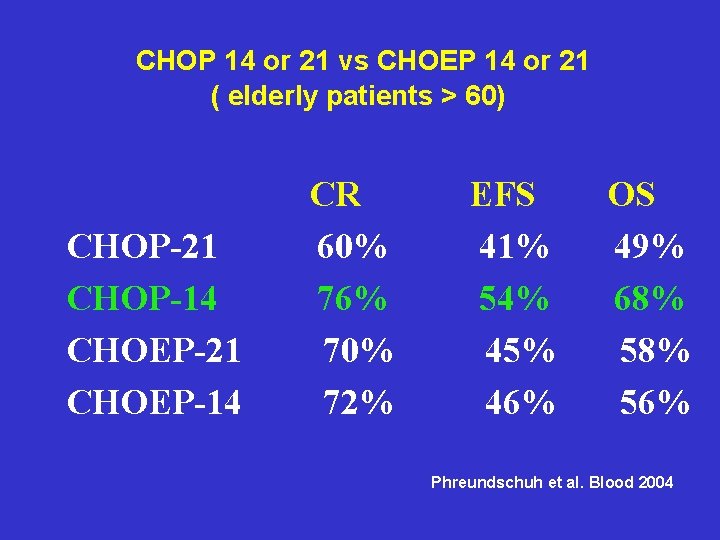
CHOP 14 or 21 vs CHOEP 14 or 21 ( elderly patients > 60) CHOP-21 CHOP-14 CHOEP-21 CHOEP-14 CR 60% 76% 70% 72% EFS 41% 54% 45% 46% OS 49% 68% 56% Phreundschuh et al. Blood 2004

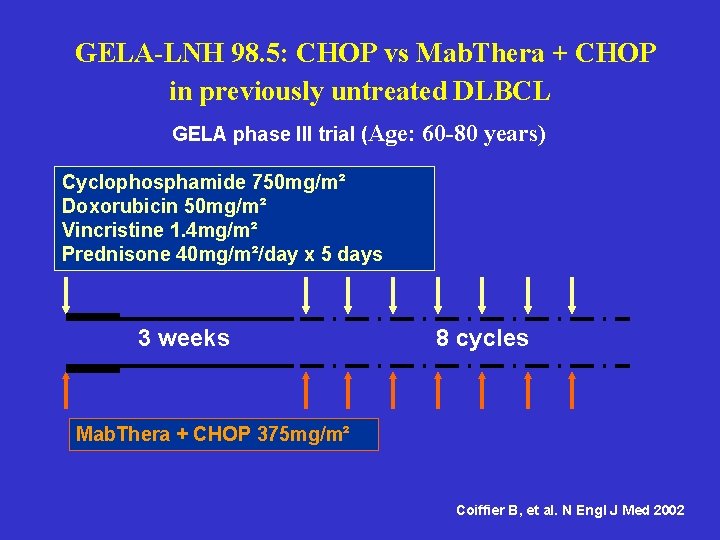
GELA-LNH 98. 5: CHOP vs Mab. Thera + CHOP in previously untreated DLBCL GELA phase III trial (Age: 60 -80 years) Cyclophosphamide 750 mg/m² Doxorubicin 50 mg/m² Vincristine 1. 4 mg/m² Prednisone 40 mg/m²/day x 5 days 3 weeks 8 cycles Mab. Thera + CHOP 375 mg/m² Coiffier B, et al. N Engl J Med 2002

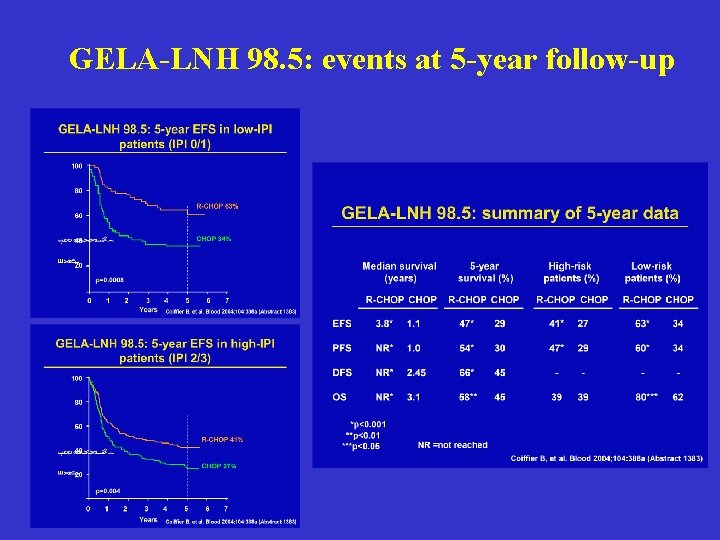
GELA-LNH 98. 5: events at 5 -year follow-up

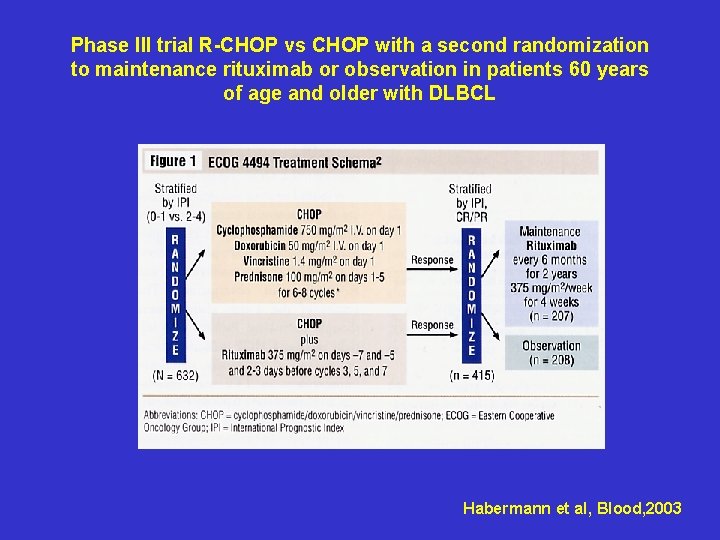
Phase III trial R-CHOP vs CHOP with a second randomization to maintenance rituximab or observation in patients 60 years of age and older with DLBCL Habermann et al, Blood, 2003

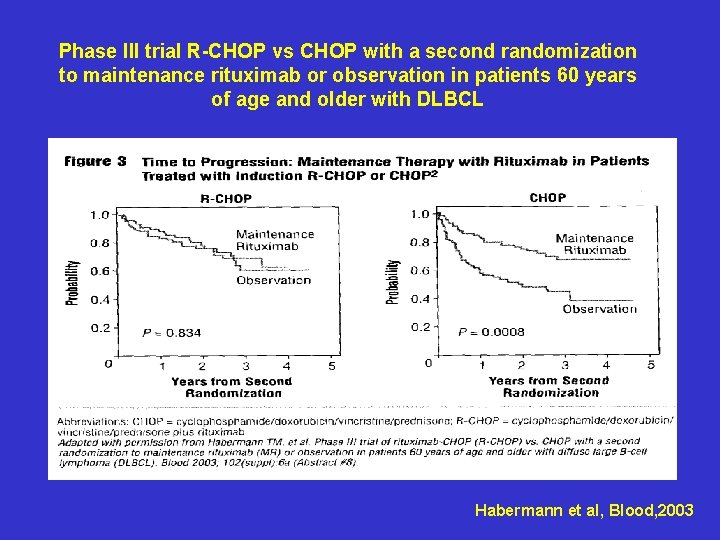
Phase III trial R-CHOP vs CHOP with a second randomization to maintenance rituximab or observation in patients 60 years of age and older with DLBCL Habermann et al, Blood, 2003

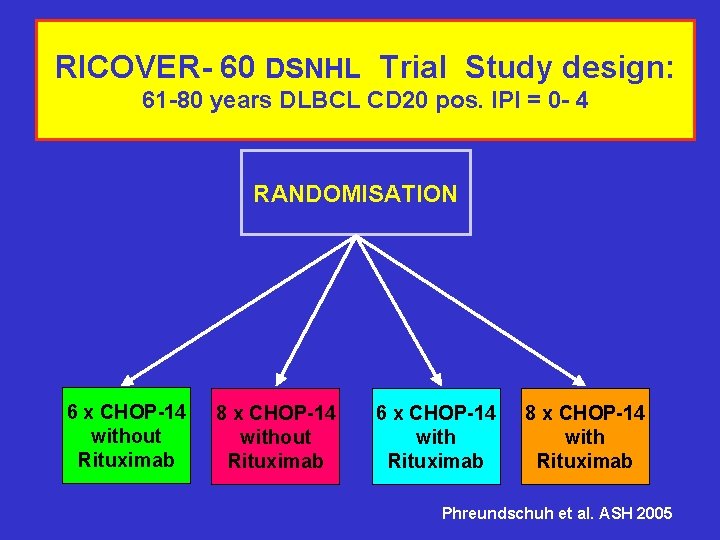
RICOVER- 60 DSNHL Trial Study design: 61 -80 years DLBCL CD 20 pos. IPI = 0 - 4 RANDOMISATION 6 x CHOP-14 without Rituximab 8 x CHOP-14 without Rituximab 6 x CHOP-14 with Rituximab 8 x CHOP-14 with Rituximab Phreundschuh et al. ASH 2005

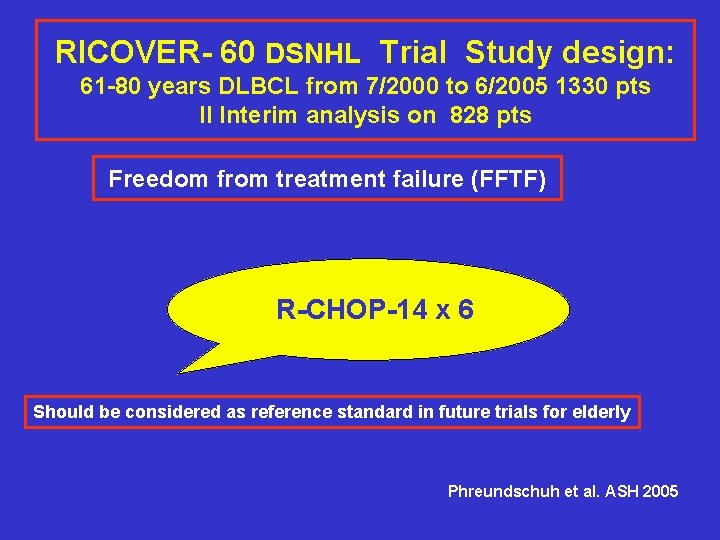
RICOVER- 60 DSNHL Trial Study design: 61 -80 years DLBCL from 7/2000 to 6/2005 1330 pts II Interim analysis on 828 pts Freedom from treatment failure (FFTF) R-CHOP-14 x 6 Should be considered as reference standard in future trials for elderly Phreundschuh et al. ASH 2005

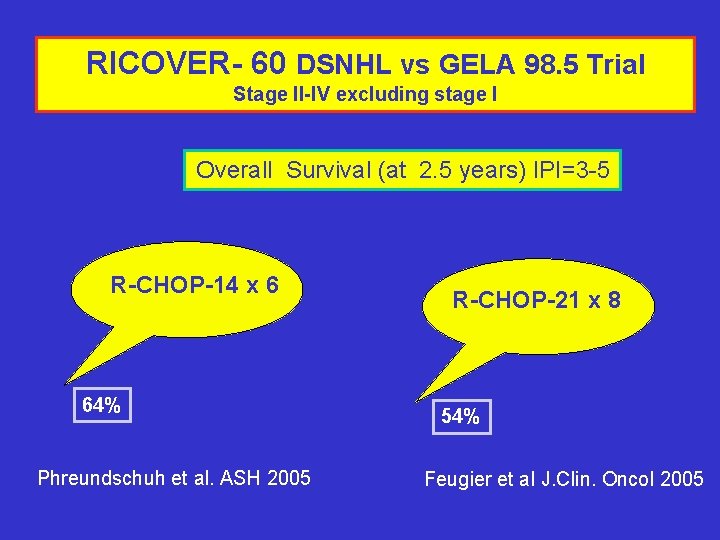
RICOVER- 60 DSNHL vs GELA 98. 5 Trial Stage II-IV excluding stage I Overall Survival (at 2. 5 years) IPI=3 -5 R-CHOP-14 x 6 64% Phreundschuh et al. ASH 2005 R-CHOP-21 x 8 54% Feugier et al J. Clin. Oncol 2005

TREATMENT OF DIFFUSE LARGE B LYMPHOMAS Young IPI 0, 1 IPI 2, 3 low-risk Young Elderly high-risk ≤ 60 years >60 years

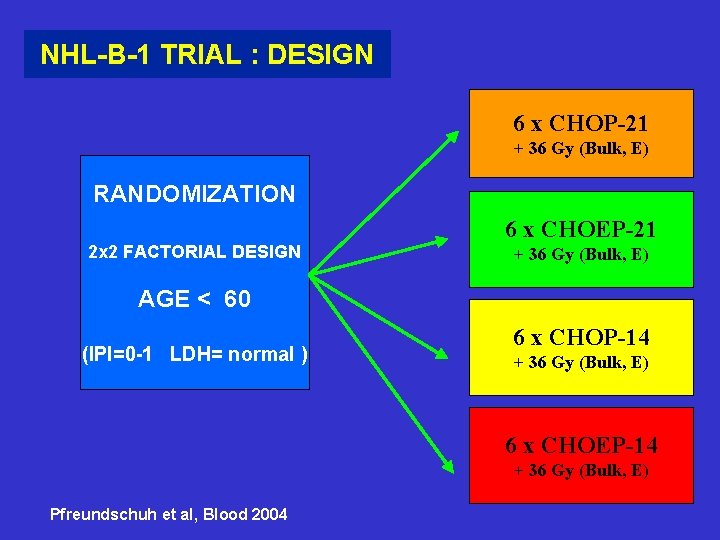
NHL-B-1 TRIAL : DESIGN 6 x CHOP-21 + 36 Gy (Bulk, E) RANDOMIZATION 2 x 2 FACTORIAL DESIGN 6 x CHOEP-21 + 36 Gy (Bulk, E) AGE < 60 (IPI=0 -1 LDH= normal ) 6 x CHOP-14 + 36 Gy (Bulk, E) 6 x CHOEP-14 + 36 Gy (Bulk, E) Pfreundschuh et al, Blood 2004

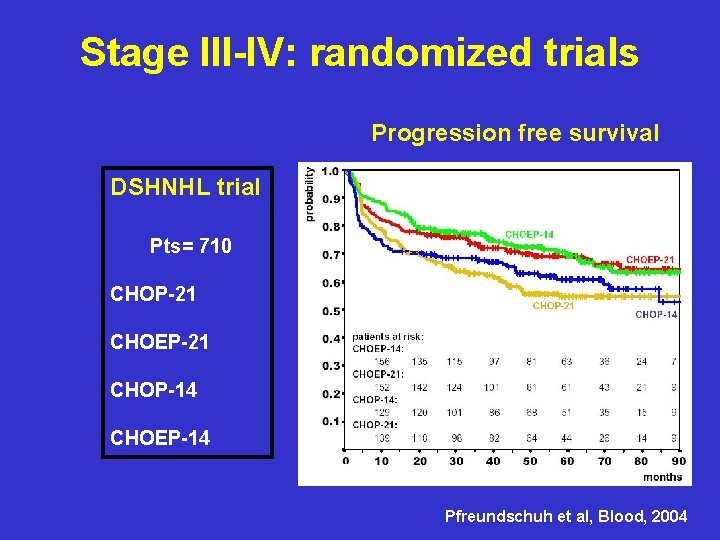
Stage III-IV: randomized trials Progression free survival DSHNHL trial Pts= 710 CHOP-21 CHOEP-21 CHOP-14 CHOEP-14 Pfreundschuh et al, Blood, 2004

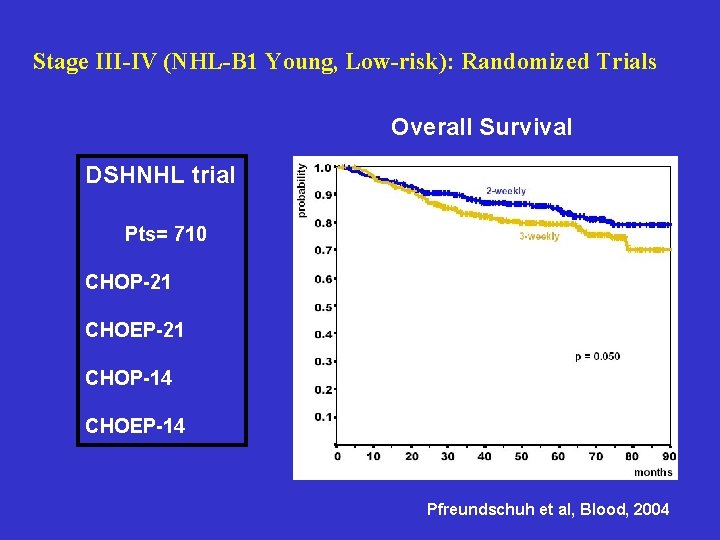
Stage III-IV (NHL-B 1 Young, Low-risk): Randomized Trials Overall Survival DSHNHL trial Pts= 710 CHOP-21 CHOEP-21 CHOP-14 CHOEP-14 Pfreundschuh et al, Blood, 2004

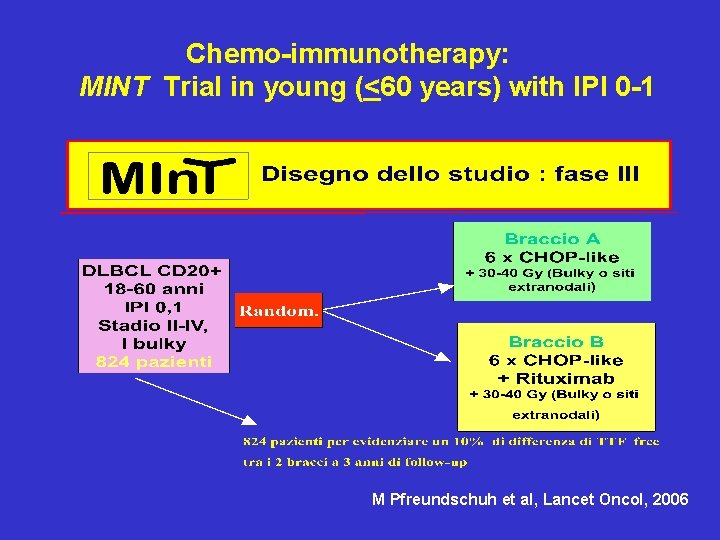
Chemo-immunotherapy: MINT Trial in young (<60 years) with IPI 0 -1 M Pfreundschuh et al, Lancet Oncol, 2006

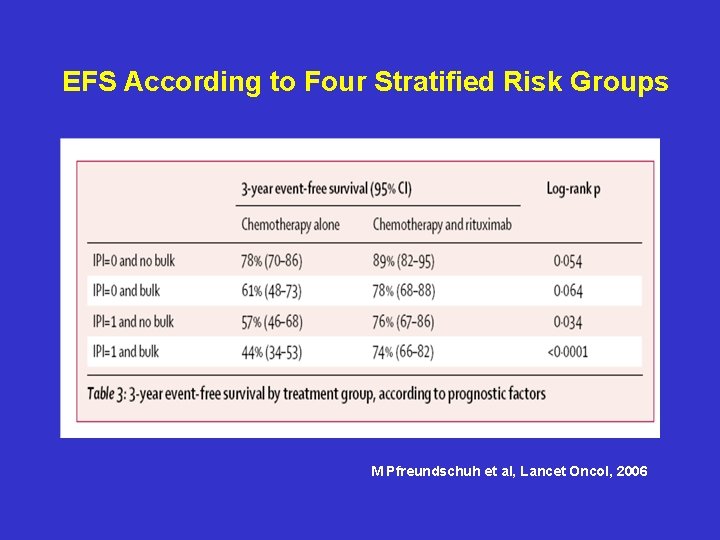
EFS According to Four Stratified Risk Groups M Pfreundschuh et al, Lancet Oncol, 2006

TREATMENT OF DIFFUSE LARGE B LYMPHOMAS Young IPI 0, 1 IPI 2, 3 low-risk Young Elderly high-risk ≤ 60 years >60 years

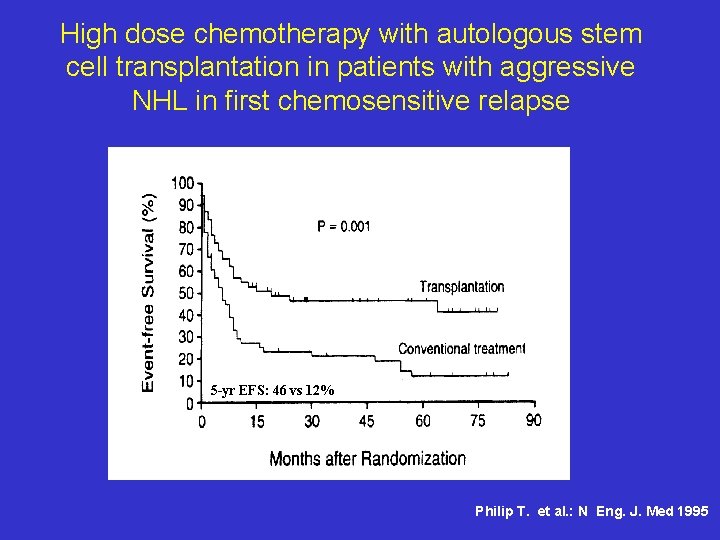
High dose chemotherapy with autologous stem cell transplantation in patients with aggressive NHL in first chemosensitive relapse 5 -yr EFS: 46 vs 12% Philip T. et al. : N Eng. J. Med 1995

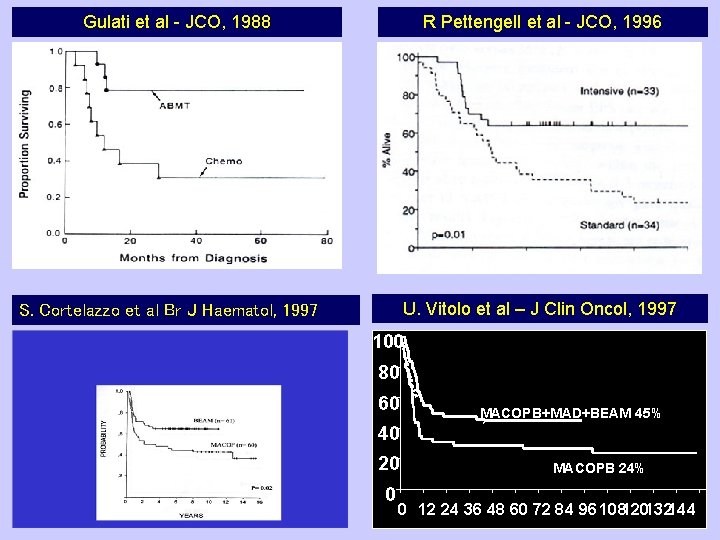
Gulati et al - JCO, 1988 R Pettengell et al - JCO, 1996 U. Vitolo et al – J Clin Oncol, 1997 S. Cortelazzo et al Br J Haematol, 1997 100 80 60 MACOPB+MAD+BEAM 45% 40 20 0 MACOPB 24% 0 12 24 36 48 60 72 84 96108120132144

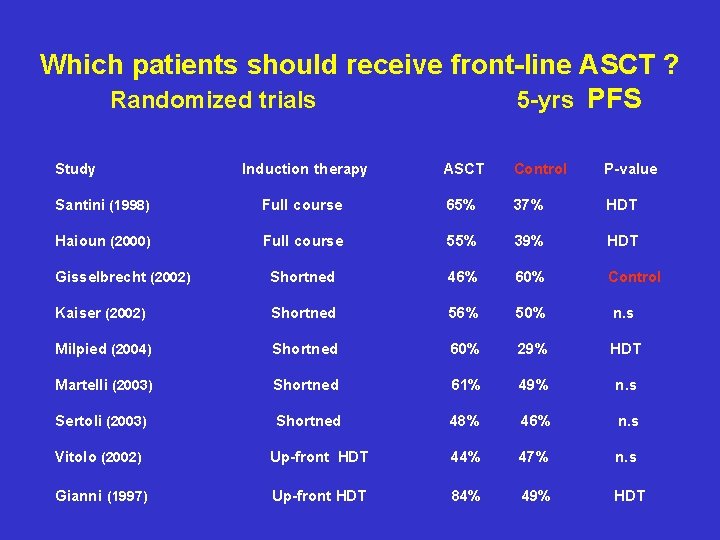
Which patients should receive front-line ASCT ? Randomized trials 5 -yrs PFS Study Induction therapy ASCT Control P-value Santini (1998) Full course 65% 37% HDT Haioun (2000) Full course 55% 39% HDT Gisselbrecht (2002) Shortned 46% 60% Control Kaiser (2002) Shortned 56% 50% n. s Milpied (2004) Shortned 60% 29% HDT Martelli (2003) Shortned 61% 49% n. s Sertoli (2003) Shortned 48% 46% n. s Vitolo (2002) Up-front HDT 44% 47% n. s Gianni (1997) Up-front HDT 84% 49% HDT

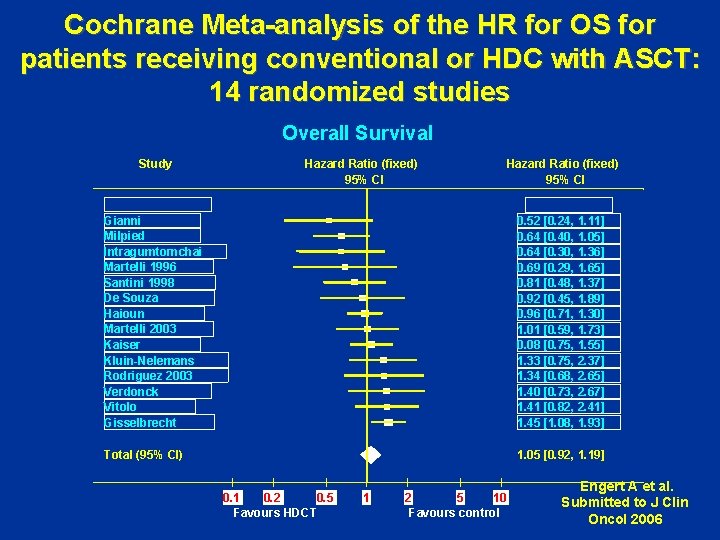
Cochrane Meta-analysis of the HR for OS for patients receiving conventional or HDC with ASCT: 14 randomized studies Overall Survival Study Hazard Ratio (fixed) 95% CI Gianni Milpied Intragumtornchai Martelli 1996 Santini 1998 De Souza Haioun Martelli 2003 Kaiser Kluin-Nelemans Rodriguez 2003 Verdonck Vitolo Gisselbrecht 0. 52 [0. 24, 1. 11] 0. 64 [0. 40, 1. 05] 0. 64 [0. 30, 1. 36] 0. 69 [0. 29, 1. 65] 0. 81 [0. 48, 1. 37] 0. 92 [0. 45, 1. 89] 0. 96 [0. 71, 1. 30] 1. 01 [0. 59, 1. 73] 0. 08 [0. 75, 1. 55] 1. 33 [0. 75, 2. 37] 1. 34 [0. 68, 2. 65] 1. 40 [0. 73, 2. 67] 1. 41 [0. 82, 2. 41] 1. 45 [1. 08, 1. 93] Total (95% CI) 1. 05 [0. 92, 1. 19] 0. 1 0. 2 0. 5 Favours HDCT 1 2 5 10 Favours control Engert A et al. Submitted to J Clin Oncol 2006

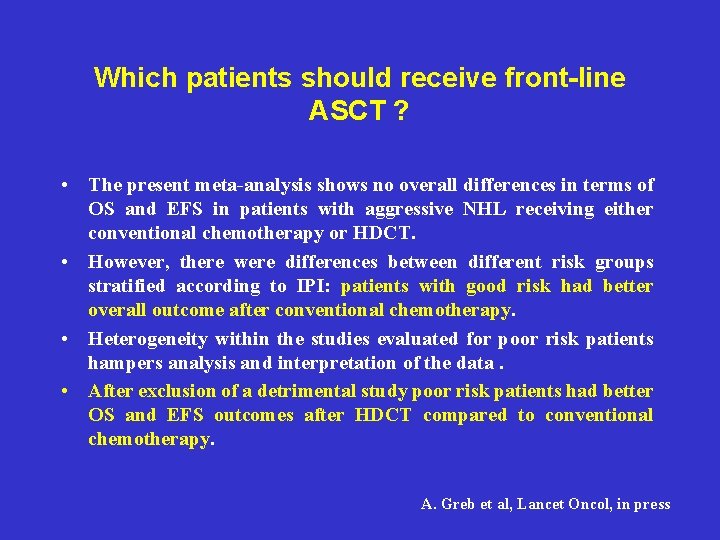
Which patients should receive front-line ASCT ? • The present meta-analysis shows no overall differences in terms of OS and EFS in patients with aggressive NHL receiving either conventional chemotherapy or HDCT. • However, there were differences between different risk groups stratified according to IPI: patients with good risk had better overall outcome after conventional chemotherapy. • Heterogeneity within the studies evaluated for poor risk patients hampers analysis and interpretation of the data. • After exclusion of a detrimental study poor risk patients had better OS and EFS outcomes after HDCT compared to conventional chemotherapy. A. Greb et al, Lancet Oncol, in press

Recommendations (Practice Guidelines from the SIE, SIES, GITMO) • Patients with stage III-IV disease should receive frontline chemoimmunotherapy with CHOP, CHOP-like or third-generation chemotherapy plus rituximab (grade A/B) • Patients with an intermediate-high/high IPI score and who are less than 65 years old may receive a frontline HDT/auto. SCT, but only within an approved study protocol. Patients enrolled into HDT/auto. SCT program should receive non-abbreviated debulking treatment (grade B) Barosi et al, Haematologica, 2006

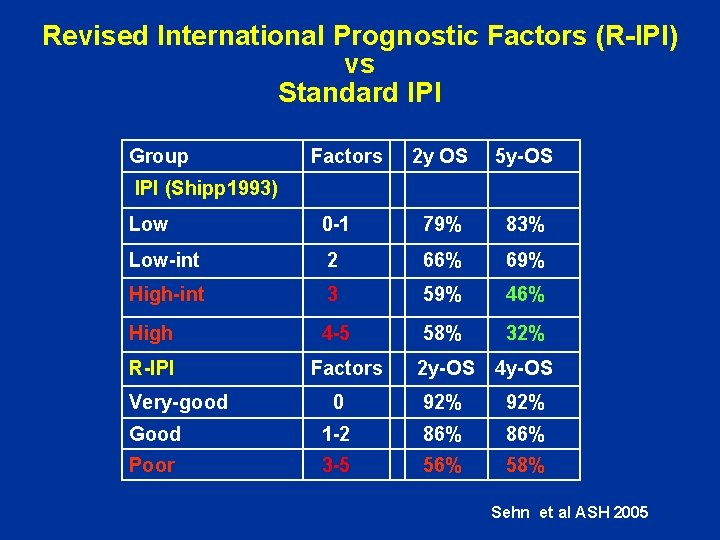
Revised International Prognostic Factors (R-IPI) vs Standard IPI Group Factors 2 y OS 5 y-OS IPI (Shipp 1993) Low 0 -1 79% 83% Low-int 2 66% 69% High-int 3 59% 46% High 4 -5 58% 32% 2 y-OS 4 y-OS 0 92% Good 1 -2 86% Poor 3 -5 56% 58% R-IPI Very-good Factors Sehn et al ASH 2005

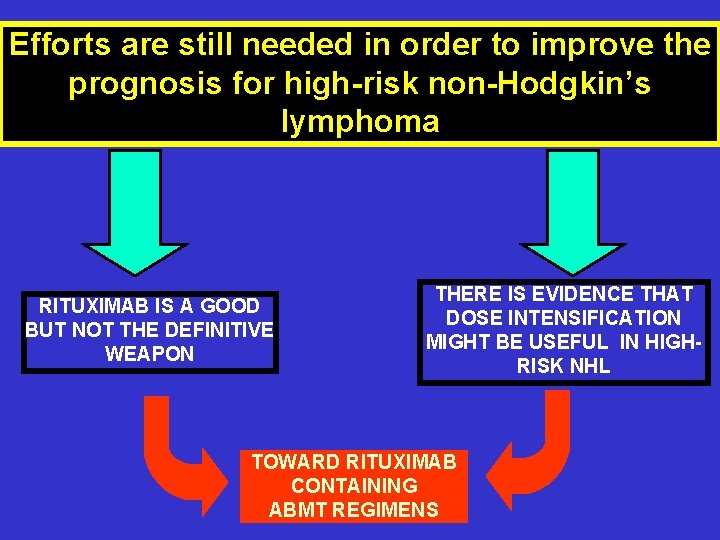
Efforts are still needed in order to improve the prognosis for high-risk non-Hodgkin’s lymphoma RITUXIMAB IS A GOOD BUT NOT THE DEFINITIVE WEAPON THERE IS EVIDENCE THAT DOSE INTENSIFICATION MIGHT BE USEFUL IN HIGHRISK NHL TOWARD RITUXIMAB CONTAINING ABMT REGIMENS

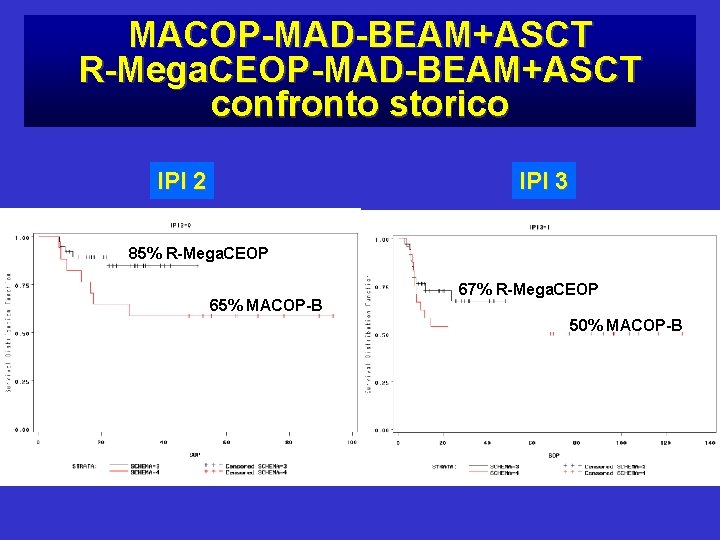
MACOP-MAD-BEAM+ASCT R-Mega. CEOP-MAD-BEAM+ASCT confronto storico IPI 2 IPI 3 85% R-Mega. CEOP 65% MACOP-B 67% R-Mega. CEOP 50% MACOP-B

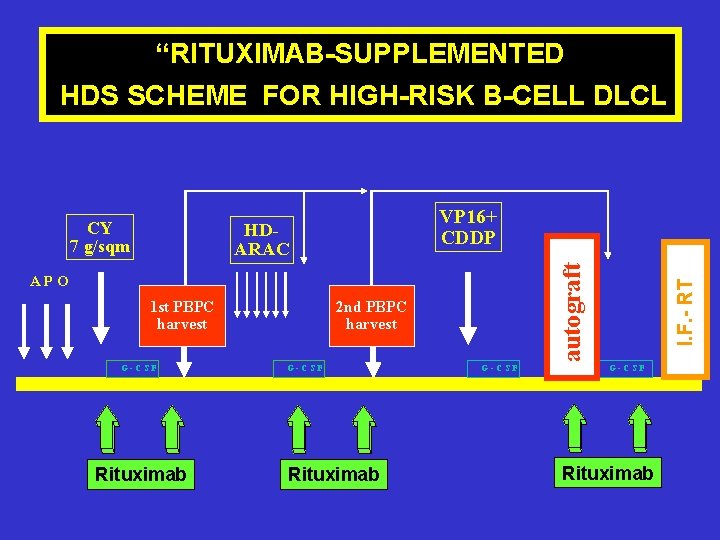
“RITUXIMAB-SUPPLEMENTED HDS SCHEME FOR HIGH-RISK B-CELL DLCL VP 16+ CDDP APO 1 st PBPC harvest G-CSF Rituximab 2 nd PBPC harvest G-CSF Rituximab G-CSF I. F. - RT HDARAC autograft CY 7 g/sqm G-CSF Rituximab


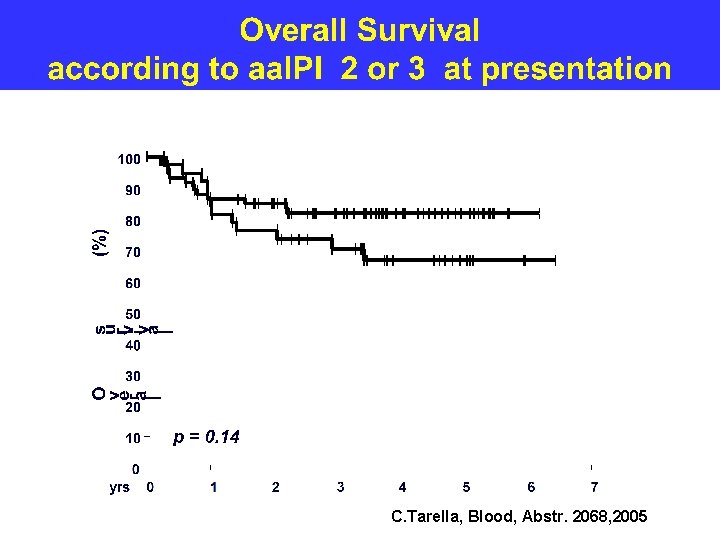
C. Tarella, Blood, Abstr. 2068, 2005

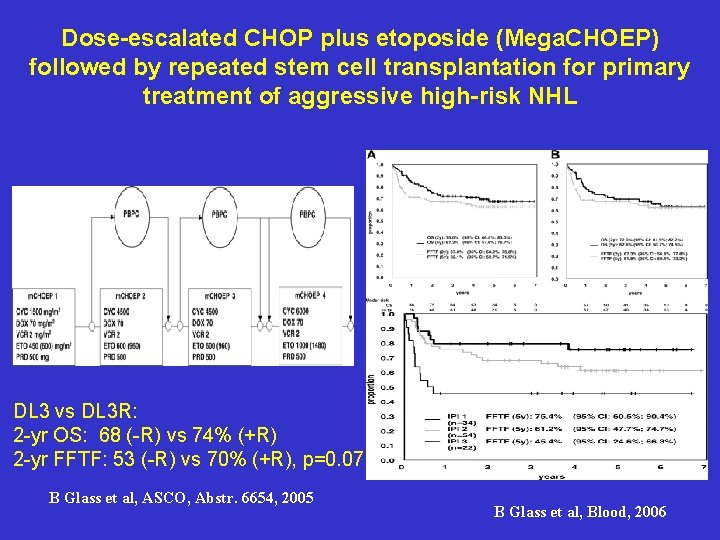
Dose-escalated CHOP plus etoposide (Mega. CHOEP) followed by repeated stem cell transplantation for primary treatment of aggressive high-risk NHL DL 3 vs DL 3 R: 2 -yr OS: 68 (-R) vs 74% (+R) 2 -yr FFTF: 53 (-R) vs 70% (+R), p=0. 07 B Glass et al, ASCO, Abstr. 6654, 2005 B Glass et al, Blood, 2006

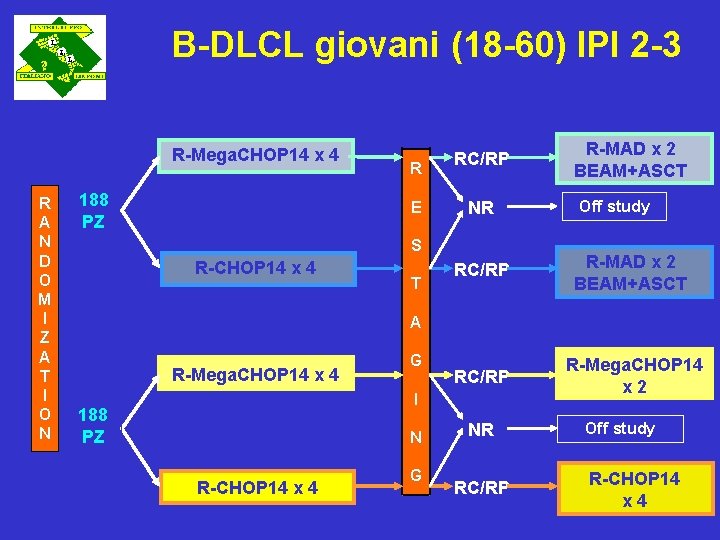
B-DLCL giovani (18 -60) IPI 2 -3 R-Mega. CHOP 14 x 4 R A N D O M I Z A T I O N 188 PZ R E RC/RP NR S R-CHOP 14 x 4 T R-MAD x 2 BEAM+ASCT Off study RC/RP R-MAD x 2 BEAM+ASCT RC/RP R-Mega. CHOP 14 x 2 A R-Mega. CHOP 14 x 4 G I 188 PZ N R-CHOP 14 x 4 G NR RC/RP Off study R-CHOP 14 x 4

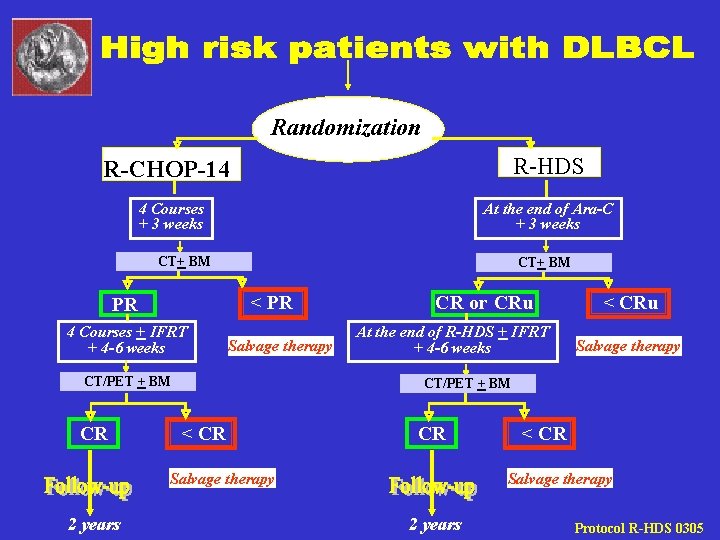
Randomization R-HDS R-CHOP-14 4 Courses + 3 weeks At the end of Ara-C + 3 weeks CT+ BM < PR PR 4 Courses + IFRT + 4 -6 weeks Salvage therapy CT/PET + BM CR CR or CRu At the end of R-HDS + IFRT + 4 -6 weeks Salvage therapy CT/PET + BM < CR CR Salvage therapy 2 years < CRu < CR Salvage therapy 2 years Protocol R-HDS 0305

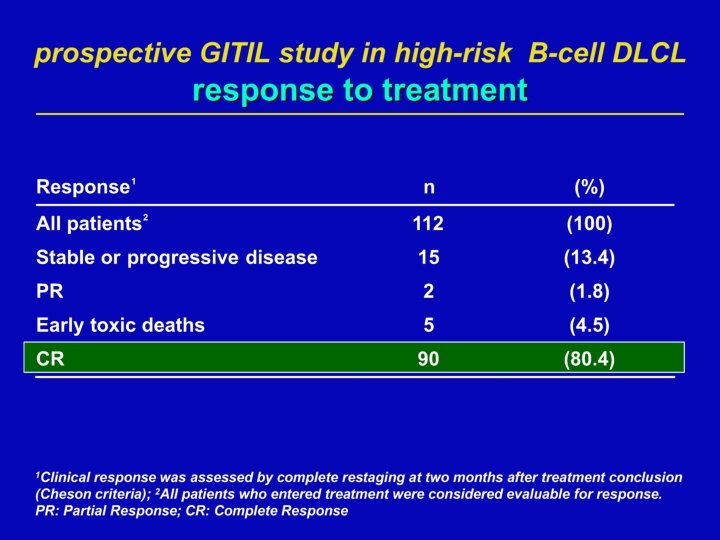
IIL-DLCL 04 GITIL-RHDS DLBCL cure R-CHOP 14 R-HDC R-HDS