Convegno Interregionale SIE Triveneto Verona 29 maggio 2008











- Slides: 49

Convegno Interregionale SIE Triveneto Verona, 29 maggio 2008 Aggiornamento Sindromi Mieloproliferative Croniche non LMC Francesco Rodeghiero Dipartimento di Terapie Cellulari ed Ematologia Ospedale San Bortolo - Vicenza

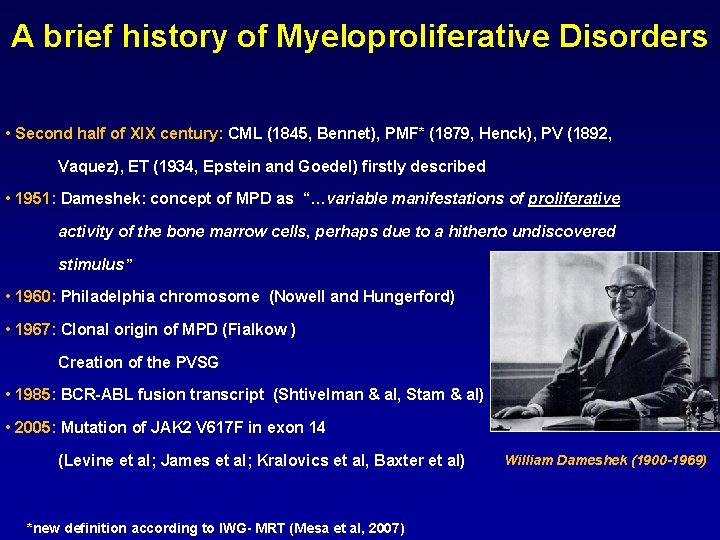
A brief history of Myeloproliferative Disorders • Second half of XIX century: CML (1845, Bennet), PMF* (1879, Henck), PV (1892, Vaquez), ET (1934, Epstein and Goedel) firstly described • 1951: Dameshek: concept of MPD as “…variable manifestations of proliferative activity of the bone marrow cells, perhaps due to a hitherto undiscovered stimulus” • 1960: Philadelphia chromosome (Nowell and Hungerford) • 1967: Clonal origin of MPD (Fialkow ) Creation of the PVSG • 1985: BCR-ABL fusion transcript (Shtivelman & al, Stam & al) • 2005: Mutation of JAK 2 V 617 F in exon 14 (Levine et al; James et al; Kralovics et al, Baxter et al) *new definition according to IWG- MRT (Mesa et al, 2007) William Dameshek (1900 -1969)

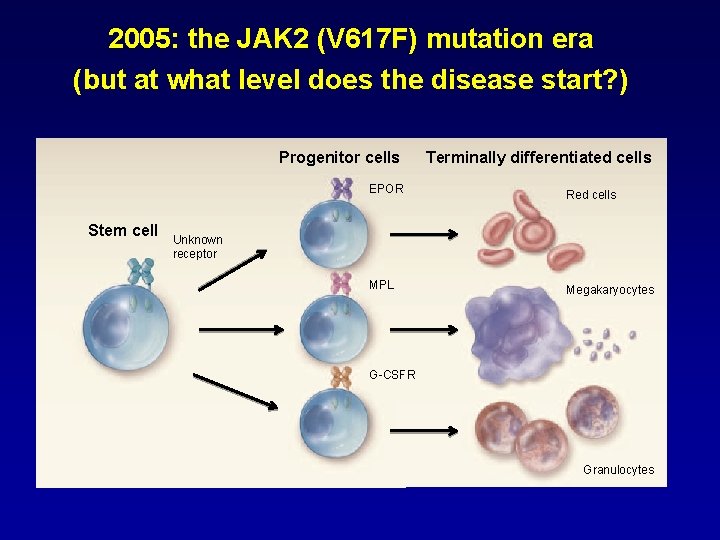
2005: the JAK 2 (V 617 F) mutation era (but at what level does the disease start? ) Progenitor cells Stem cell Terminally differentiated cells EPOR Red cells MPL Megakaryocytes Unknown receptor G-CSFR Granulocytes

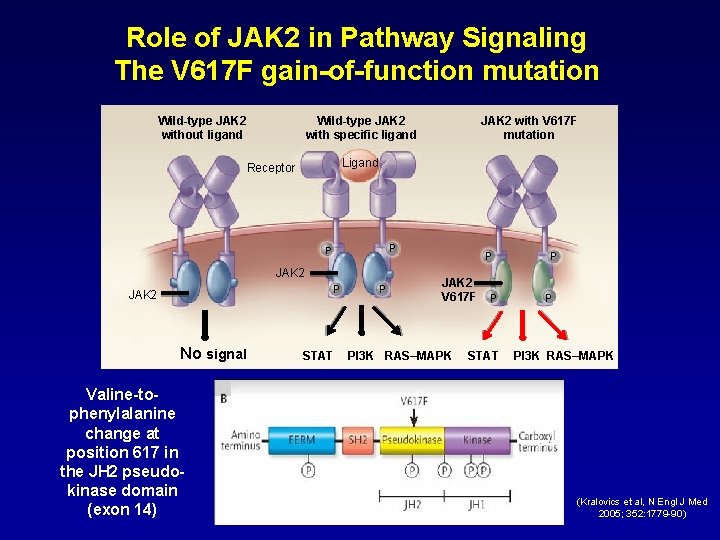
Role of JAK 2 in Pathway Signaling The V 617 F gain-of-function mutation Wild-type JAK 2 without ligand Wild-type JAK 2 with specific ligand Ligand Receptor P P JAK 2 No signal Valine-tophenylalanine change at position 617 in the JH 2 pseudokinase domain (exon 14) JAK 2 with V 617 F mutation STAT P P JAK 2 V 617 F PI 3 K RAS–MAPK P STAT P P PI 3 K RAS–MAPK (Kralovics et al, N Engl J Med 2005; 352: 1779 -90)

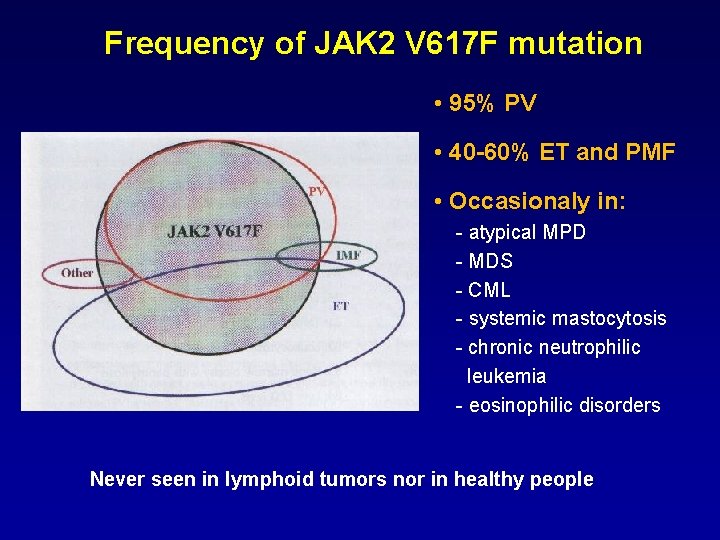
Frequency of JAK 2 V 617 F mutation • 95% PV • 40 -60% ET and PMF • Occasionaly in: - atypical MPD - MDS - CML - systemic mastocytosis - chronic neutrophilic leukemia - eosinophilic disorders Never seen in lymphoid tumors nor in healthy people

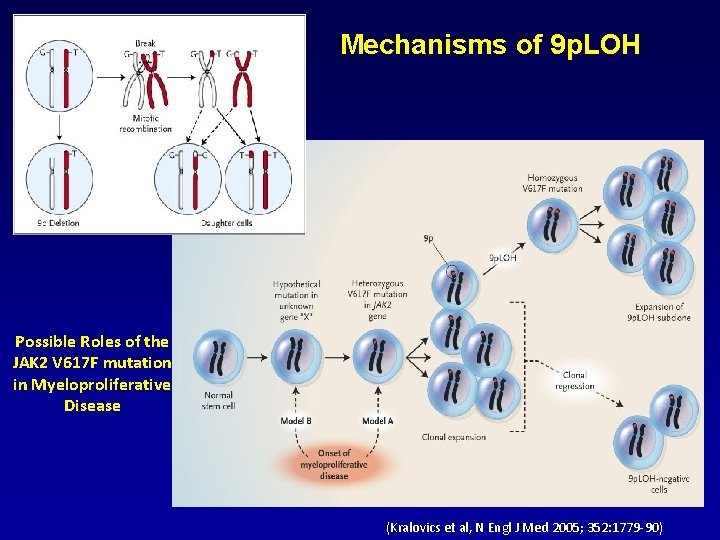
Mechanisms of 9 p. LOH Possible Roles of the JAK 2 V 617 F mutation in Myeloproliferative Disease (Kralovics et al, N Engl J Med 2005; 352: 1779 -90)

Other somatic mutations in MPDs • 3% PV patients JAK 2 V 617 F neg present a mutation in exon 12 of the JAK 2 • 5% PMF patients and 1% ET patients present mutations of the C-MPLgene (exon 10, W 515 L or W 515 K, S 505 N)

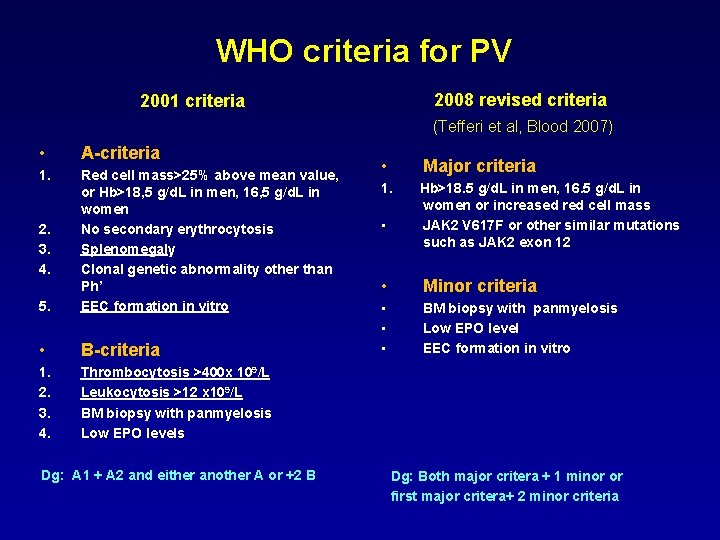
WHO criteria for PV 2008 revised criteria 2001 criteria (Tefferi et al, Blood 2007) • A-criteria 1. 5. Red cell mass>25% above mean value, or Hb>18, 5 g/d. L in men, 16, 5 g/d. L in women No secondary erythrocytosis Splenomegaly Clonal genetic abnormality other than Ph’ EEC formation in vitro • B-criteria 1. 2. 3. 4. Thrombocytosis >400 x 109/L Leukocytosis >12 x 109/L BM biopsy with panmyelosis Low EPO levels 2. 3. 4. Dg: A 1 + A 2 and either another A or +2 B • Major criteria 1. Hb>18. 5 g/d. L in men, 16. 5 g/d. L in women or increased red cell mass JAK 2 V 617 F or other similar mutations such as JAK 2 exon 12 • • Minor criteria • • • BM biopsy with panmyelosis Low EPO level EEC formation in vitro Dg: Both major critera + 1 minor or first major critera+ 2 minor criteria

WHO criteria for ET 2008 revised criteria 2001 criteria • Positive criteria • Sustained platelet count >600 x 109/L BM with proliferation of the MK (enlarged, mature MK) • • Criteria of exclusion • No evidence of PV, CML, CIMF, MDS No reactive thrombocytosis • Dg: all criteria (Tefferi et al, Blood 2007) • • Sustained platelet count >450 x 109/L BM biopsy with proliferation of the MK lineage (increased n. of enlarged, mature MK) No evidence of PV, PMF, CML, MDS or other myeloid neoplasm JAK 2 V 617 F or other clonal marker, or in its absence, no reactive thrombocytosis Dg: all criteria

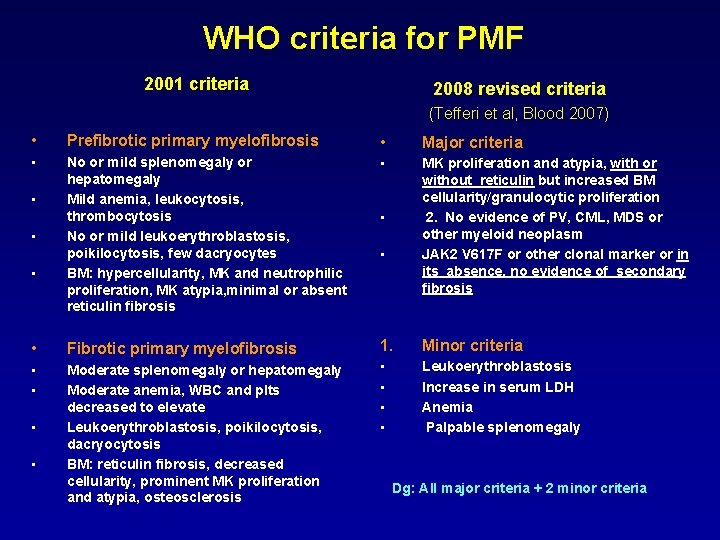
WHO criteria for PMF 2001 criteria 2008 revised criteria (Tefferi et al, Blood 2007) • Prefibrotic primary myelofibrosis • Major criteria • No or mild splenomegaly or hepatomegaly Mild anemia, leukocytosis, thrombocytosis No or mild leukoerythroblastosis, poikilocytosis, few dacryocytes BM: hypercellularity, MK and neutrophilic proliferation, MK atypia, minimal or absent reticulin fibrosis • MK proliferation and atypia, with or without reticulin but increased BM cellularity/granulocytic proliferation 2. No evidence of PV, CML, MDS or other myeloid neoplasm JAK 2 V 617 F or other clonal marker or in its absence, no evidence of secondary fibrosis • Fibrotic primary myelofibrosis 1. Minor criteria • • Moderate splenomegaly or hepatomegaly Moderate anemia, WBC and plts decreased to elevate Leukoerythroblastosis, poikilocytosis, dacryocytosis BM: reticulin fibrosis, decreased cellularity, prominent MK proliferation and atypia, osteosclerosis • • Leukoerythroblastosis Increase in serum LDH Anemia Palpable splenomegaly • • Dg: All major criteria + 2 minor criteria

Major criticisms of revised WHO diagnostic criteria (Spivak and Silver, Blood 2008) • Hb or Ht alone cannot substitute for red cell mass and plasma volume determinations • Since JAK 2 causes a ‘PV-like’ phenotype in ET pts, red cell mass and plasma volume must be required in isolated trombocytosis. • Since JAK 2 is the leading factor for classification, EPO and EEC assay (never standardized) are redundant • Marrow morphology reflects a particular stage in MPS evolution, so it is a non specific moving target with respect to phenotype • No differences between pts labeled as ‘prefibrotic MF’ or ‘true ET’ in clinical and laboratory features, JAK 2 status, survival, thrombosis and hemorrhage. • Histologic criteria in WHO classification are unreliable (Wilkins et al, Blood 2008)

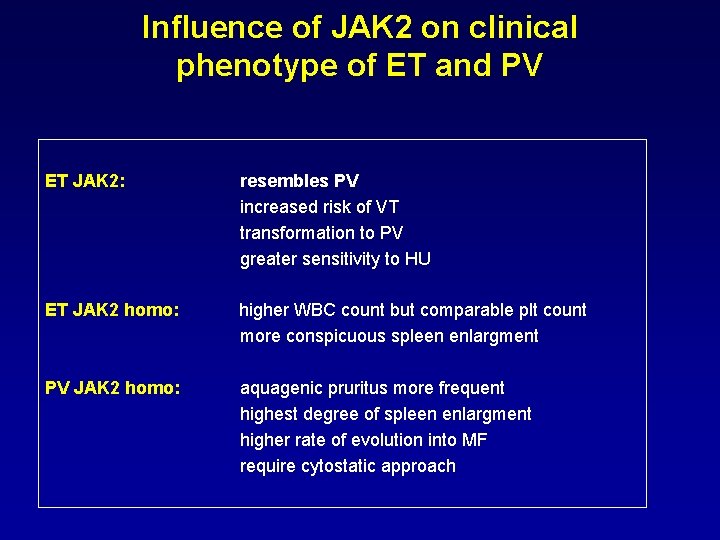
Influence of JAK 2 on clinical phenotype of ET and PV ET JAK 2: resembles PV increased risk of VT transformation to PV greater sensitivity to HU ET JAK 2 homo: higher WBC count but comparable plt count more conspicuous spleen enlargment PV JAK 2 homo: aquagenic pruritus more frequent highest degree of spleen enlargment higher rate of evolution into MF require cytostatic approach

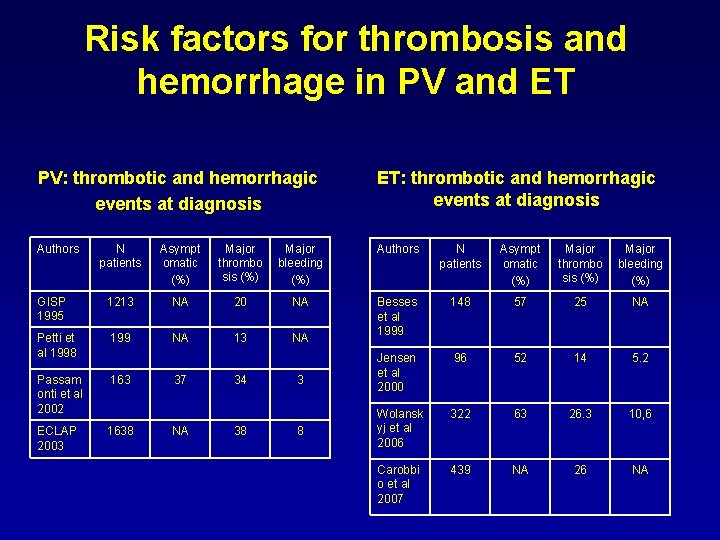
Risk factors for thrombosis and hemorrhage in PV and ET PV: thrombotic and hemorrhagic events at diagnosis ET: thrombotic and hemorrhagic events at diagnosis Authors N patients Asympt omatic (%) Major thrombo sis (%) Major bleeding (%) GISP 1995 1213 NA 20 NA 148 57 25 NA Petti et al 1998 199 NA 13 NA Besses et al 1999 96 52 14 5. 2 Passam onti et al 2002 163 Jensen et al 2000 322 63 26. 3 10, 6 ECLAP 2003 1638 Wolansk yj et al 2006 Carobbi o et al 2007 439 NA 26 NA 37 NA 34 38 3 8

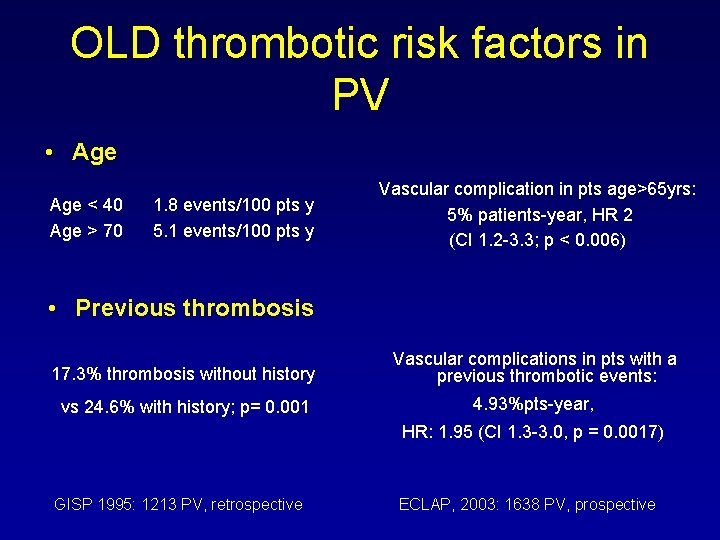
OLD thrombotic risk factors in PV • Age < 40 Age > 70 1. 8 events/100 pts y 5. 1 events/100 pts y Vascular complication in pts age>65 yrs: 5% patients-year, HR 2 (CI 1. 2 -3. 3; p < 0. 006) • Previous thrombosis 17. 3% thrombosis without history Vascular complications in pts with a previous thrombotic events: vs 24. 6% with history; p= 0. 001 4. 93%pts-year, HR: 1. 95 (CI 1. 3 -3. 0, p = 0. 0017) GISP 1995: 1213 PV, retrospective ECLAP, 2003: 1638 PV, prospective

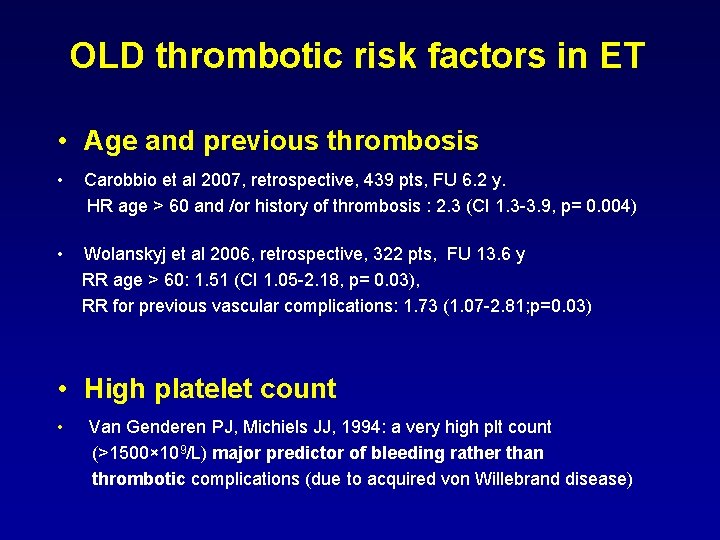
OLD thrombotic risk factors in ET • Age and previous thrombosis • Carobbio et al 2007, retrospective, 439 pts, FU 6. 2 y. HR age > 60 and /or history of thrombosis : 2. 3 (CI 1. 3 -3. 9, p= 0. 004) • Wolanskyj et al 2006, retrospective, 322 pts, FU 13. 6 y RR age > 60: 1. 51 (CI 1. 05 -2. 18, p= 0. 03), RR for previous vascular complications: 1. 73 (1. 07 -2. 81; p=0. 03) • High platelet count • Van Genderen PJ, Michiels JJ, 1994: a very high plt count (>1500× 109/L) major predictor of bleeding rather than thrombotic complications (due to acquired von Willebrand disease)

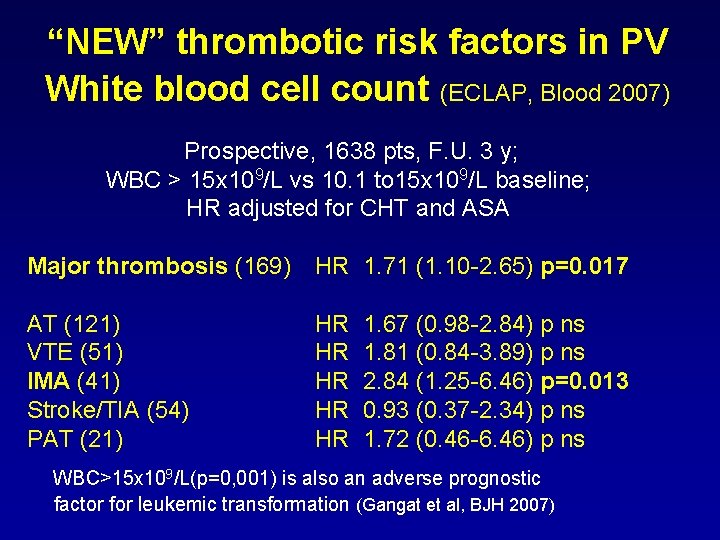
“NEW” thrombotic risk factors in PV White blood cell count (ECLAP, Blood 2007) Prospective, 1638 pts, F. U. 3 y; WBC > 15 x 109/L vs 10. 1 to 15 x 109/L baseline; HR adjusted for CHT and ASA Major thrombosis (169) HR 1. 71 (1. 10 -2. 65) p=0. 017 AT (121) VTE (51) IMA (41) Stroke/TIA (54) PAT (21) HR HR HR 1. 67 (0. 98 -2. 84) p ns 1. 81 (0. 84 -3. 89) p ns 2. 84 (1. 25 -6. 46) p=0. 013 0. 93 (0. 37 -2. 34) p ns 1. 72 (0. 46 -6. 46) p ns WBC>15 x 109/L(p=0, 001) is also an adverse prognostic factor for leukemic transformation (Gangat et al, BJH 2007)

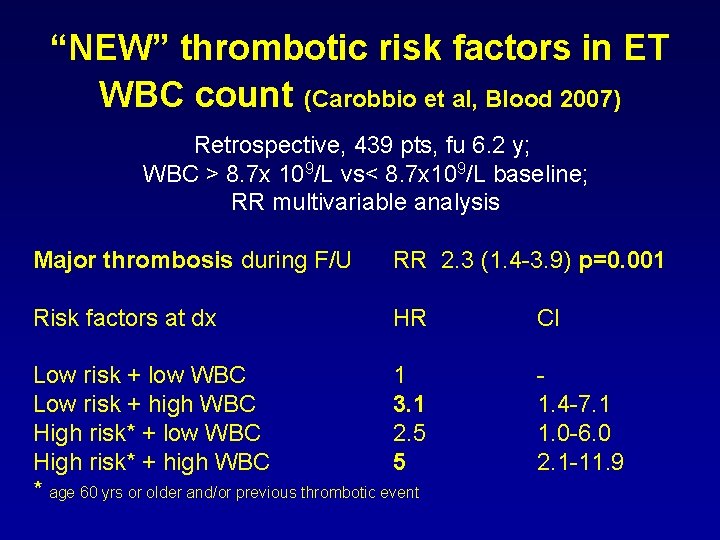
“NEW” thrombotic risk factors in ET WBC count (Carobbio et al, Blood 2007) Retrospective, 439 pts, fu 6. 2 y; WBC > 8. 7 x 109/L vs< 8. 7 x 109/L baseline; RR multivariable analysis Major thrombosis during F/U RR 2. 3 (1. 4 -3. 9) p=0. 001 Risk factors at dx HR Low risk + low WBC 1 Low risk + high WBC 3. 1 High risk* + low WBC 2. 5 High risk* + high WBC 5 * age 60 yrs or older and/or previous thrombotic event CI 1. 4 -7. 1 1. 0 -6. 0 2. 1 -11. 9

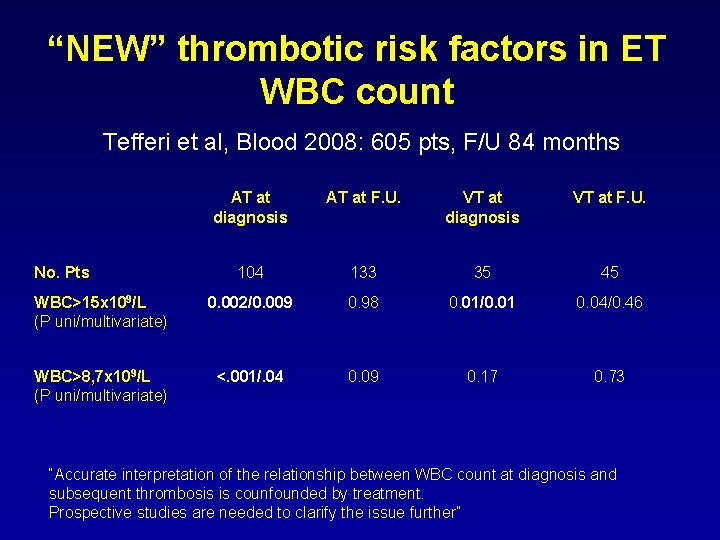
“NEW” thrombotic risk factors in ET WBC count Tefferi et al, Blood 2008: 605 pts, F/U 84 months AT at diagnosis AT at F. U. VT at diagnosis VT at F. U. 104 133 35 45 WBC>15 x 109/L (P uni/multivariate) 0. 002/0. 009 0. 98 0. 01/0. 01 0. 04/0. 46 WBC>8, 7 x 109/L (P uni/multivariate) <. 001/. 04 0. 09 0. 17 0. 73 No. Pts “Accurate interpretation of the relationship between WBC count at diagnosis and subsequent thrombosis is counfounded by treatment. Prospective studies are needed to clarify the issue further”

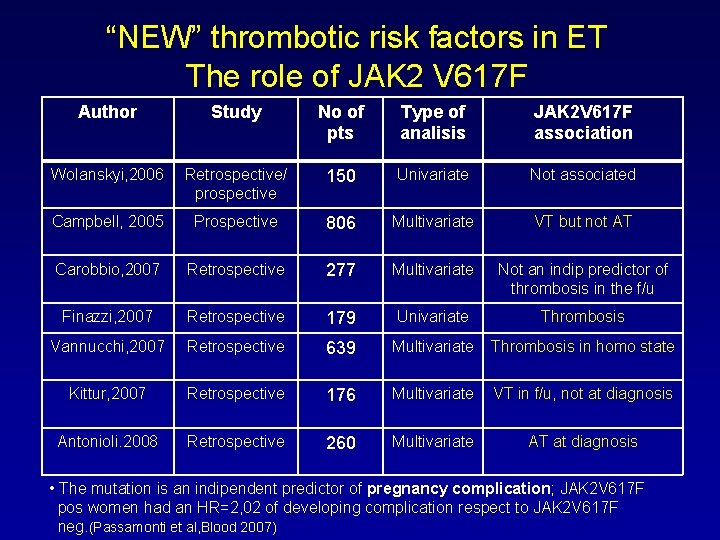
“NEW” thrombotic risk factors in ET The role of JAK 2 V 617 F Author Study No of pts Type of analisis JAK 2 V 617 F association Wolanskyi, 2006 Retrospective/ prospective 150 Univariate Not associated Campbell, 2005 Prospective 806 Multivariate VT but not AT Carobbio, 2007 Retrospective 277 Multivariate Not an indip predictor of thrombosis in the f/u Finazzi, 2007 Retrospective 179 Univariate Thrombosis Vannucchi, 2007 Retrospective 639 Multivariate Thrombosis in homo state Kittur, 2007 Retrospective 176 Multivariate VT in f/u, not at diagnosis Antonioli. 2008 Retrospective 260 Multivariate AT at diagnosis • The mutation is an indipendent predictor of pregnancy complication; JAK 2 V 617 F pos women had an HR=2, 02 of developing complication respect to JAK 2 V 617 F neg. (Passamonti et al, Blood 2007)

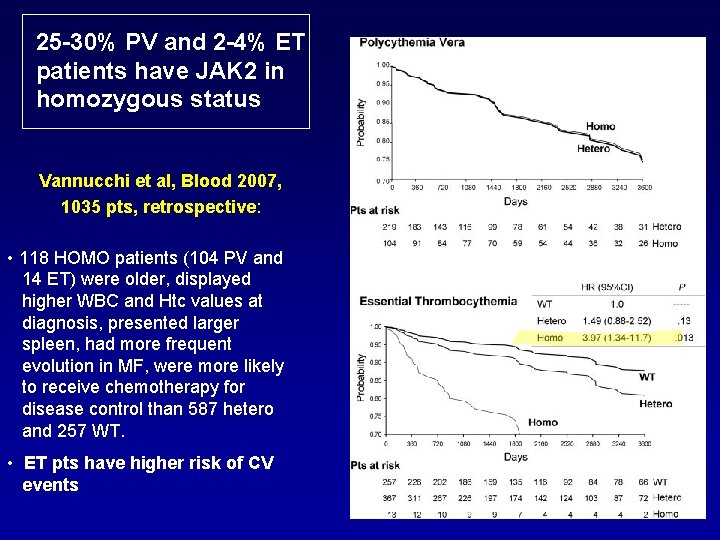
25 -30% PV and 2 -4% ET patients have JAK 2 in homozygous status Vannucchi et al, Blood 2007, 1035 pts, retrospective: • 118 HOMO patients (104 PV and 14 ET) were older, displayed higher WBC and Htc values at diagnosis, presented larger spleen, had more frequent evolution in MF, were more likely to receive chemotherapy for disease control than 587 hetero and 257 WT. • ET pts have higher risk of CV events

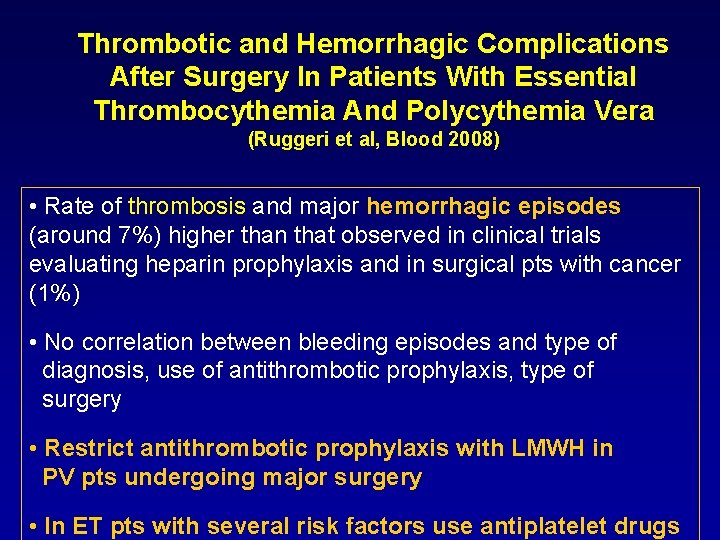
Thrombotic and Hemorrhagic Complications After Surgery In Patients With Essential Thrombocythemia And Polycythemia Vera (Ruggeri et al, Blood 2008) • Rate of thrombosis and major hemorrhagic episodes (around 7%) higher than that observed in clinical trials evaluating heparin prophylaxis and in surgical pts with cancer (1%) • No correlation between bleeding episodes and type of diagnosis, use of antithrombotic prophylaxis, type of surgery • Restrict antithrombotic prophylaxis with LMWH in PV pts undergoing major surgery • In ET pts with several risk factors use antiplatelet drugs

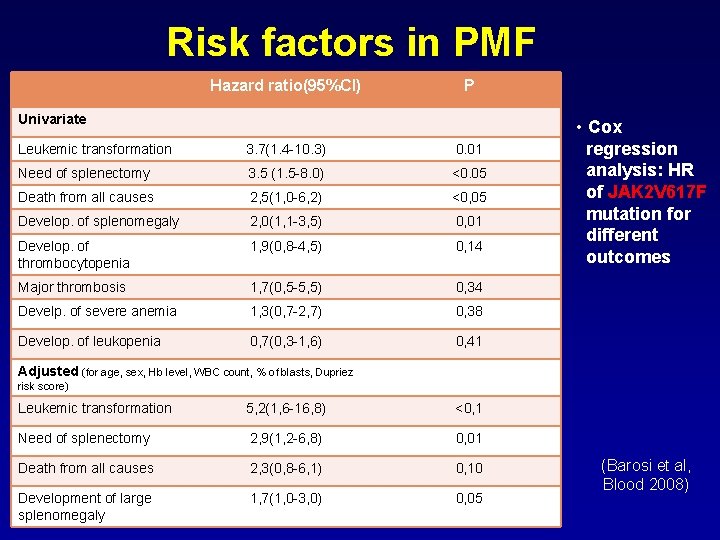
Risk factors in PMF Hazard ratio(95%CI) P Univariate Leukemic transformation 3. 7(1. 4 -10. 3) 0. 01 Need of splenectomy 3. 5 (1. 5 -8. 0) <0. 05 Death from all causes 2, 5(1, 0 -6, 2) <0, 05 Develop. of splenomegaly 2, 0(1, 1 -3, 5) 0, 01 Develop. of thrombocytopenia 1, 9(0, 8 -4, 5) 0, 14 Major thrombosis 1, 7(0, 5 -5, 5) 0, 34 Develp. of severe anemia 1, 3(0, 7 -2, 7) 0, 38 Develop. of leukopenia 0, 7(0, 3 -1, 6) 0, 41 • Cox regression analysis: HR of JAK 2 V 617 F mutation for different outcomes Adjusted (for age, sex, Hb level, WBC count, % of blasts, Dupriez risk score) Leukemic transformation 5, 2(1, 6 -16, 8) <0, 1 Need of splenectomy 2, 9(1, 2 -6, 8) 0, 01 Death from all causes 2, 3(0, 8 -6, 1) 0, 10 Development of large splenomegaly 1, 7(1, 0 -3, 0) 0, 05 (Barosi et al, Blood 2008)

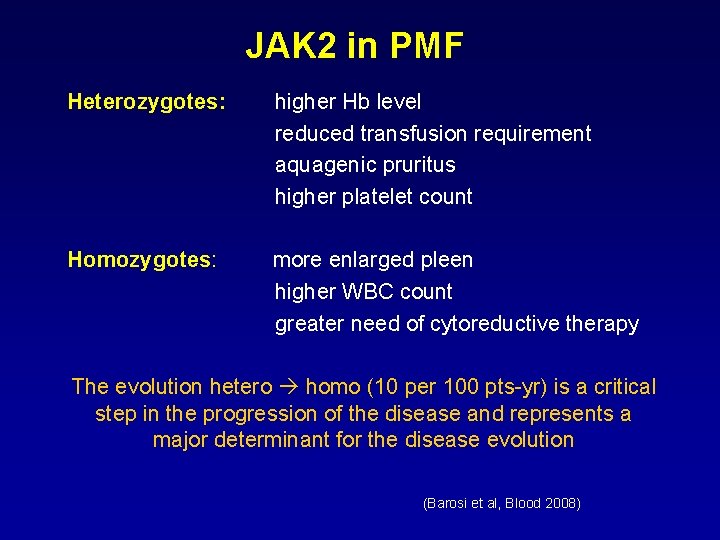
JAK 2 in PMF Heterozygotes: higher Hb level reduced transfusion requirement aquagenic pruritus higher platelet count Homozygotes: more enlarged pleen higher WBC count greater need of cytoreductive therapy The evolution hetero homo (10 per 100 pts-yr) is a critical step in the progression of the disease and represents a major determinant for the disease evolution (Barosi et al, Blood 2008)

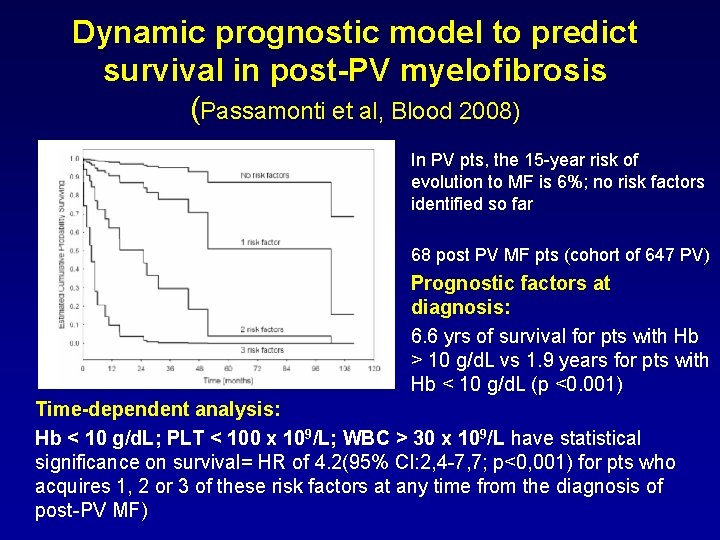
Dynamic prognostic model to predict survival in post-PV myelofibrosis (Passamonti et al, Blood 2008) In PV pts, the 15 -year risk of evolution to MF is 6%; no risk factors identified so far 68 post PV MF pts (cohort of 647 PV) Prognostic factors at diagnosis: 6. 6 yrs of survival for pts with Hb > 10 g/d. L vs 1. 9 years for pts with Hb < 10 g/d. L (p <0. 001) Time-dependent analysis: Hb < 10 g/d. L; PLT < 100 x 109/L; WBC > 30 x 109/L have statistical significance on survival= HR of 4. 2(95% CI: 2, 4 -7, 7; p<0, 001) for pts who acquires 1, 2 or 3 of these risk factors at any time from the diagnosis of post-PV MF)

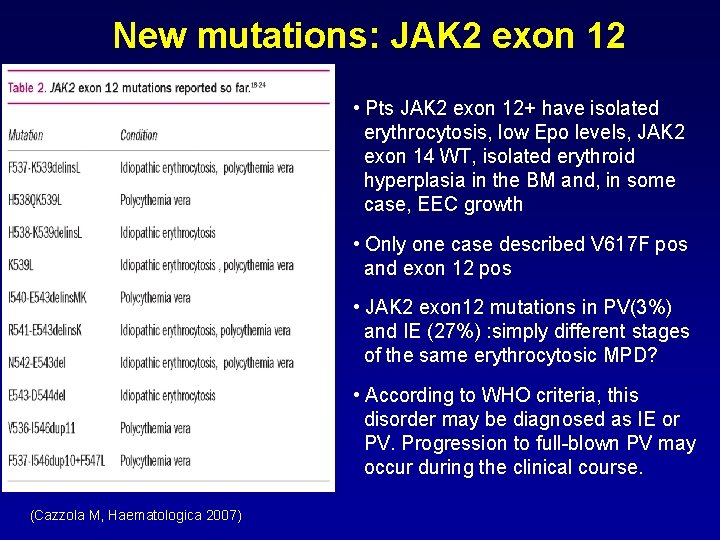
New mutations: JAK 2 exon 12 • Pts JAK 2 exon 12+ have isolated erythrocytosis, low Epo levels, JAK 2 exon 14 WT, isolated erythroid hyperplasia in the BM and, in some case, EEC growth • Only one case described V 617 F pos and exon 12 pos • JAK 2 exon 12 mutations in PV(3%) and IE (27%) : simply different stages of the same erythrocytosic MPD? • According to WHO criteria, this disorder may be diagnosed as IE or PV. Progression to full-blown PV may occur during the clinical course. (Cazzola M, Haematologica 2007)

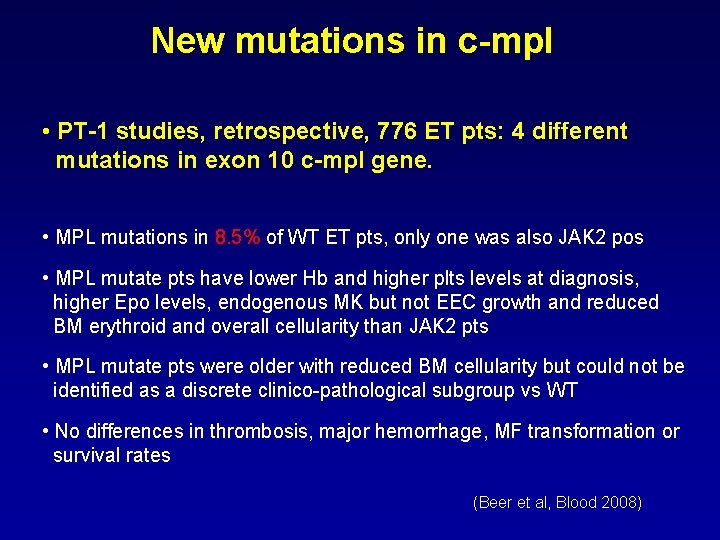
New mutations in c-mpl • PT-1 studies, retrospective, 776 ET pts: 4 different mutations in exon 10 c-mpl gene. • MPL mutations in 8. 5% of WT ET pts, only one was also JAK 2 pos • MPL mutate pts have lower Hb and higher plts levels at diagnosis, higher Epo levels, endogenous MK but not EEC growth and reduced BM erythroid and overall cellularity than JAK 2 pts • MPL mutate pts were older with reduced BM cellularity but could not be identified as a discrete clinico-pathological subgroup vs WT • No differences in thrombosis, major hemorrhage, MF transformation or survival rates (Beer et al, Blood 2008)

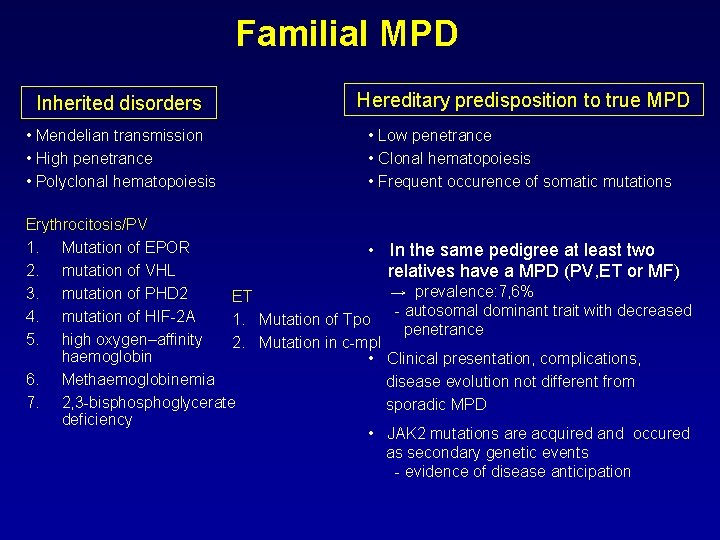
Familial MPD Inherited disorders Hereditary predisposition to true MPD • Mendelian transmission • High penetrance • Polyclonal hematopoiesis • Low penetrance • Clonal hematopoiesis • Frequent occurence of somatic mutations Erythrocitosis/PV 1. Mutation of EPOR • In the same pedigree at least two 2. mutation of VHL relatives have a MPD (PV, ET or MF) → prevalence: 7, 6% 3. mutation of PHD 2 ET - autosomal dominant trait with decreased 4. mutation of HIF-2 A 1. Mutation of Tpo penetrance 5. high oxygen–affinity 2. Mutation in c-mpl haemoglobin • Clinical presentation, complications, 6. Methaemoglobinemia disease evolution not different from 7. 2, 3 -bisphoglycerate sporadic MPD deficiency • JAK 2 mutations are acquired and occured as secondary genetic events - evidence of disease anticipation

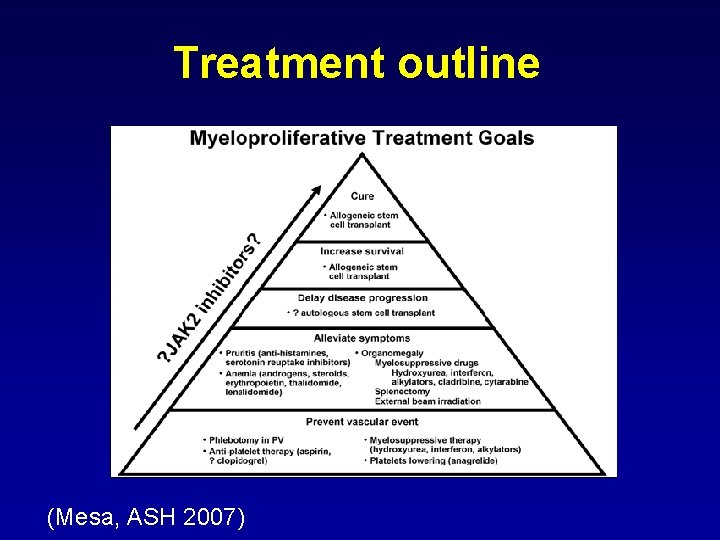
Treatment outline (Mesa, ASH 2007)

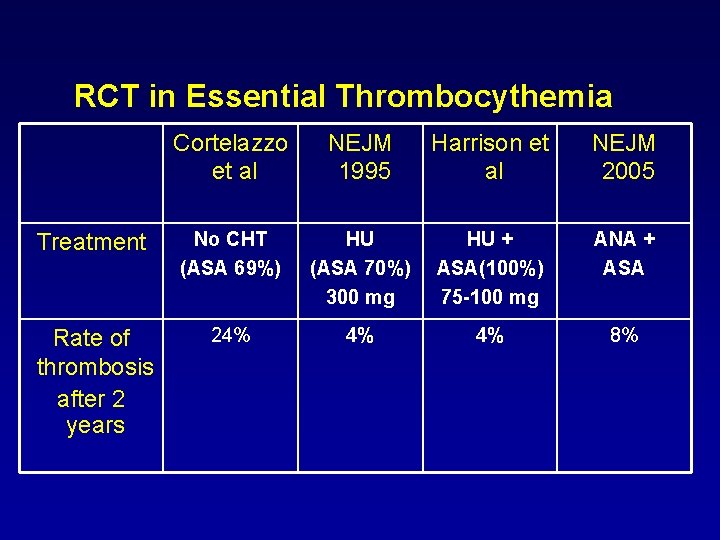
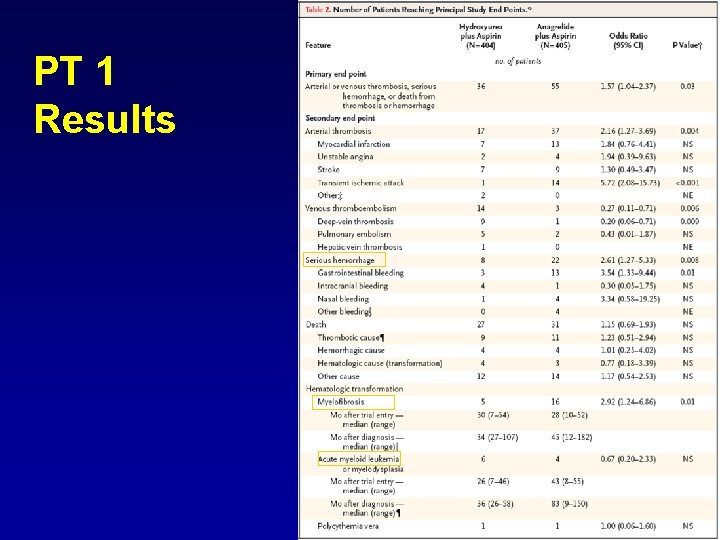
RCT in Essential Thrombocythemia Cortelazzo et al NEJM 1995 Harrison et al NEJM 2005 Treatment No CHT (ASA 69%) HU (ASA 70%) 300 mg HU + ASA(100%) 75 -100 mg ANA + ASA Rate of thrombosis after 2 years 24% 4% 4% 8%

PT 1 Results

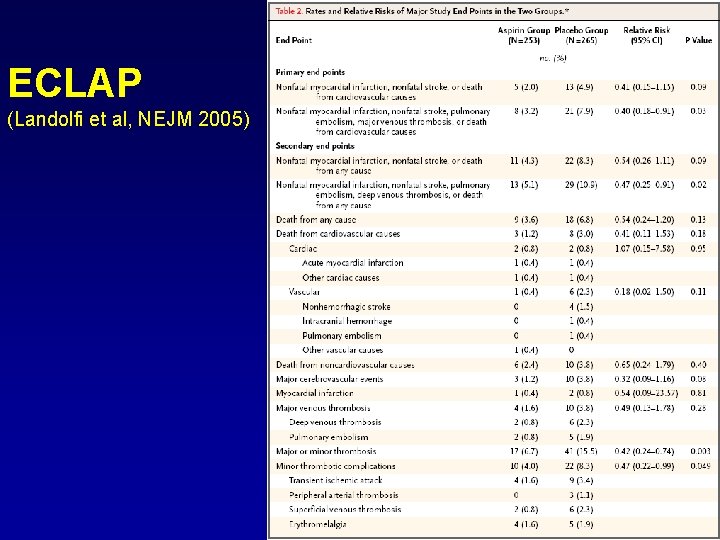
ECLAP (Landolfi et al, NEJM 2005)

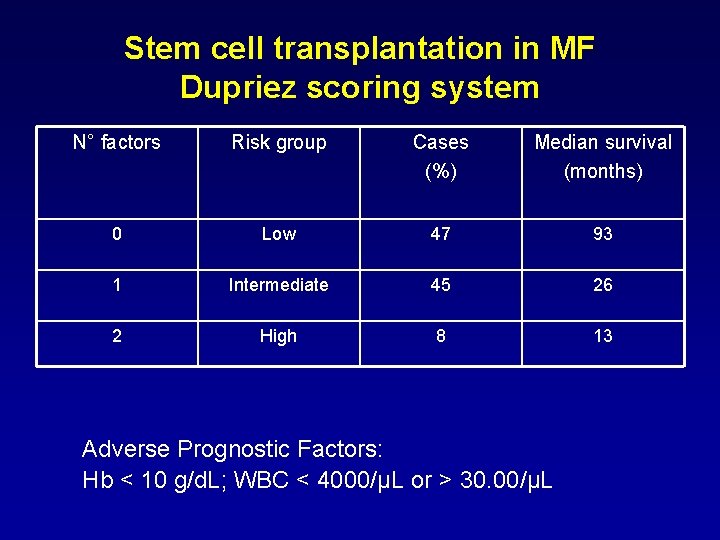
Stem cell transplantation in MF Dupriez scoring system N° factors Risk group Cases (%) Median survival (months) 0 Low 47 93 1 Intermediate 45 26 2 High 8 13 Adverse Prognostic Factors: Hb < 10 g/d. L; WBC < 4000/µL or > 30. 00/µL

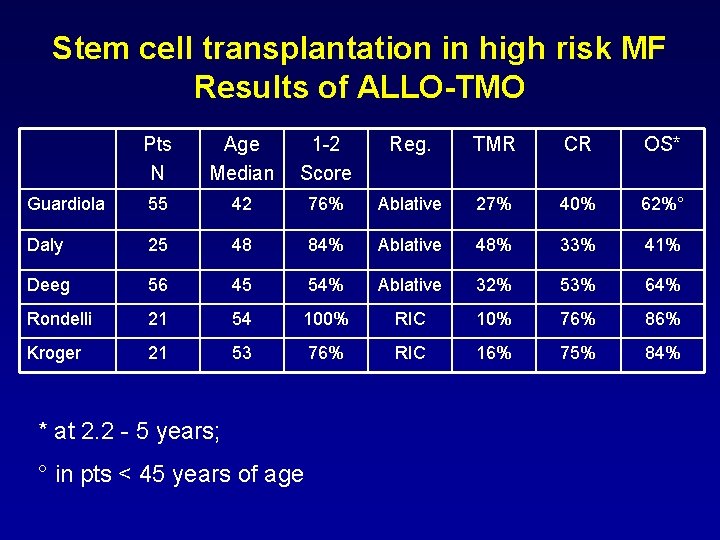
Stem cell transplantation in high risk MF Results of ALLO-TMO Pts N Age Median 1 -2 Score Reg. TMR CR OS* Guardiola 55 42 76% Ablative 27% 40% 62%° Daly 25 48 84% Ablative 48% 33% 41% Deeg 56 45 54% Ablative 32% 53% 64% Rondelli 21 54 100% RIC 10% 76% 86% Kroger 21 53 76% RIC 16% 75% 84% * at 2. 2 - 5 years; ° in pts < 45 years of age

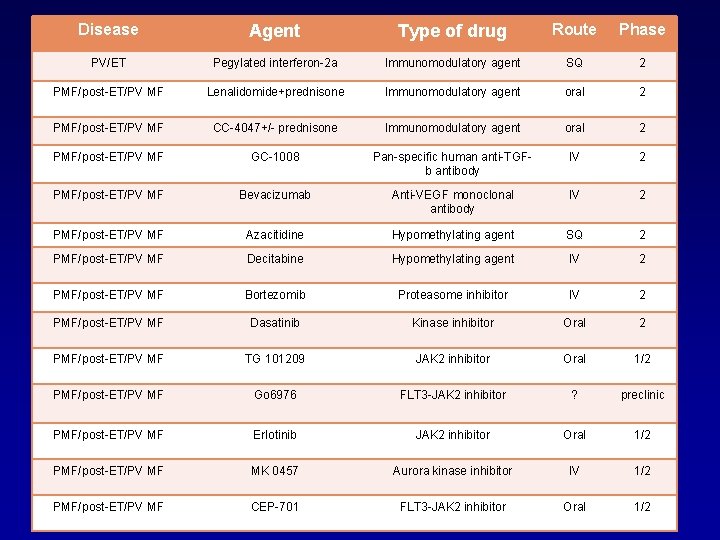
Disease Agent Type of drug Route Phase PV/ET Pegylated interferon-2 a Immunomodulatory agent SQ 2 PMF/post-ET/PV MF Lenalidomide+prednisone Immunomodulatory agent oral 2 PMF/post-ET/PV MF CC-4047+/- prednisone Immunomodulatory agent oral 2 PMF/post-ET/PV MF GC-1008 Pan-specific human anti-TGFb antibody IV 2 PMF/post-ET/PV MF Bevacizumab Anti-VEGF monoclonal antibody IV 2 PMF/post-ET/PV MF Azacitidine Hypomethylating agent SQ 2 PMF/post-ET/PV MF Decitabine Hypomethylating agent IV 2 PMF/post-ET/PV MF Bortezomib Proteasome inhibitor IV 2 PMF/post-ET/PV MF Dasatinib Kinase inhibitor Oral 2 PMF/post-ET/PV MF TG 101209 JAK 2 inhibitor Oral 1/2 PMF/post-ET/PV MF Go 6976 FLT 3 -JAK 2 inhibitor ? preclinic PMF/post-ET/PV MF Erlotinib JAK 2 inhibitor Oral 1/2 PMF/post-ET/PV MF MK 0457 Aurora kinase inhibitor IV 1/2 PMF/post-ET/PV MF CEP-701 FLT 3 -JAK 2 inhibitor Oral 1/2

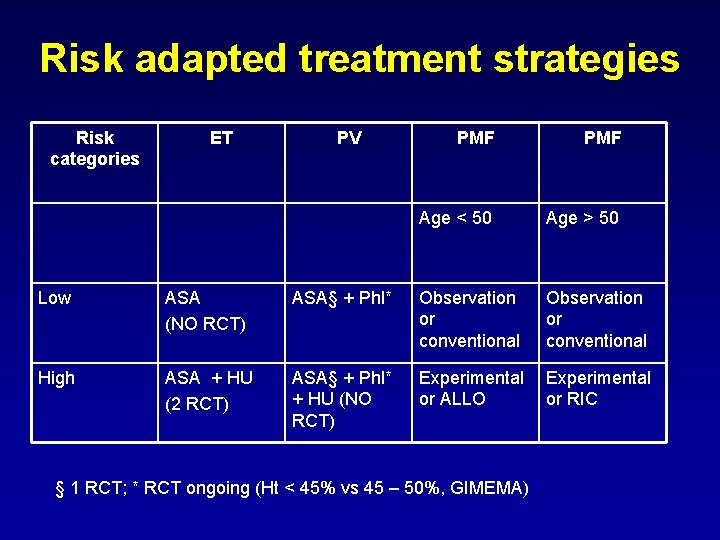
Risk adapted treatment strategies Risk categories ET PV PMF Age < 50 Age > 50 Low ASA (NO RCT) ASA§ + Phl* Observation or conventional High ASA + HU (2 RCT) ASA§ + Phl* + HU (NO RCT) Experimental or ALLO Experimental or RIC § 1 RCT; * RCT ongoing (Ht < 45% vs 45 – 50%, GIMEMA)

Acknowledgments • Marco Ruggeri • Elena Albiero • Martina Bernardi • Silvia Finotto



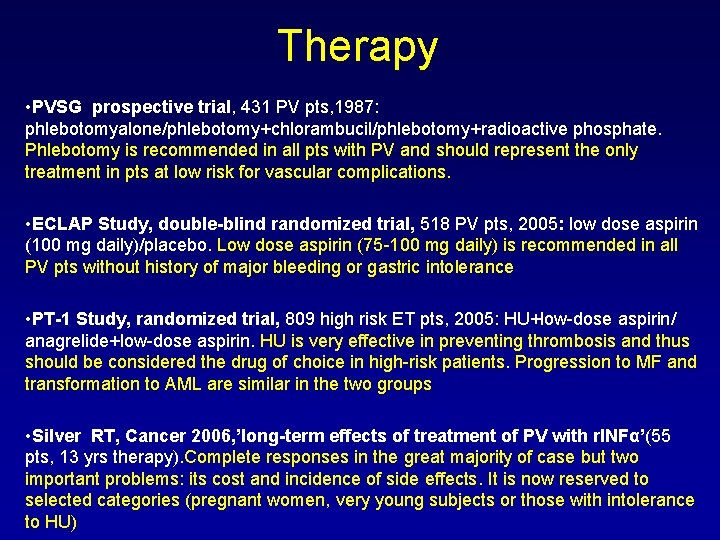
Therapy • PVSG prospective trial, 431 PV pts, 1987: phlebotomyalone/phlebotomy+chlorambucil/phlebotomy+radioactive phosphate. Phlebotomy is recommended in all pts with PV and should represent the only treatment in pts at low risk for vascular complications. • ECLAP Study, double-blind randomized trial, 518 PV pts, 2005: low dose aspirin (100 mg daily)/placebo. Low dose aspirin (75 -100 mg daily) is recommended in all PV pts without history of major bleeding or gastric intolerance • PT-1 Study, randomized trial, 809 high risk ET pts, 2005: HU+low-dose aspirin/ anagrelide+low-dose aspirin. HU is very effective in preventing thrombosis and thus should be considered the drug of choice in high-risk patients. Progression to MF and transformation to AML are similar in the two groups • Silver RT, Cancer 2006, ’long-term effects of treatment of PV with r. INFα’(55 pts, 13 yrs therapy). Complete responses in the great majority of case but two important problems: its cost and incidence of side effects. It is now reserved to selected categories (pregnant women, very young subjects or those with intolerance to HU)

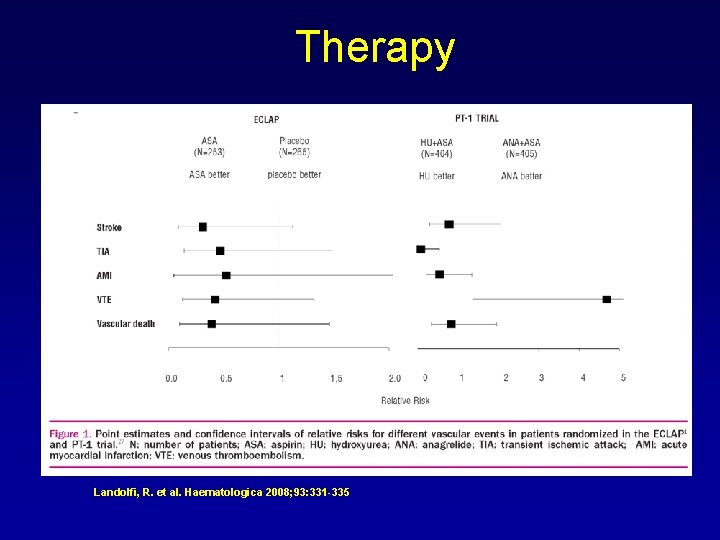
Therapy Landolfi, R. et al. Haematologica 2008; 93: 331 -335

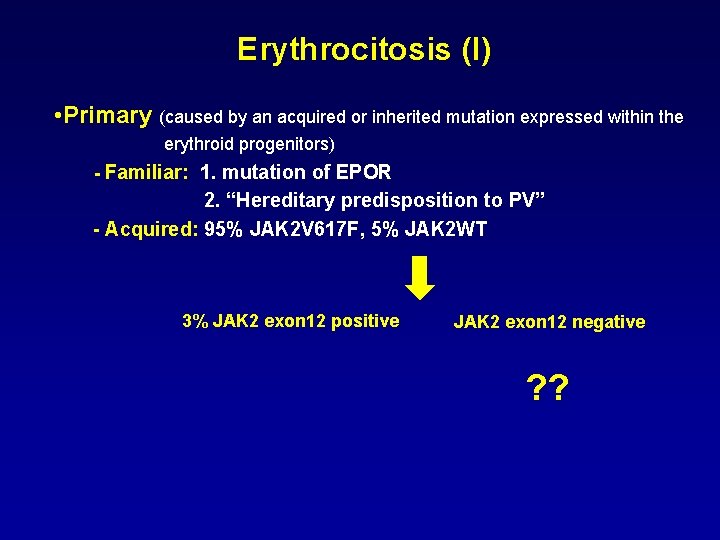
Erythrocitosis (I) • Primary (caused by an acquired or inherited mutation expressed within the erythroid progenitors) - Familiar: 1. mutation of EPOR 2. “Hereditary predisposition to PV” - Acquired: 95% JAK 2 V 617 F, 5% JAK 2 WT 3% JAK 2 exon 12 positive JAK 2 exon 12 negative ? ?

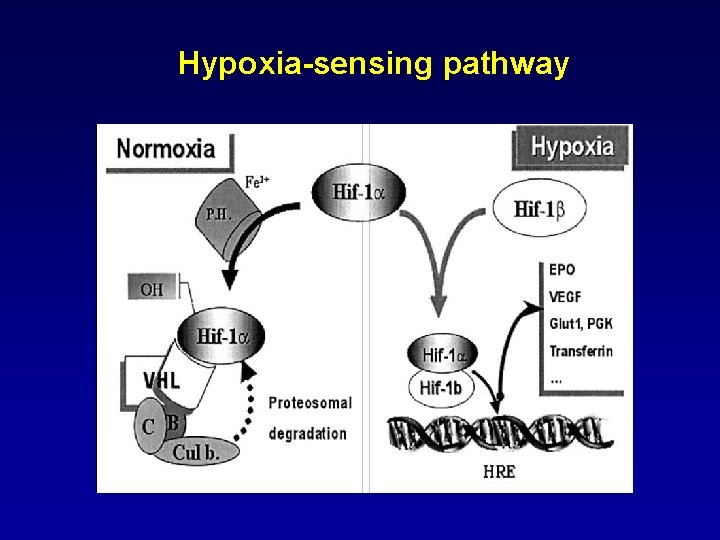
Erythrocitosis (II) • Secondary(caused by circulating plasma factors stimulating erythropoiesis) - Congenital/Familiar: 1. mutation of VHL 2. mutation of PHD 2 3. mutation of HIF-2 A 4. high oxygen–affinity haemoglobin 5. methaemoglobinemia 6. 2, 3 -bisphoglycerate deficiency - Acquired: 1. Hipoxia-driven 2. Oxygen-indipendent

Hypoxia-sensing pathway


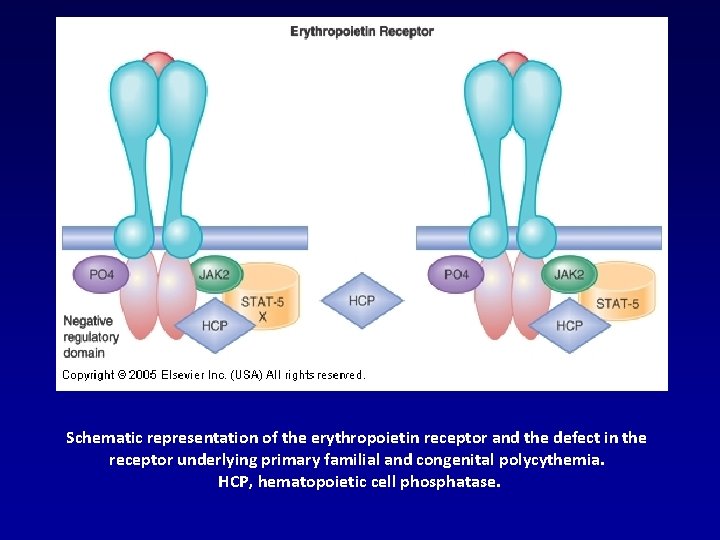
Schematic representation of the erythropoietin receptor and the defect in the receptor underlying primary familial and congenital polycythemia. HCP, hematopoietic cell phosphatase.

The 2008 WHO classification for Myeloid Neoplasms • 1. Acute myeloid leukemia • 2. Myelodysplastic syndromes (MDS) • 3. Myeloproliferative neoplasms (MPN) 3. 1 Chronic myelogenous leukemia 3. 2 Polycythemia vera 3. 3 Essential thrombocythemia 3. 4 Primary myelofibrosis 3. 5 Chronic neutrophilic leukemia 3. 6 Chronic eosinophilic leukemia, not otherwise categorized 3. 7 Hypereosinophilic syndrome 3. 8 Mast cell disease 3. 9 MPNs, unclassifiable • 4. MDS/MPN 4. 1 Chronic myelomonocytic leukemia 4. 2 Juvenile myelomonocytic leukemia 4. 3 Atypical chronic myeloid leukemia 4. 4 MDS/MPN, unclassifiable • 5. Myeloid neoplasms associated with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR 1 5. 1 Myeloid neoplasms associated with PDGFRA rearrangement 5. 2 Myeloid neoplasms associated with PDGFRB rearrangement 5. 3 Myeloid neoplasms associated with FGFR 1 rearrangement (8 p 11 myeloproliferative syndrome)

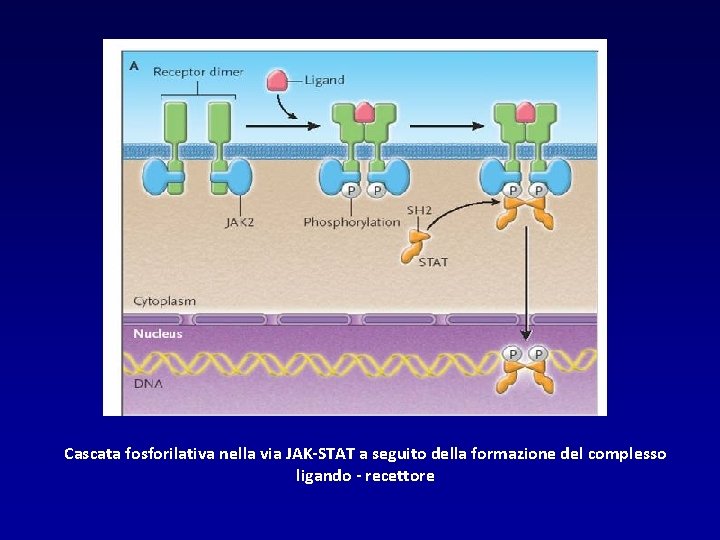
Cascata fosforilativa nella via JAK-STAT a seguito della formazione del complesso ligando - recettore


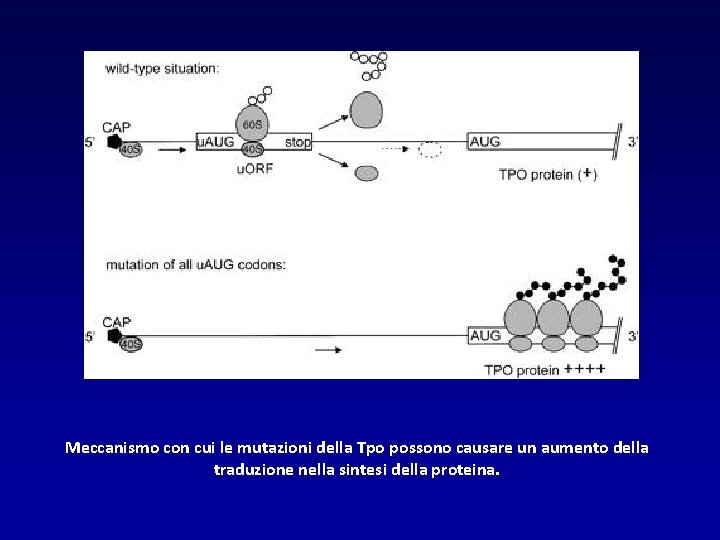
Meccanismo con cui le mutazioni della Tpo possono causare un aumento della traduzione nella sintesi della proteina.
 Prepositioner
Prepositioner Wir - ihr - sie
Wir - ihr - sie Akkusativ ich du er sie es
Akkusativ ich du er sie es Convegno origami e didattica
Convegno origami e didattica Convegno ecclesiale di firenze documento finale
Convegno ecclesiale di firenze documento finale Sbobinatura convegno
Sbobinatura convegno 2008 2008
2008 2008 Maggio kattar reviews
Maggio kattar reviews Isomorfismo coercitivo
Isomorfismo coercitivo Sante et securite du travail
Sante et securite du travail Sintesi 5 maggio
Sintesi 5 maggio La guerra di piero spiegazione
La guerra di piero spiegazione Similitudine 5 maggio
Similitudine 5 maggio Ich du er sie es wir ihr sie
Ich du er sie es wir ihr sie Redemittel zum telefonieren
Redemittel zum telefonieren 2.person singular
2.person singular Ich bin du bist er sie es ist wir sind ihr seid sie sind
Ich bin du bist er sie es ist wir sind ihr seid sie sind Dutch verona
Dutch verona Liceo scientifico galilei verona
Liceo scientifico galilei verona Strada le grazie 15 verona
Strada le grazie 15 verona Liceo fracastoro
Liceo fracastoro Gmail azienda ospedaliera verona
Gmail azienda ospedaliera verona Kelly yeomans
Kelly yeomans Manuela peruzzi
Manuela peruzzi Verona crisis
Verona crisis Hbw verona
Hbw verona Diocesi di verona
Diocesi di verona What term does the chorus use to describe the lovers?
What term does the chorus use to describe the lovers? Ufficio scolastico provinciale di verona
Ufficio scolastico provinciale di verona Cai seniores verona
Cai seniores verona Micro macro verona
Micro macro verona Indovinello veronese
Indovinello veronese Wikitravel verona
Wikitravel verona Ddl diamond
Ddl diamond Negrar verona
Negrar verona Verona
Verona Gianluca verona rinati
Gianluca verona rinati Two households
Two households Gianenrico senna
Gianenrico senna Two households both alike in dignity
Two households both alike in dignity Www.liceomontanari.edu.it
Www.liceomontanari.edu.it Magistrale scienze motorie verona
Magistrale scienze motorie verona Gianluca verona rinati
Gianluca verona rinati Dsv saima avandero
Dsv saima avandero O romeo romeo
O romeo romeo Romeo and juliet two households
Romeo and juliet two households Romeo and juliet prologue translated
Romeo and juliet prologue translated Iaa verona
Iaa verona Grest cornedo
Grest cornedo Iso 9001:2008 certification in mumbai
Iso 9001:2008 certification in mumbai