CONTENTS INTRODUCTION PHYSICAL PROPERTIES ACID VALUE IDENTIFICATION QUANTIFICATION




















- Slides: 58


CONTENTS Ø Ø Ø INTRODUCTION PHYSICAL PROPERTIES ACID VALUE IDENTIFICATION & QUANTIFICATION OF FA’S SEPERATION OF FATTY ACIDS SAPONIFICATION VALUE ESTER VALUE IODINE VALUE PEROXIDE VALUE ESTIMATION OF OILS ACETYL VALUE HYDROXYL VALUE

FATS These compounds are so named because the first isolated member was from fat. Ø The general formula is R-COOH, R being an alkyl group with a short or long chain. Ø

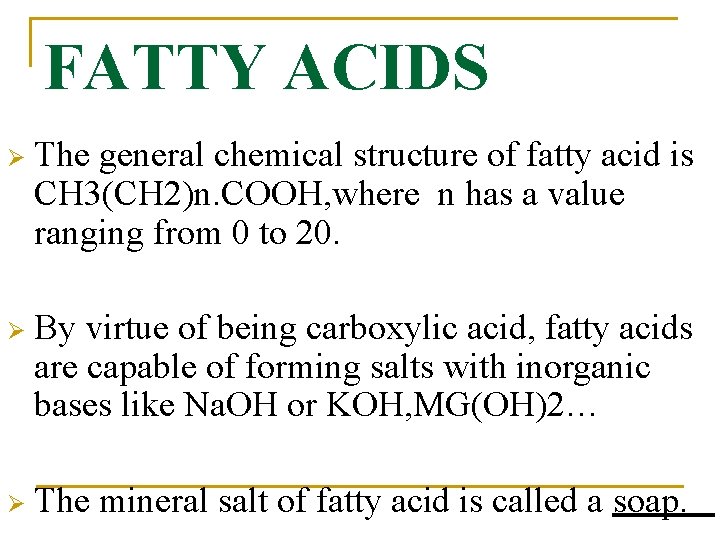
FATTY ACIDS Ø The general chemical structure of fatty acid is CH 3(CH 2)n. COOH, where n has a value ranging from 0 to 20. Ø By virtue of being carboxylic acid, fatty acids are capable of forming salts with inorganic bases like Na. OH or KOH, MG(OH)2… Ø The mineral salt of fatty acid is called a soap.

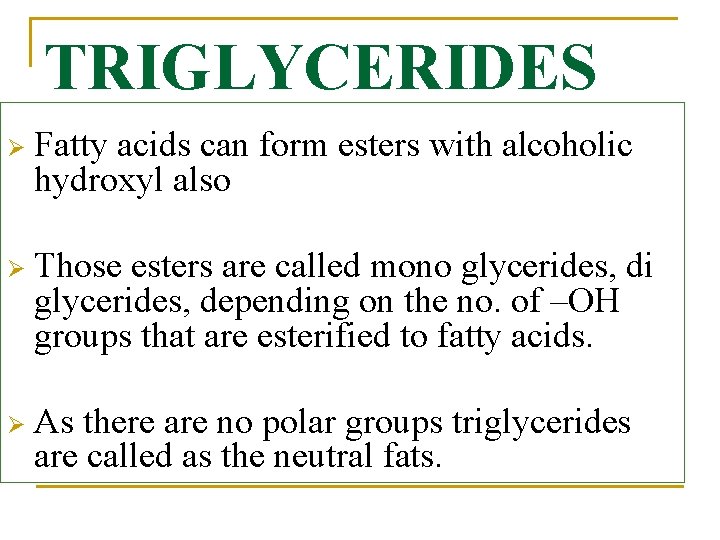
TRIGLYCERIDES Ø Fatty acids can form esters with alcoholic hydroxyl also Ø Those esters are called mono glycerides, di glycerides, depending on the no. of –OH groups that are esterified to fatty acids. Ø As there are no polar groups triglycerides are called as the neutral fats.

PHYSICAL PROPERTIES Ø They are colorless, odorless, tasteless substances than water in which they are insoluble but soluble in organic solvents. Ø The melting point of fats are higher than their solidification points.

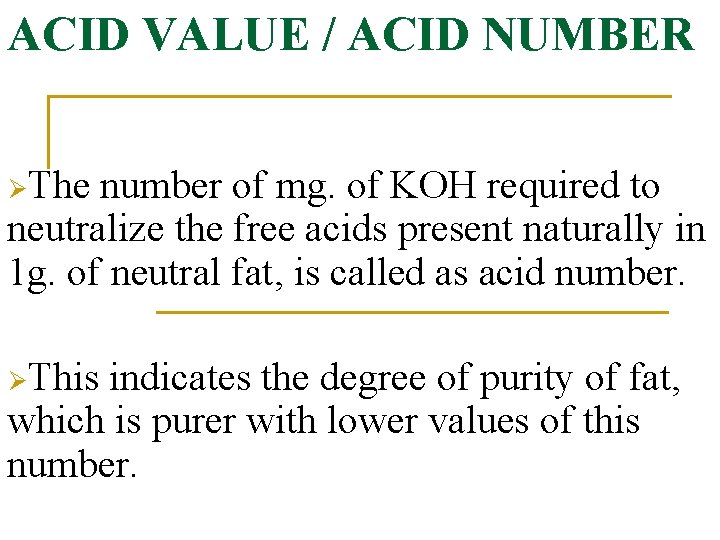
ACID VALUE / ACID NUMBER ØThe number of mg. of KOH required to neutralize the free acids present naturally in 1 g. of neutral fat, is called as acid number. ØThis indicates the degree of purity of fat, which is purer with lower values of this number.

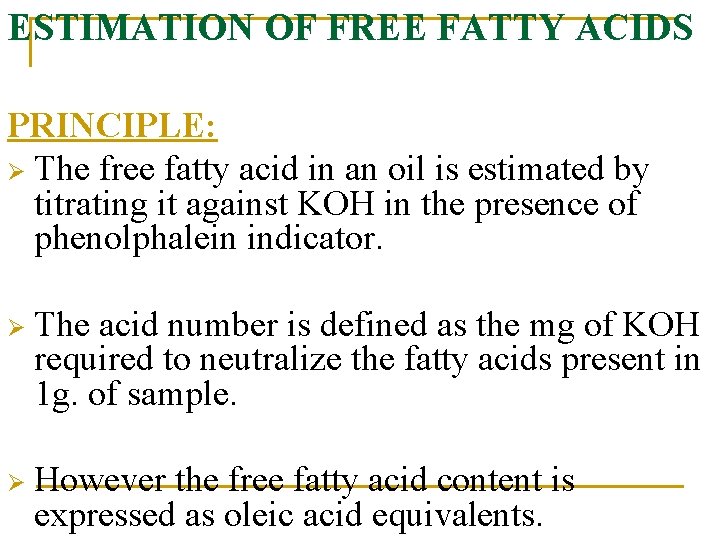
ESTIMATION OF FREE FATTY ACIDS PRINCIPLE: Ø The free fatty acid in an oil is estimated by titrating it against KOH in the presence of phenolphalein indicator. Ø The acid number is defined as the mg of KOH required to neutralize the fatty acids present in 1 g. of sample. Ø However the free fatty acid content is expressed as oleic acid equivalents.

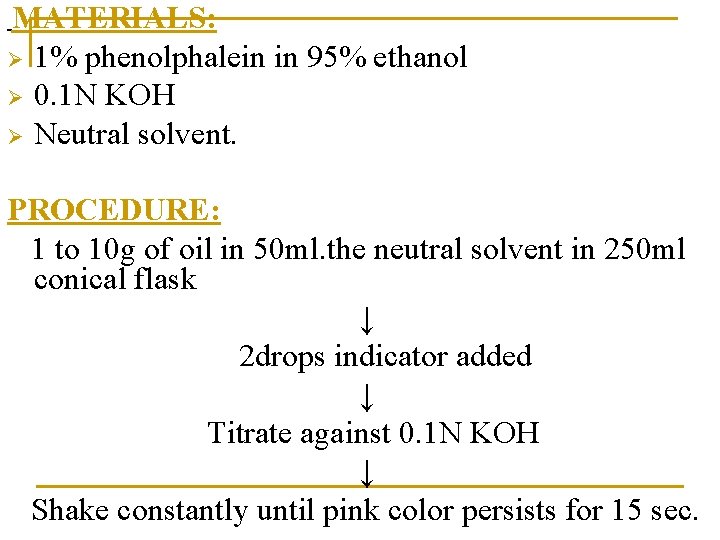
MATERIALS: Ø 1% phenolphalein in 95% ethanol Ø 0. 1 N KOH Ø Neutral solvent. PROCEDURE: 1 to 10 g of oil in 50 ml. the neutral solvent in 250 ml conical flask ↓ 2 drops indicator added ↓ Titrate against 0. 1 N KOH ↓ Shake constantly until pink color persists for 15 sec.

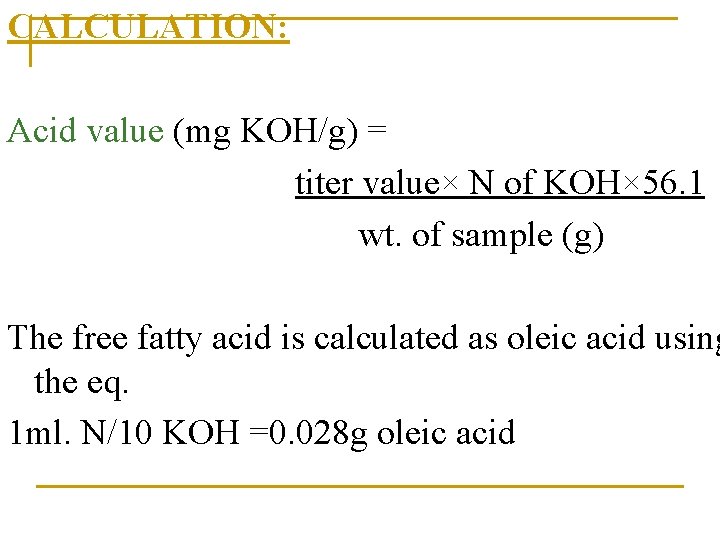
CALCULATION: Acid value (mg KOH/g) = titer value× N of KOH× 56. 1 wt. of sample (g) The free fatty acid is calculated as oleic acid using the eq. 1 ml. N/10 KOH =0. 028 g oleic acid

IDENTIFICATION & QUANTIFICATION OF FATTY ACIDS PRINCIPLE: Ø FA are made volatile by converting them into methyl esters. The conversion of FA’s in methyl esters is carried out directly by trans esterification. Ø The esters are identification & quantified by injecting into GLC & comparing with a set of std esters.

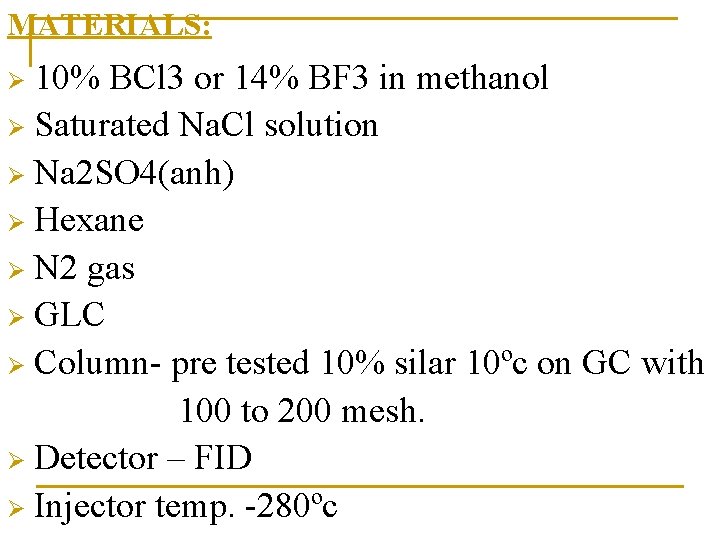
MATERIALS: 10% BCl 3 or 14% BF 3 in methanol Ø Saturated Na. Cl solution Ø Na 2 SO 4(anh) Ø Hexane Ø N 2 gas Ø GLC Ø Column- pre tested 10% silar 10ºc on GC with 100 to 200 mesh. Ø Detector – FID Ø Injector temp. -280ºc Ø

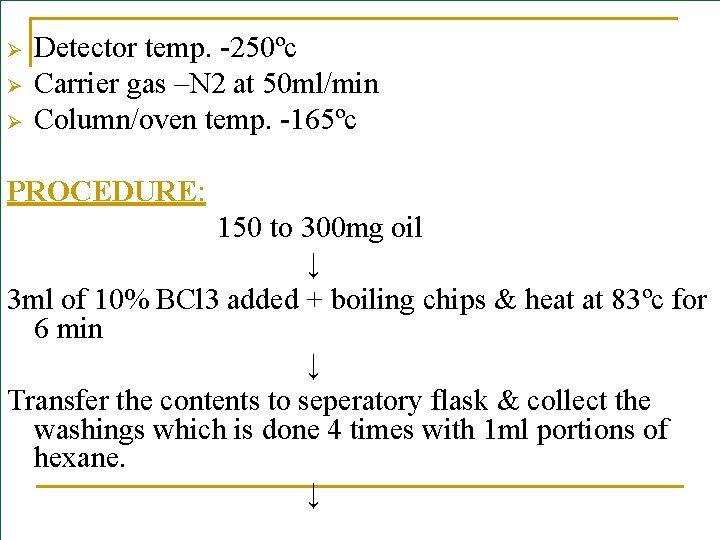
Ø Ø Ø Detector temp. -250ºc Carrier gas –N 2 at 50 ml/min Column/oven temp. -165ºc PROCEDURE: 150 to 300 mg oil ↓ 3 ml of 10% BCl 3 added + boiling chips & heat at 83ºc for 6 min ↓ Transfer the contents to seperatory flask & collect the washings which is done 4 times with 1 ml portions of hexane. ↓

↓ Shake & allow to separate ↓ 4 ml sat. Na. Cl solution added ↓ Shake & collect hexane layer over anh. Na 2 SO 4 ↓ Rinse funnel with 2 ml hexane& collect the hexane layer ↓ Filter the hexane extract ↓ Reduce the vol. to 2 -3 ml by drying with N 2 gas steam ↓

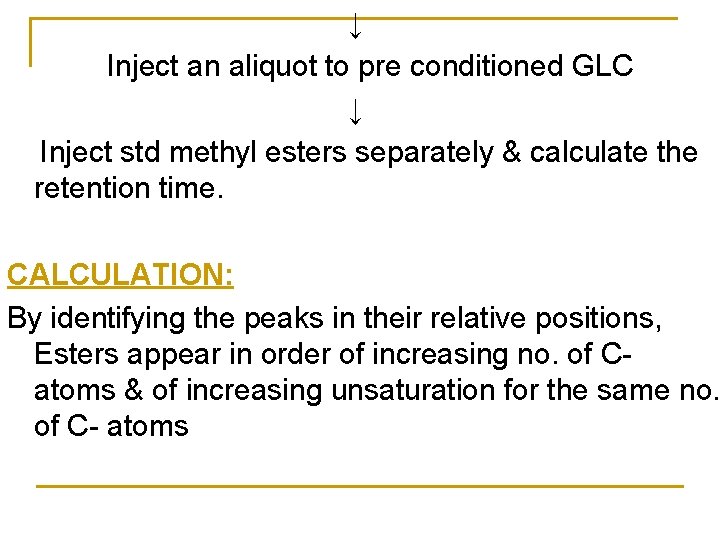
↓ Inject an aliquot to pre conditioned GLC ↓ Inject std methyl esters separately & calculate the retention time. CALCULATION: By identifying the peaks in their relative positions, Esters appear in order of increasing no. of Catoms & of increasing unsaturation for the same no. of C- atoms

DETERMINATION OF THE ACID VALUE OF A FAT PRINCIPLE: Ø During storage, fats may become rancid as a result of peroxide formation at the double bonds by atm. O 2 & hydrolysis by micro organisms with the liberation of free acids. Ø The amt of free acid gives an indication of the age & quality of the fat.

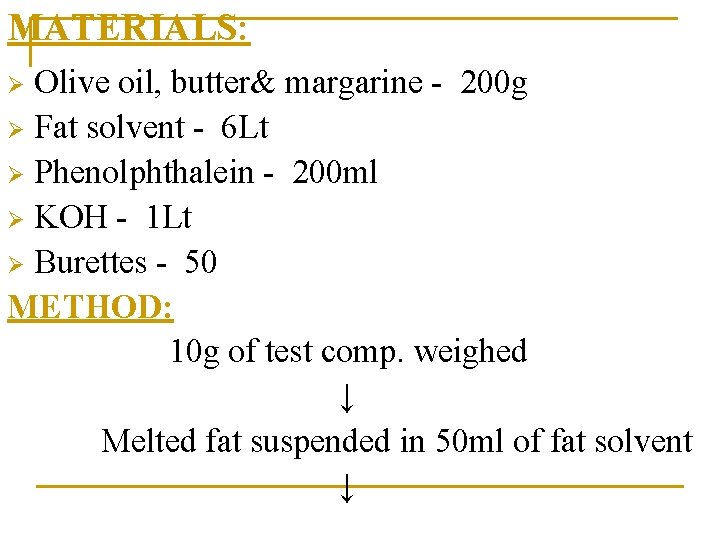
MATERIALS: Olive oil, butter& margarine - 200 g Ø Fat solvent - 6 Lt Ø Phenolphthalein - 200 ml Ø KOH - 1 Lt Ø Burettes - 50 METHOD: 10 g of test comp. weighed ↓ Melted fat suspended in 50 ml of fat solvent ↓ Ø

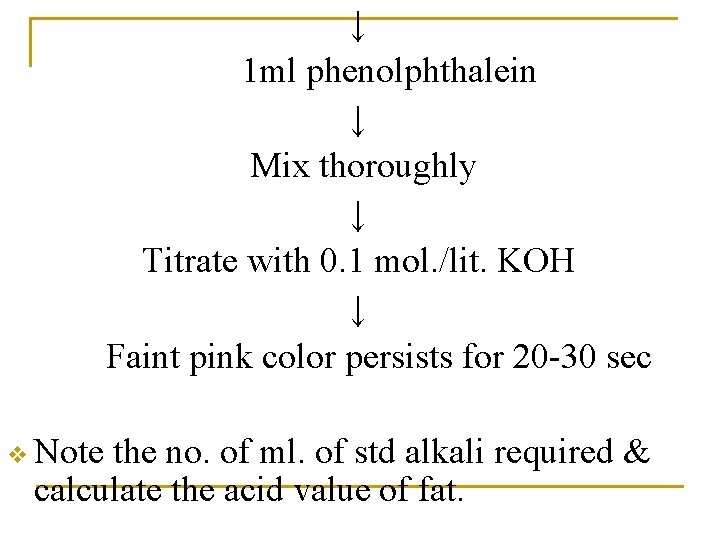
↓ 1 ml phenolphthalein ↓ Mix thoroughly ↓ Titrate with 0. 1 mol. /lit. KOH ↓ Faint pink color persists for 20 -30 sec v Note the no. of ml. of std alkali required & calculate the acid value of fat.

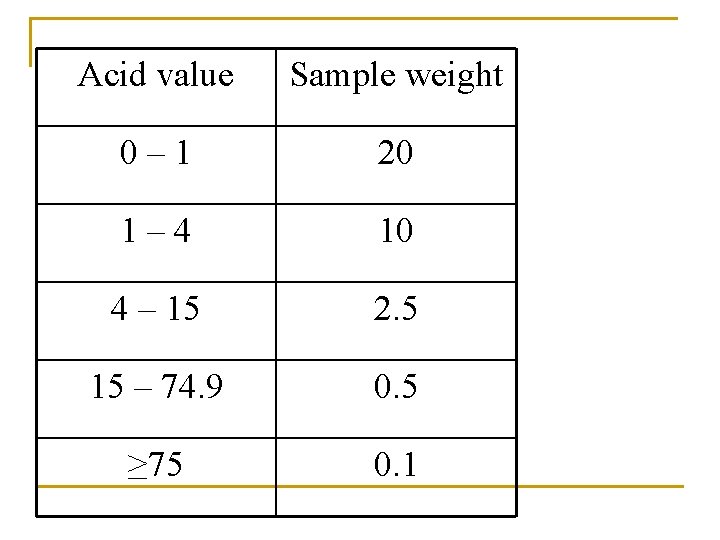
Acid value Sample weight 0– 1 20 1– 4 10 4 – 15 2. 5 15 – 74. 9 0. 5 ≥ 75 0. 1

SEPERATION OF FATTY ACIDS BY REVERSE PHASE PAPER CHROMATOGRAPHY Ø In this method, the S. P is the organic solvent adsorbed to an inactive supporting material. Ø The M. P immiscible with the first is aq. Ø Whatmaan no. 1 & 3 filter paper of size 20× 20 cm is dipped in the 10% liq. paraffin in pet. Ether.

The std FA’s samples are applied to the paper in the followed manner: Ø Saturated FA’s (2 -5µg each): lauric acid, myristic acid, palmatic acid, stearic acid. Ø Unsaturated FA’s (1 -3µg each): linolenic acid, oleic acid. Ø Develop the papers in the chromatographic chambers saturated with the solvents like acetone-water 80: 20 or acetic acid-acetonitrile 1: 1 v/v saturated with liq. paraffin in pet. Ether. Ø After development the papers are hanged to room temp. for detecting the spots with phosphomolybdic acid reagent. Ø Heat in hot air oven at 120ºc for 10 -15 min. by identifying the FA content of sample.

SAPONIFICATION VALUE PRINCIPLE: Ø The saponification value is the no. of mg. of KOH required to neutralize the FA’s resulting from the complete hydrolysis of 1 g. Of fat. Ø The saponification value gives an indication of the nature of the FA’s in the fat since longer the C-chains the less acid is liberated per gram of fat hydrolyzed.

MATERIALS: Fats – 20 g Ø Fat solvent(95% ethanol , ether) – 1 Lt Ø Alcoholic KOH – 3 Lt Ø Reflux condenser - 100 Ø Boiling water bath - 100 Ø Phenolphthalein – 50 ml Ø HCl – 3 Lt Ø Burettes – 100 Ø Conical flasks - 100 Ø

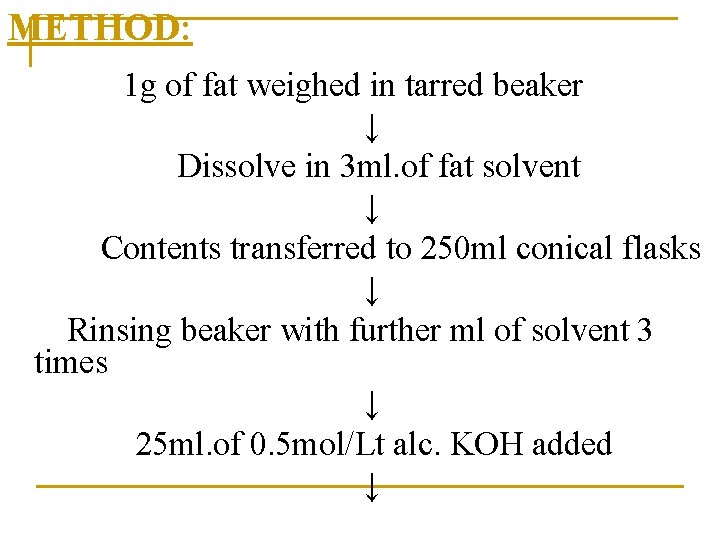
METHOD: 1 g of fat weighed in tarred beaker ↓ Dissolve in 3 ml. of fat solvent ↓ Contents transferred to 250 ml conical flasks ↓ Rinsing beaker with further ml of solvent 3 times ↓ 25 ml. of 0. 5 mol/Lt alc. KOH added ↓

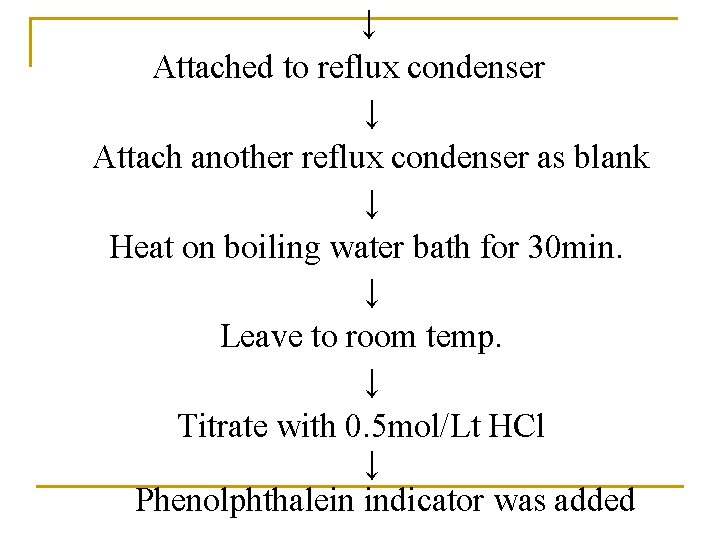
↓ Attached to reflux condenser ↓ Attach another reflux condenser as blank ↓ Heat on boiling water bath for 30 min. ↓ Leave to room temp. ↓ Titrate with 0. 5 mol/Lt HCl ↓ Phenolphthalein indicator was added

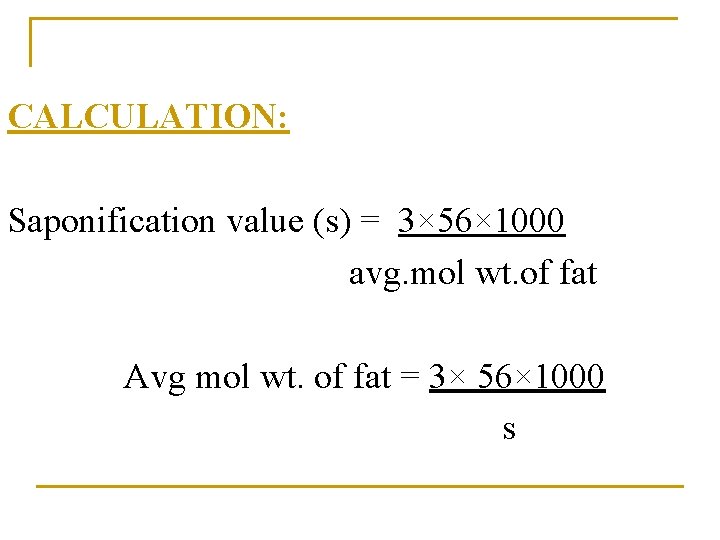
CALCULATION: Saponification value (s) = 3× 56× 1000 avg. mol wt. of fat Avg mol wt. of fat = 3× 56× 1000 s

DETERMINATION OF ESTER VALUE PRINCIPLE: Ø The ester value is the no. of mg. of KOH required to saponify the esters present in 1 g. Of the substance. Ø Ester value = saponification value-acid value.

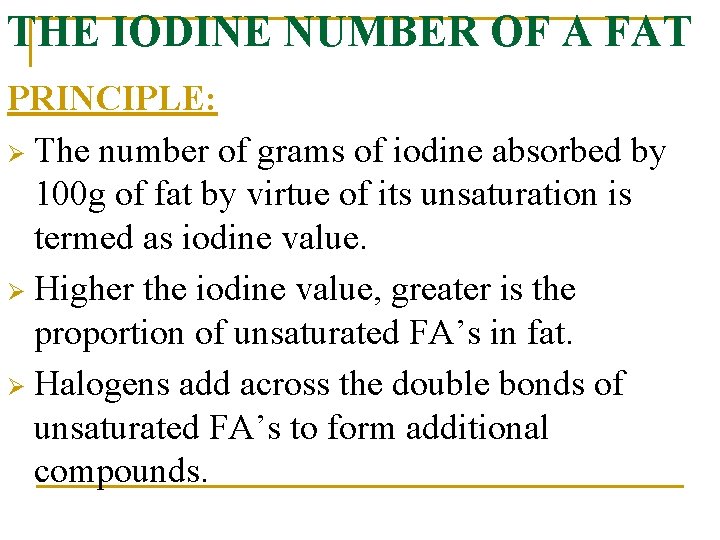
THE IODINE NUMBER OF A FAT PRINCIPLE: Ø The number of grams of iodine absorbed by 100 g of fat by virtue of its unsaturation is termed as iodine value. Ø Higher the iodine value, greater is the proportion of unsaturated FA’s in fat. Ø Halogens add across the double bonds of unsaturated FA’s to form additional compounds.

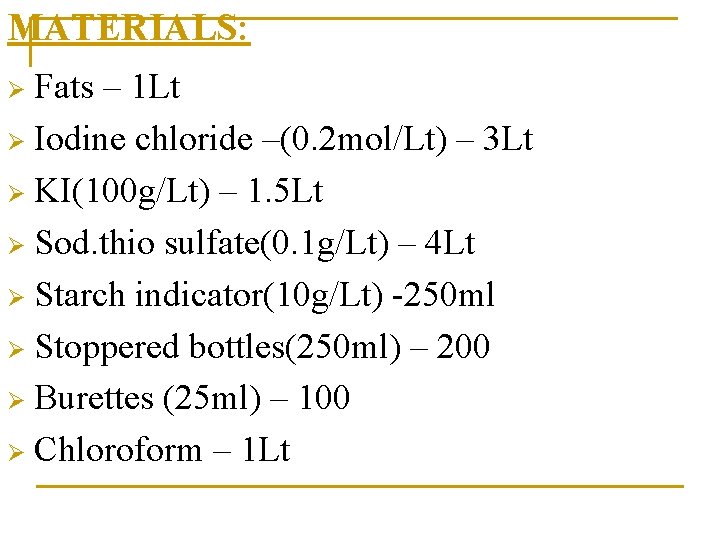
MATERIALS: Fats – 1 Lt Ø Iodine chloride –(0. 2 mol/Lt) – 3 Lt Ø KI(100 g/Lt) – 1. 5 Lt Ø Sod. thio sulfate(0. 1 g/Lt) – 4 Lt Ø Starch indicator(10 g/Lt) -250 ml Ø Stoppered bottles(250 ml) – 200 Ø Burettes (25 ml) – 100 Ø Chloroform – 1 Lt Ø

METHOD: Pipette out 10 ml of fat solution ↓ 25 ml of iodine chloride solution added ↓ Leave to stand in dark for 1 hr. Shaking thoroughly ↓ Rinse the stoppers, necks with 50 ml water ↓ 10 ml. KI solution added ↓

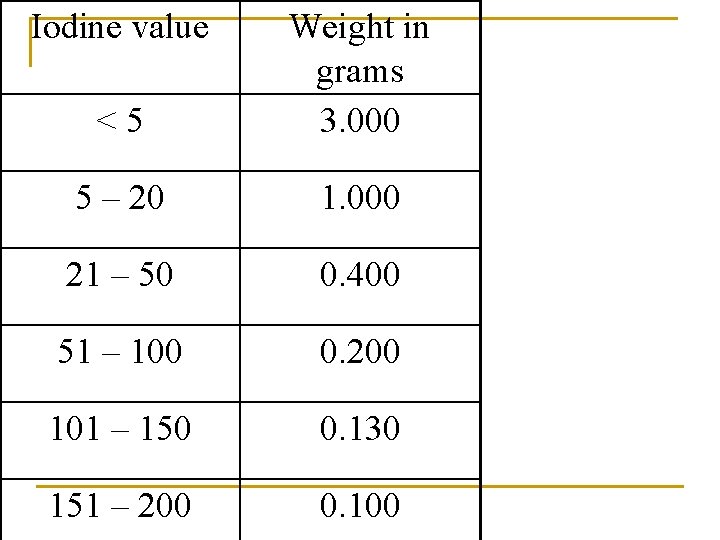
When the solution is pale straw color add 1 ml. Of starch solution until blue color disappears ↓ Bottles must be shaken thoroughly to ensure that all iodine is removed from chloroform layer. CALCULATION: Iodine number = (B-T) × 6. 35 g per 100 g of fat

Iodine value <5 Weight in grams 3. 000 5 – 20 1. 000 21 – 50 0. 400 51 – 100 0. 200 101 – 150 0. 130 151 – 200 0. 100

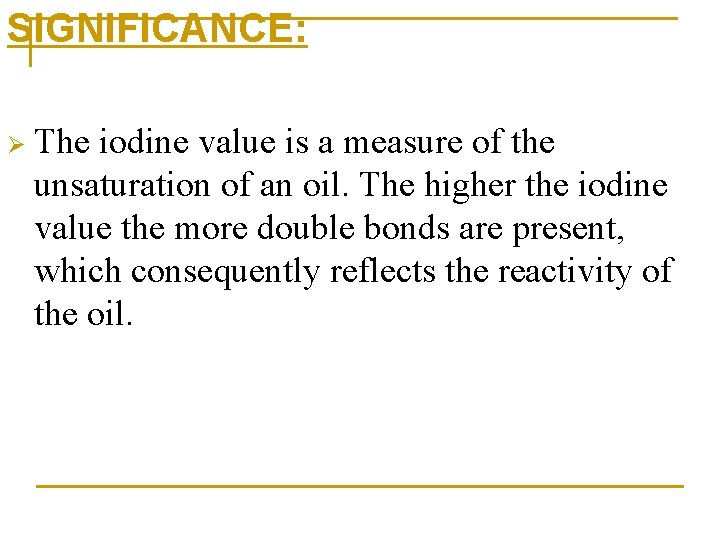
SIGNIFICANCE: Ø The iodine value is a measure of the unsaturation of an oil. The higher the iodine value the more double bonds are present, which consequently reflects the reactivity of the oil.

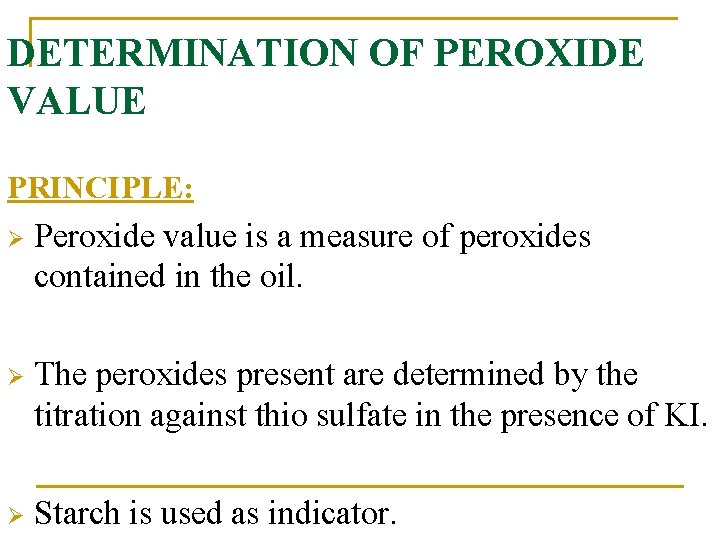
DETERMINATION OF PEROXIDE VALUE PRINCIPLE: Ø Peroxide value is a measure of peroxides contained in the oil. Ø The peroxides present are determined by the titration against thio sulfate in the presence of KI. Ø Starch is used as indicator.

MATERIALS: Ø Solvent mixture – mix 2 vol. of glacial acetic acid with 1 vol. of chloroform Ø 5% KI solution Ø 1% starch solution Ø N/500 sod. Thio sulfate solution

PROCEDURE: 1 g of oil/fat are weighed in dry boiling tube ↓ 1 g of powdered KI & 20 ml of solvent mixture ↓ Place the tube in boiling water for 30 sec. ↓ Transfer the contents to conical flask containing 20 ml of 5% KI solution ↓ Wash the tube with 25 ml water twice ↓

↓ Titrate against N/500 sod. Thio sulfate solution until yellow color disappears ↓ 0. 5 ml starch added ↓ Shake vigorously till blue color just disappears. CALCULATION: Peroxide value = S × N × 1000 sample (g)

ESTIMATION OF OIL IN OIL SEEDS PRINCIPLE: Ø Oil from known quantity of the seed is extracted with pet. Ether. Ø It is then distilled off completely & dried. MATERIALS: Ø Pet. Ether(40 -160°c) Ø Whatmaan no. 2 filter paper Ø Absorbent cotton Ø Soxhlet apparatus.

PROCEDURE: Fold a piece of filter paper in such a way hold the seed meal. ↓ Cotton wool is placed at the top to distribute evenly ↓ Place the sample packet in butt tubes of soxhlet apparatus ↓ Extract with pet. Ether for 6 hrs without heating ↓ Cool & dismantle the apparatus ↓

↓ Evaporate the ether on steam or water bath until no odour remains ↓ Cool to room temp. ↓ Carefully remove the dirt outside the flask & weigh ↓ Repeat heating until constant wt. is obtained.

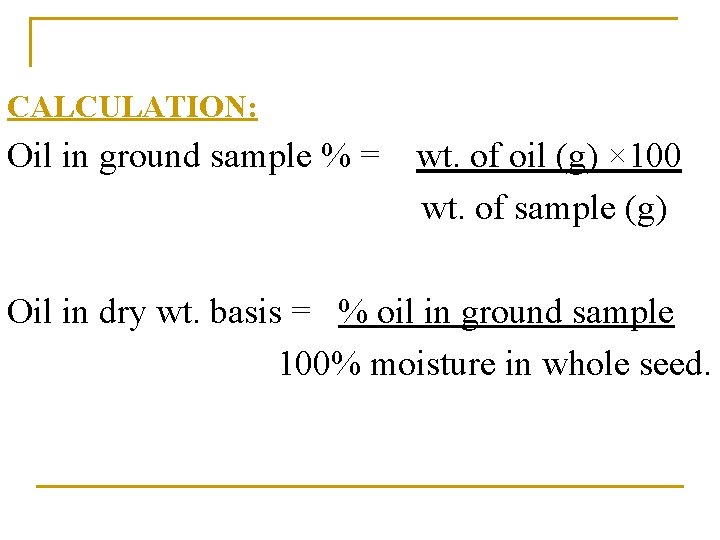
CALCULATION: Oil in ground sample % = wt. of oil (g) × 100 wt. of sample (g) Oil in dry wt. basis = % oil in ground sample 100% moisture in whole seed.

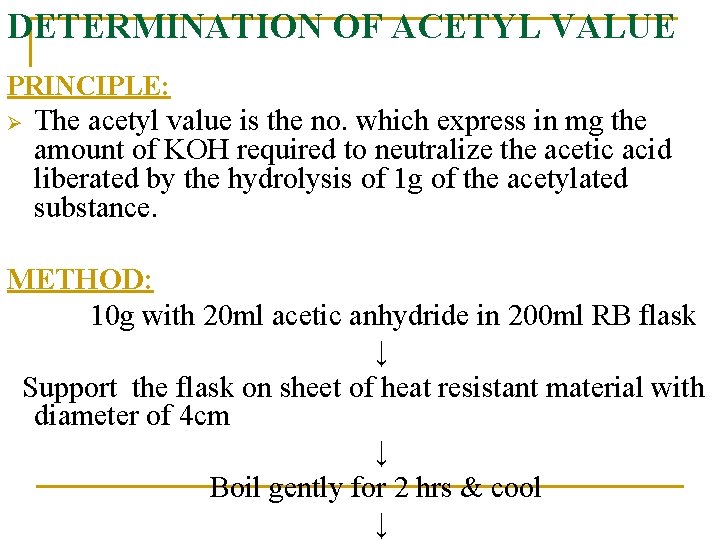
DETERMINATION OF ACETYL VALUE PRINCIPLE: Ø The acetyl value is the no. which express in mg the amount of KOH required to neutralize the acetic acid liberated by the hydrolysis of 1 g of the acetylated substance. METHOD: 10 g with 20 ml acetic anhydride in 200 ml RB flask ↓ Support the flask on sheet of heat resistant material with diameter of 4 cm ↓ Boil gently for 2 hrs & cool ↓

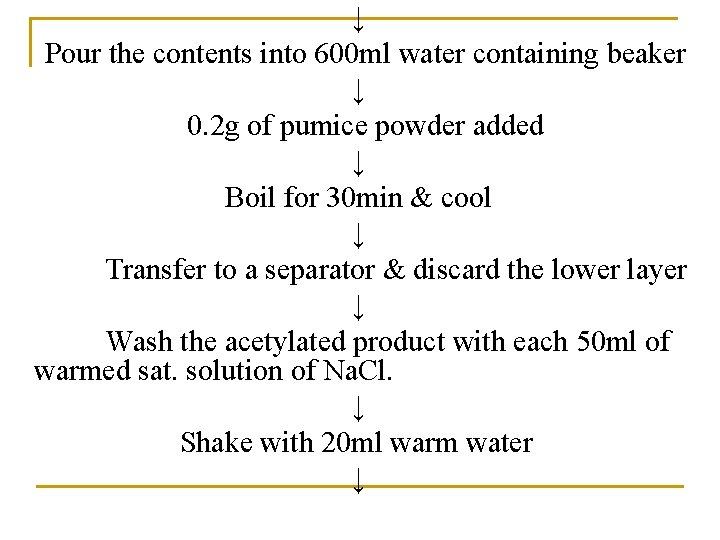
↓ Pour the contents into 600 ml water containing beaker ↓ 0. 2 g of pumice powder added ↓ Boil for 30 min & cool ↓ Transfer to a separator & discard the lower layer ↓ Wash the acetylated product with each 50 ml of warmed sat. solution of Na. Cl. ↓ Shake with 20 ml warm water ↓

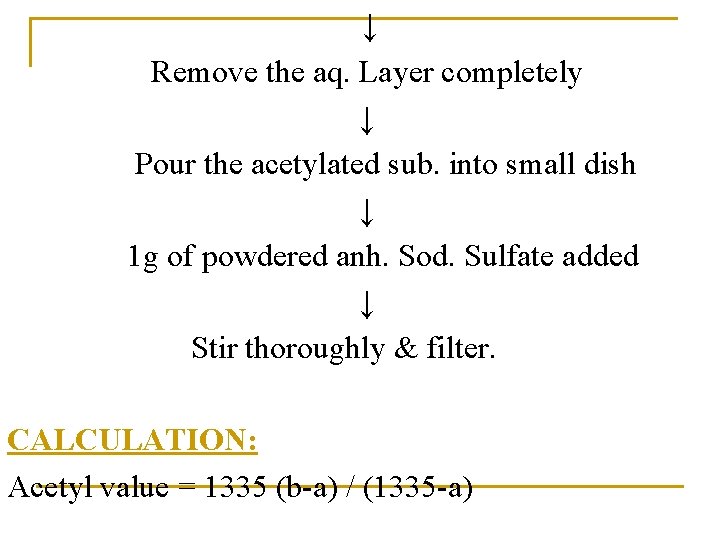
↓ Remove the aq. Layer completely ↓ Pour the acetylated sub. into small dish ↓ 1 g of powdered anh. Sod. Sulfate added ↓ Stir thoroughly & filter. CALCULATION: Acetyl value = 1335 (b-a) / (1335 -a)

DETERMINATION OF HYDROXYL VALUE PRINCIPLE: Ø The hydroxyl value is the no. of mg of KOH required to neutralize the acid combined by acetylation in 1 g of the substance. METHOD: Accurately weigh the substance in 150 ml acetylation flask ↓ Pyridine acetic anhydride reagent added ↓

↓ Boil for 1 hr on water bath & maintain it to 3 cm above the level of liquid in flask ↓ 5 ml water added from top of the condenser ↓ If cloudiness is observed add pyridine to produce clear liquid ↓ Shake & replace the flask for 10 min & cool ↓ Rinse the walls & condenser with 5 ml ethanol ↓ Titrate with 0. 5 M ethanolic KOH using dilute phenolphthalein as indicator.

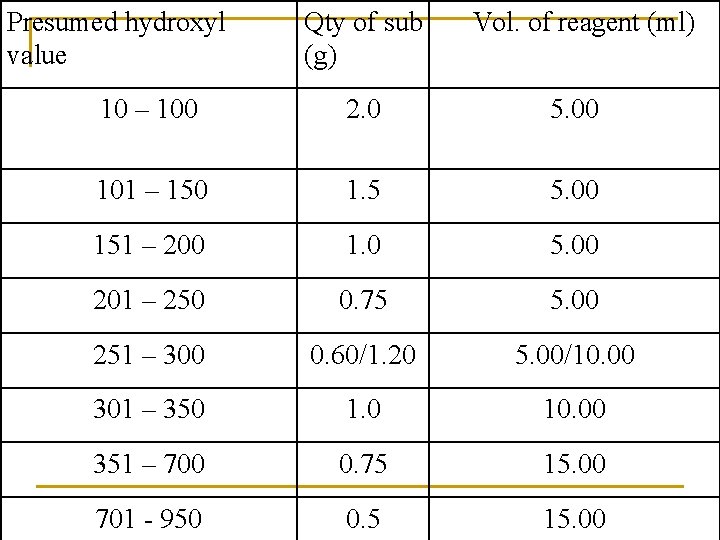
Presumed hydroxyl value Qty of sub (g) Vol. of reagent (ml) 10 – 100 2. 0 5. 00 101 – 150 1. 5 5. 00 151 – 200 1. 0 5. 00 201 – 250 0. 75 5. 00 251 – 300 0. 60/1. 20 5. 00/10. 00 301 – 350 1. 0 10. 00 351 – 700 0. 75 15. 00 701 - 950 0. 5 15. 00

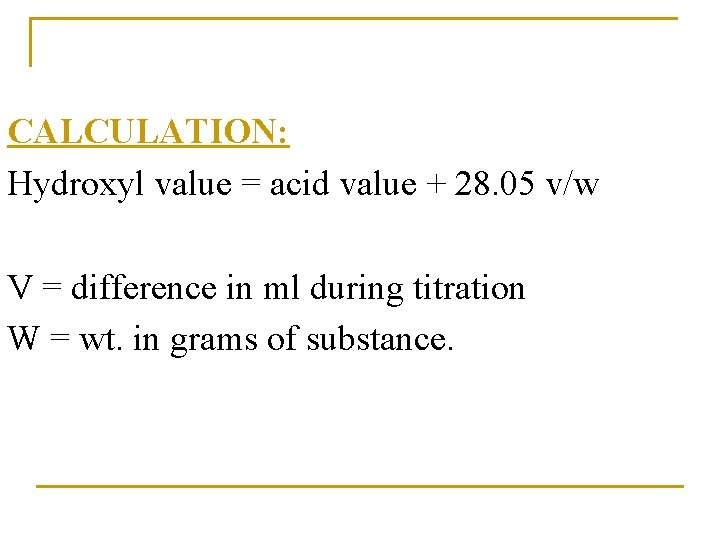
CALCULATION: Hydroxyl value = acid value + 28. 05 v/w V = difference in ml during titration W = wt. in grams of substance.

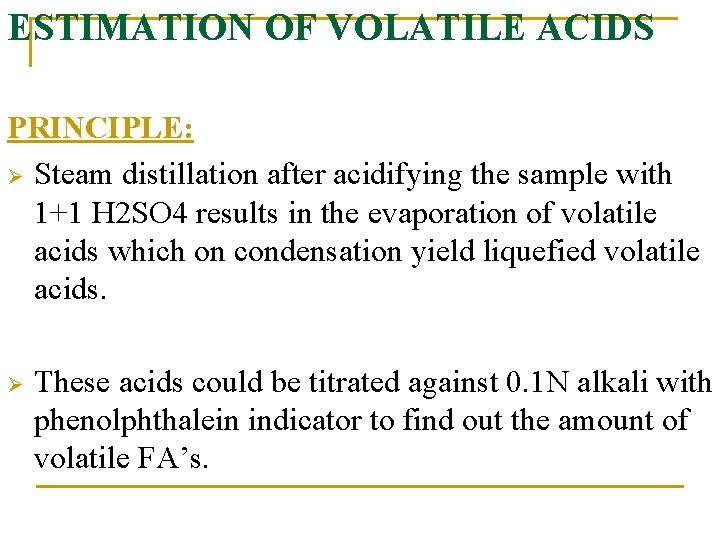
ESTIMATION OF VOLATILE ACIDS PRINCIPLE: Ø Steam distillation after acidifying the sample with 1+1 H 2 SO 4 results in the evaporation of volatile acids which on condensation yield liquefied volatile acids. Ø These acids could be titrated against 0. 1 N alkali with phenolphthalein indicator to find out the amount of volatile FA’s.

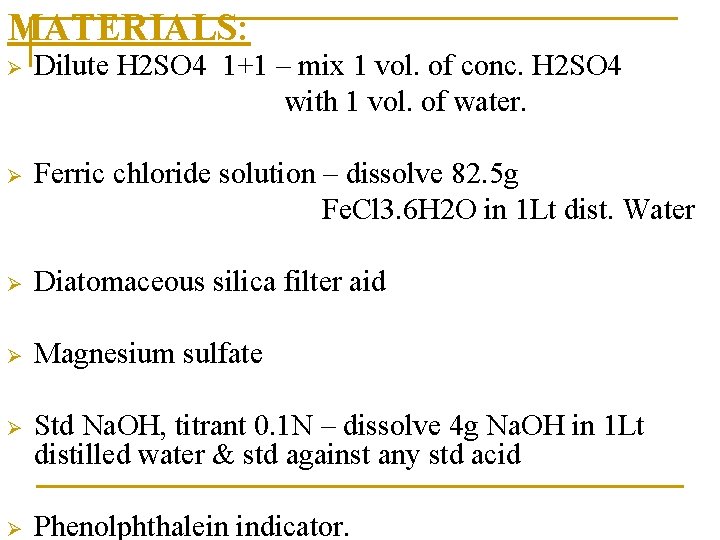
MATERIALS: Ø Dilute H 2 SO 4 1+1 – mix 1 vol. of conc. H 2 SO 4 with 1 vol. of water. Ø Ferric chloride solution – dissolve 82. 5 g Fe. Cl 3. 6 H 2 O in 1 Lt dist. Water Ø Diatomaceous silica filter aid Ø Magnesium sulfate Ø Std Na. OH, titrant 0. 1 N – dissolve 4 g Na. OH in 1 Lt distilled water & std against any std acid Ø Phenolphthalein indicator.

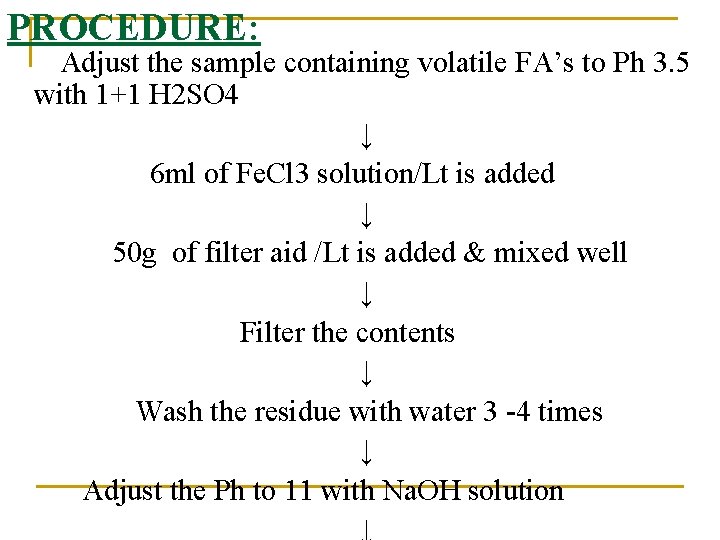
PROCEDURE: Adjust the sample containing volatile FA’s to Ph 3. 5 with 1+1 H 2 SO 4 ↓ 6 ml of Fe. Cl 3 solution/Lt is added ↓ 50 g of filter aid /Lt is added & mixed well ↓ Filter the contents ↓ Wash the residue with water 3 -4 times ↓ Adjust the Ph to 11 with Na. OH solution ↓

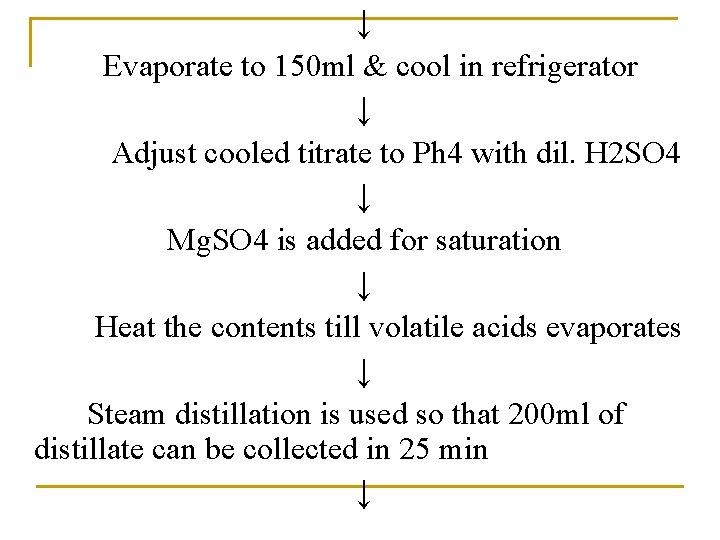
↓ Evaporate to 150 ml & cool in refrigerator ↓ Adjust cooled titrate to Ph 4 with dil. H 2 SO 4 ↓ Mg. SO 4 is added for saturation ↓ Heat the contents till volatile acids evaporates ↓ Steam distillation is used so that 200 ml of distillate can be collected in 25 min ↓

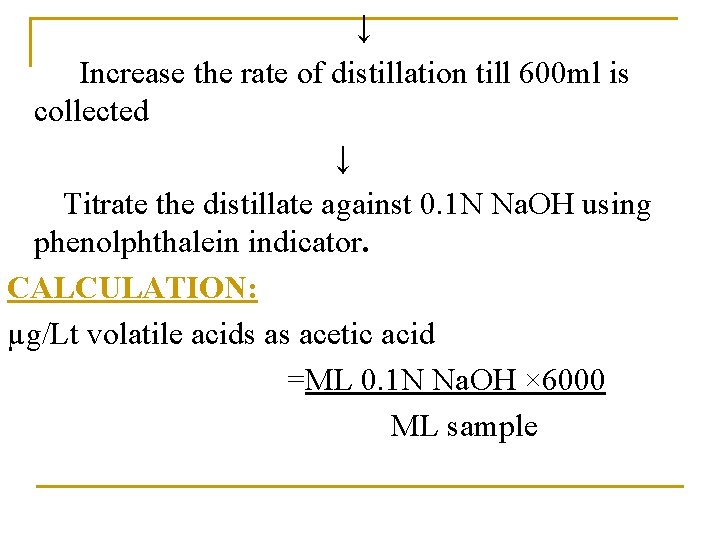
↓ Increase the rate of distillation till 600 ml is collected ↓ Titrate the distillate against 0. 1 N Na. OH using phenolphthalein indicator. CALCULATION: µg/Lt volatile acids as acetic acid =ML 0. 1 N Na. OH × 6000 ML sample

THIN LAYER CHROMATOGRAPHY OF PHOSPHOLIPIDS Ø The preferred solvent for phospholipids separation is chloroform-methanol-acetic acid-water(25: 15: 4: 2). Ø The phospholipids on exposure to iodine vapors, absorb iodine & visible as brown spots on yellow background Ø These are not stable, however iodine in chloroform solution(1%conc) can be sprayed on.

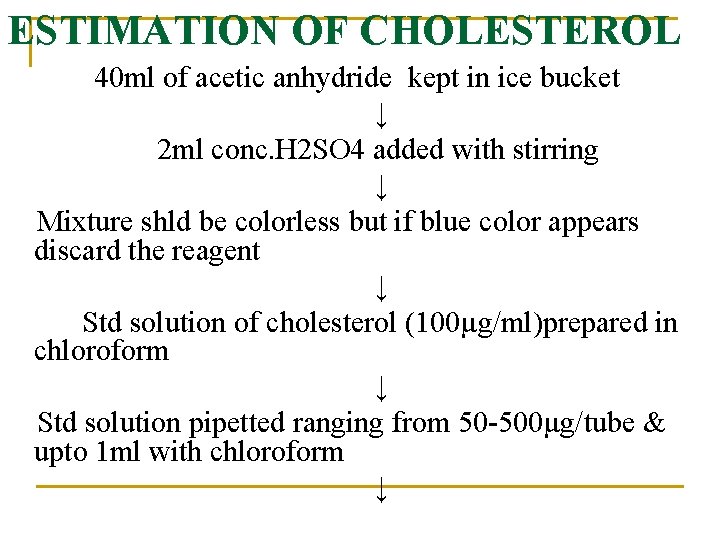
ESTIMATION OF CHOLESTEROL 40 ml of acetic anhydride kept in ice bucket ↓ 2 ml conc. H 2 SO 4 added with stirring ↓ Mixture shld be colorless but if blue color appears discard the reagent ↓ Std solution of cholesterol (100µg/ml)prepared in chloroform ↓ Std solution pipetted ranging from 50 -500µg/tube & upto 1 ml with chloroform ↓

↓ 5 ml reagent added to each tube & mixed well ↓ If rosy red to blue or greenish blue color is noticed tubes covered with dark cloth for 15 min. ↓ Absorbance-640 nm.

SIGNIFICANCE OF FATS Ø Ø They are the concentrated source of energy as 1 g of fat contributes 9 kilo calories of energy. They are good source of vitamin A, D, E, K. They impart special flavor & texture to our food which increases palatability. They are used by the body to make prostaglandins involved in vital physiological functions.

D. J. SRAVANTHI M. GLORY HEPSIBAH P. INDIRA M. PHARM (ANALYSIS)