Acid rain The formation of acid rain Acid










- Slides: 14

Acid rain

The formation of acid rain • Acid rain occurs mostly in the Northern Hemisphere the more industrialized, half of the globe. • Winds can sweep up emissions from high smokestacks and carry pollutants far from their original sources, crossing national borders in the process. • Acid rain do not have the range of greenhouse gases, but it is a transboundary, and therefore a international, issue.

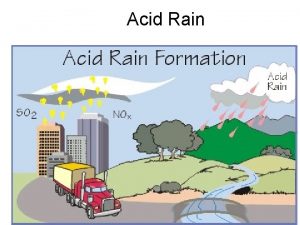
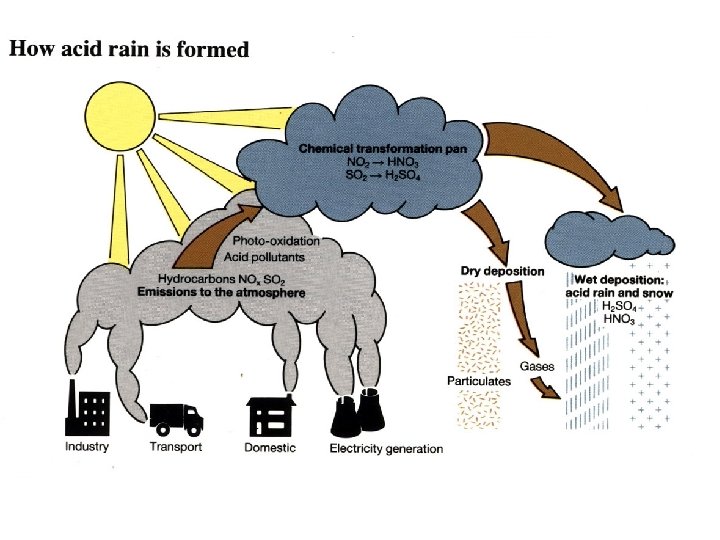
The formation of acid rain • Acid rain, also known as acid deposition, is caused by emissions of sulfur dioxide (SO 2) and nitrogen oxides (NOx) from electricity generation, industry, transport factories, and domestic fires. • Natural sources like volcanoes, forest fires and lightning strikes can also add to the man made pollution.

The formation of acid rain • SO 2 and NOx become acids when they enter the atmosphere and react with water vapor. The resulting sulfuric and nitric acids can fall as wet or dry depositions. • Wet deposition is precipitation: acid rain, snow, sleet or fog. • Dry deposition falls as acidic particulates or gases


The Effects of Acid Rain • Acid rain dissolves helpful minerals and nutrients like calcium, magnesium and potassium before trees can absorb them. • Forests rely on their soil's buffering capacity to protect them from acid rain. Acidic waters draw out soil toxins like aluminum. Trees take in the poisonous substances, and runoff dumps it in lakes, rivers and streams. Acid rain rarely kills a forest outright but instead stunts its growth through years of soil degradation. Nutrient deprivation and exposure to toxins make trees more likely to topple in storms or die in cold weather. • Even trees in well buffered soil can weaken in harsh acid fog. High elevation forests soak in acidic clouds, which strip leaves of nutrients and break down trees' ability to resist cold.

Materials and Finishes • Acid rain has the ability to break down and deteriorate stone and metal, the most durable of materials. Old buildings, monuments and tombstones bear the signs of acidic corrosion and deterioration. Acid deposition speeds up natural weathering caused by rain, sun, snow and wind. • Acid rain also deteriorate the paint on cars. Acid markings leave irregular, etched shapes on horizontal surfaces. Repainting is the only way to fix a car finish disfigured by acid rain.

Health Acid rain can kill aquatic animals, weaken trees and dissolve stone. It doesn't affect people in the same way as it does fish or plants. Acid rain feels the same as regular rain. But the sulfate and nitrate particulates of dry deposition cause asthma, bronchitis and heart problems. The NOx in acid deposition also reacts with volatile organic compounds (VOCs) to form ground level ozone. Ozone, or smog, aggravates and weakens the respiratory system.

The p. H of Acid Rain • The acidity of acid rain is expressed by using the p. H scale. • The scale defines a solution's acidity, neutrality or alkalinity based on its concentration of hydrogen ions. • Acids have a high concentration of hydrogen ions and a low p. H. The scale ranges from zero to 14, with pure water at a neutral 7. 0. Most water, however, is not exactly pure. Even clean, normal rain has a p. H of about 5. 6. This is because it reacts with carbon dioxide in the atmosphere and forms mildly acidic carbonic acid before it becomes rain.

Surface Waters • Surface waters and their fragile ecosystems are perhaps the most famous victims of acid rain. Most of the precipitation that enters a lake, river, stream or wetland must first pass over and seep through soil. All soil has a buffering capacity, or ability to resist changes in acidity and alkalinity. The soil's buffering capacity determines a water body's acidity. If the capacity is low, or has reached its limit, acid rain can pass through un neutralized • Even at its weakest, acid rain wrecks ecosystems by stunting sensitive plants and killing delicate aquatic eggs.

Acid deposition weakens trees and pollutes surface waters. • Most life is comfortable at a near neutral. • Plankton and invertebrates are sensitive to changes in acidity and die first. At p. H 5. 0, fish eggs degrade and young cannot develop. Adult fish and frogs can sometimes tolerate acidities as low as p. H 4. 0, but they starve as their weaker food sources die out. When acid rain disrupts the food chain, biodiversity decreases.

Coastal waters and estuaries • Nitrogen deposition from acid rain also damages coastal waters and estuaries. Nitrogen rich water supports massive algae growth and algal blooms. Bacteria decompose the dead algae, flourish themselves and soak up the water's available oxygen. Fish, shellfish, sea grass beds and coral reefs die in the algae choked, oxygen-depleted waters. • (Source: Environmental Protection Agency).

• Most acidic bodies of water do not look polluted. As decaying organic matter settles, acidified water can appear clear and blue. Some species, like rushes and moss, even thrive in acidic conditions. In acidic water diversity drops, and species left without predators often grow disturbingly large. • (Source: www. science. howstuffworks. com)

Resources • www. science. howstuffworks. com
 Rain rain go away
Rain rain go away Bergeron process diagram
Bergeron process diagram Formation initiale vs formation continue
Formation initiale vs formation continue Acid rain
Acid rain Acid rain pathway
Acid rain pathway Acid rain map
Acid rain map Acid rain reaction
Acid rain reaction Hccco2h
Hccco2h Acid rain in canada
Acid rain in canada Watch?v=mwrxc48fdjq
Watch?v=mwrxc48fdjq Acid rain in germany illustration
Acid rain in germany illustration Acid rain calcium carbonate
Acid rain calcium carbonate Acid rain facts
Acid rain facts Objectives of acid rain
Objectives of acid rain Copy the given chart in your notebook
Copy the given chart in your notebook