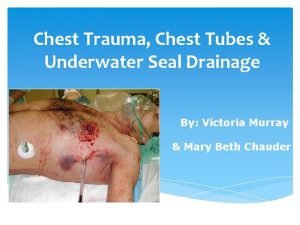
Acid rain Acid Deposition Acid Deposition Wet Dry






















- Slides: 32

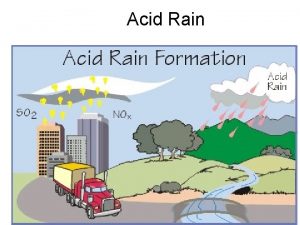
Acid rain “Acid Deposition”

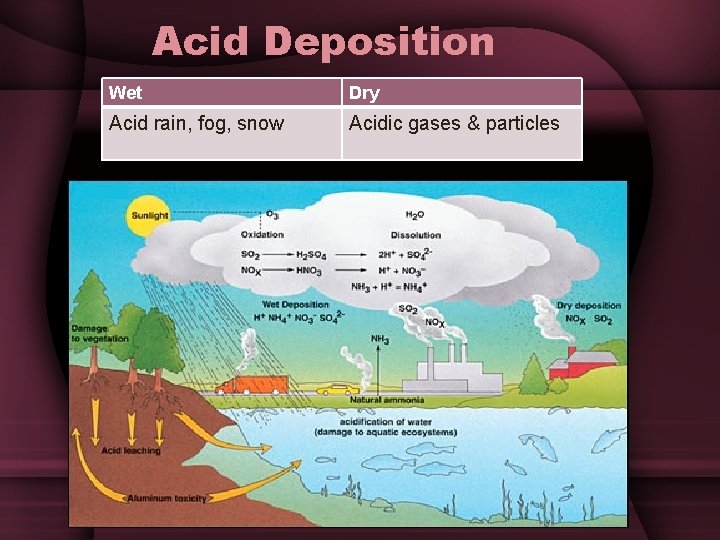
Acid Deposition Wet Dry Acid rain, fog, snow Acidic gases & particles

SOx: oxides of sulfur NOx: oxides of nitrogen THE SOURCE

Acid Deposition • Caused by: • Emissions of sulfur dioxide, nitrogen oxides & carbon dioxide –Fossil fuel combustion by automobiles –Electric utilities –Industrial facilities • Once airborne: • These pollutants can react w/water, oxygen, & oxidants to produce sulfuric acid & nitric acid • Pollutants leading to acid deposition can travel long distances, leading to political issues between states/countries

Effects of Acid Deposition • It limits the ability of lakes to support aquatic life • Can change p. H of water • Can leach Al out of soil/rock & into waterways, killing the fish by damaging their gills & disrupting their salt balance, water balance, breathing, and circulation • May damage trees & plants • Causes nutrient leaching from soil • H+ take place of important nutrients Ca, Mg & K • Causes insoluble forms of Al, zinc, mercury & copper into soluble forms, which can decrease water & nutrient uptake by plants • Leaves become discolored due to chlorosis (bleaching) & then die • More susceptible to pathogens & insects because weaker from rain • Damages agricultural crops • Erodes stone buildings (national monuments), corrodes cars, & erases writing from tombstones

• Sulfur Dioxide • NOx emissions • New technologies, like scrubbers, have decreased SO 2 emissions when burning fossil fuels • Have increased • But reduction is not enough

OZONE

Ozone, O 3 Good ozone • Bad ozone • 6 -30 miles above Earth’s surface (Stratosphere) • ABSORBS most of the Sun’s Ultraviolet-B & UV-C radiation • Provides critical protection because UV radiation is a mutagen & carcinogen • Destroyed by CFC’s • Montreal Protocol set up a time table for phasing out CFC’s & other ozone depleting substances • Troposphere • Formed when pollutants emitted by car exhaust, gasoline vapors, industrial facilities, electric utilities & chemical solvents react chemically in the presence of sunlight – NOx + Sunlight= ozone • Harmful air pollutant! • Part of photochemical smog – NOx/SOx + ozone + sunlight + VOCs= smog

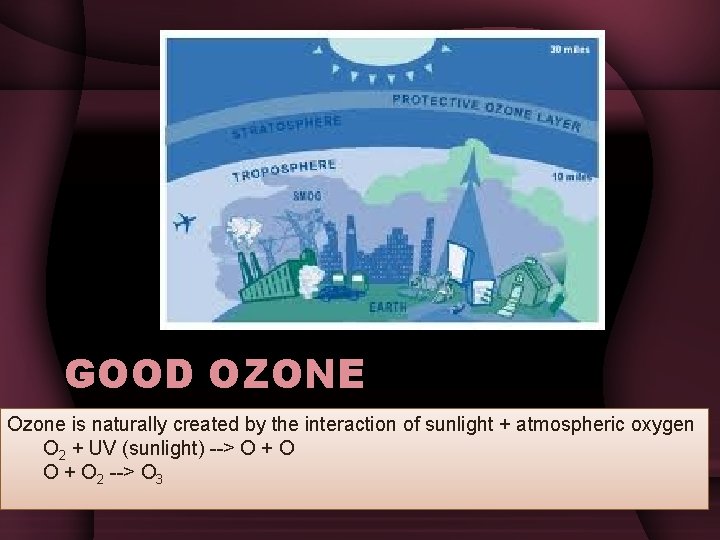
GOOD OZONE Ozone is naturally created by the interaction of sunlight + atmospheric oxygen O 2 + UV (sunlight) --> O + O 2 --> O 3

Formation of stratospheric ozone

The Sun The sun emits: • High-energy ultraviolet waves • Medium-energy waves that we see as light • Lower-energy infrared heat waves

Stratospheric Ozone • Ozone layer in stratosphere blocks about 99% of the sun’s UV-B & UV-C radiation. – UV-A passes through atmosphere w/out being absorbed & contributes to, and possibly initiates skin cancer – UV-B & UV-C have enough energy to cause potentially significant damage to the tissues & DNA of living organisms • Can also: reduce primary productivity in oceans, disrupt food chains, cause widespread effects on major food crops, decrease plant productivity O 3 + UV-B or UV-C O 2 + O


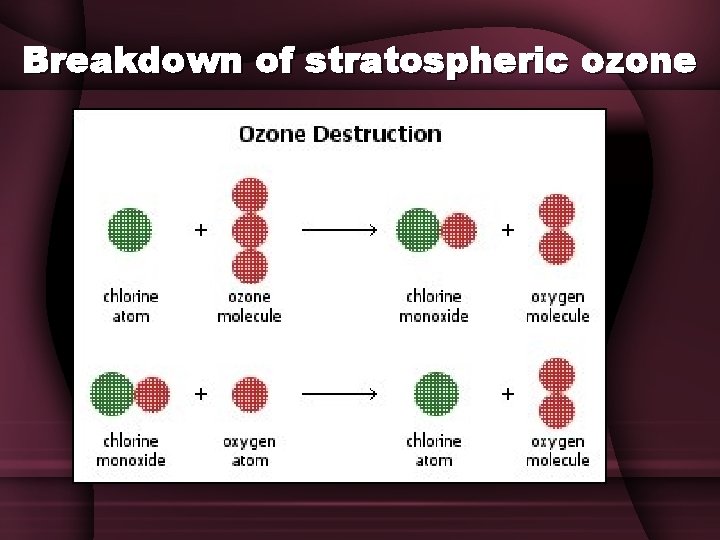
Breakdown of stratospheric ozone

Breakdown of stratospheric ozone • Certain chemical catalysts, most notably chlorine, can break down ozone. • Chlorofluorcarbons (CFCs) – Man-made – Used in refrigeration & air conditioning – Propellants in aerosol cans, like deodorant or insect spray – Blowing agents to inject air into foam products like styrofoam cups and foam insulation – Stable, nontoxic, and nonflammable – Don’t break down, so they slowly circulate to atmosphere

Breakdown of stratospheric ozone • The CFC’s migrate to the upper stratosphere where the UV radiation breaks them apart, releasing chlorine atoms. CCl 3 F + UV Cl + CCl 2 F • The chlorine breaks ozone’s bonds & pulls off one atom of oxygen • Cl + O 3 Cl. O + O 2 • Then a free oxygen pulls the oxygen atom from Cl. O, freeing the chlorine & creating one more oxygen molecule – Cl. O + O Cl + O 2 • The free chlorine molecule is ready to break down more ozone!

Breakdown of stratospheric ozone Can also be broken down by: • NOx • Bromines & other Halons – Used as fumigants for soil pests such as termites • Carbon tetrachloride – Formally used as a cleaning solvent *They don’t reach stratosphere as efficiently as CFC’s because they are more reactive in the troposphere

Halons

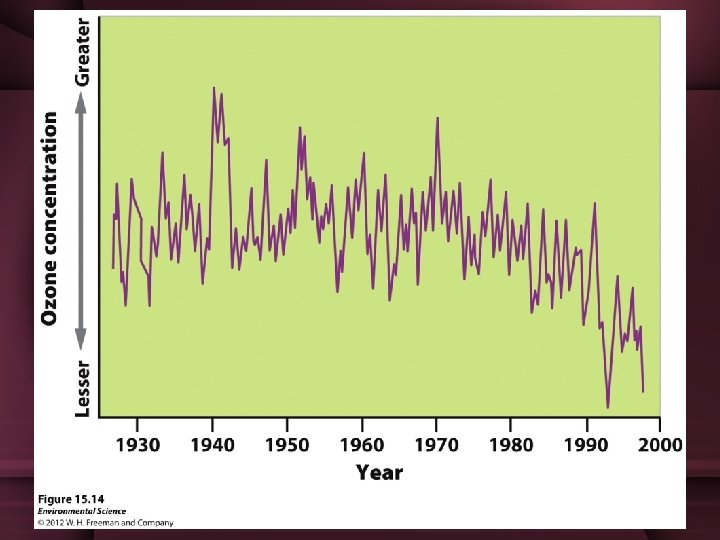
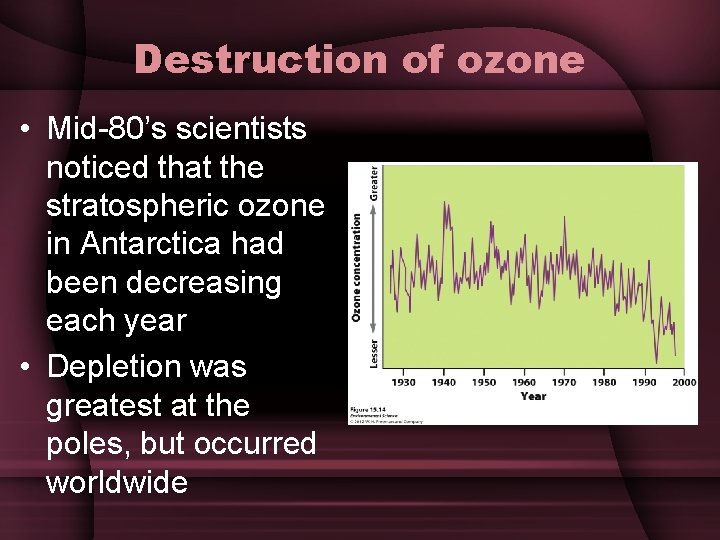
Destruction of ozone • Mid-80’s scientists noticed that the stratospheric ozone in Antarctica had been decreasing each year • Depletion was greatest at the poles, but occurred worldwide

Destruction of ozone • In Antarctic, ozone depletion was seasonal – Depletion occurred from Aug-Nov – Late winter-early spring • Caused an area of severely reduced ozone concentrations over most of Antarctica (“ozone hole”)

Destruction of ozone • Winter conditions cause a build up of ice crystals mixed with NO, which creates stable Cl 2 • When sun reappears in Spring, UV radiation breaks down this molecule into Cl again, freeing it to catalyze the destruction of the ozone. – Ozone loss is greatest in the spring • As the air continues to warm, the natural production of ozone increases as more sunlight catalyzes the combination of oxygen back into ozone. – This occurs in Antarctica’s summer (Jan-Feb)

The Result • Decreased stratospheric ozone has increased the amount of UV-B radiation that reaches the Earth’s surface. – In mid-latitude N. America, UV radiation increased by 4% from 1979 -1992 – Increase is greater at the poles • UV radiation harms cells & can reduce photosynthetic activity of plants • Significant increases of skin cancer have increased, particularly in countries near the Antarctic ozone hole such as Chile & Australia

Efforts to reduce ozone depletion • 1987, 24 nations signed an agreement called the Montreal Protocol on Substances that Deplete the Ozone Layer • Committed to reducing reduce CFC production 50% by the year 2000 – Most far-reaching environmental treaty to date because global biosphere protection was prioritized over economic self-interest • Amendments eventually signed by 180 countries requiring the elimination of CFC production & use by 1996 – In total, protocol addressed 96 ozone-depleting compounds

The Result • Concentrations of chlorine in stratosphere has stabilized at about 5 ppb and should fall to 1 ppb by 2100 • Reduction process is slow because CFC’s are not easily removed from atmosphere

Bad Ozone & Photochemical Smog “EPA says half of the United States is Breathing Excessive Levels of Smog” Maximum allowable rate of O 3 is 0. 075 parts per million over 8 hours

Criteria Air Pollutants Compound Symbol Human-derived source Effects/Impacts Ozone O 3 A secondary • Reduces lung function & pollutant formed by exacerbates respiratory symptoms the combination of (harmful to respiratory tissue) sunlight, water, • - asthma, ephysema oxygen, VOCs, and NOx • Harmful to plant tissue • Damages materials such as rubber & plastic Causes: shortness of breath, pain when inhaling, wheezing/coughing, eye/nose irritation, interferes with body’s ability to fight off infection

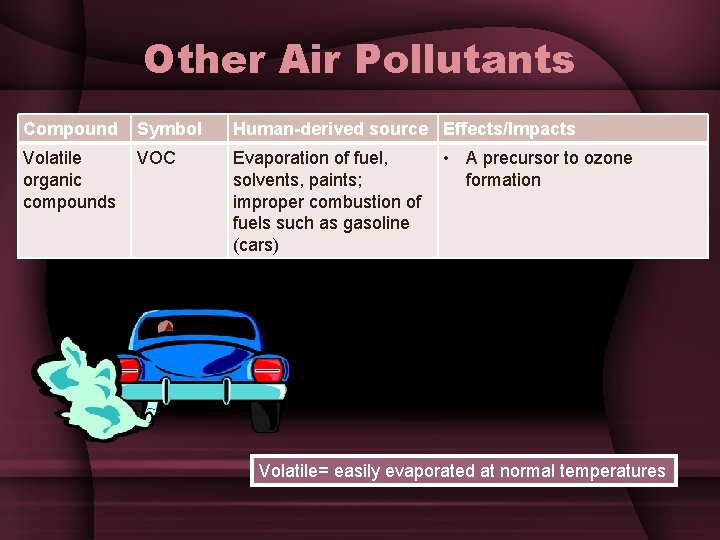
Other Air Pollutants Compound Symbol Human-derived source Effects/Impacts Volatile organic compounds VOC Evaporation of fuel, solvents, paints; improper combustion of fuels such as gasoline (cars) • A precursor to ozone formation Volatile= easily evaporated at normal temperatures

Smog formation • The term "smog" was first used in London during the early 1900's to describe the combination of smoke and fog. • What we typically call "smog" today is a mixture of pollutants but is primarily made up of ground-level ozone. • Smog is often thought of as an urban problem but its not limited to these areas. Trees & shrubs produce VOCs that can contribute to photochemical smog, as do forest fires that begin naturally in rural areas.

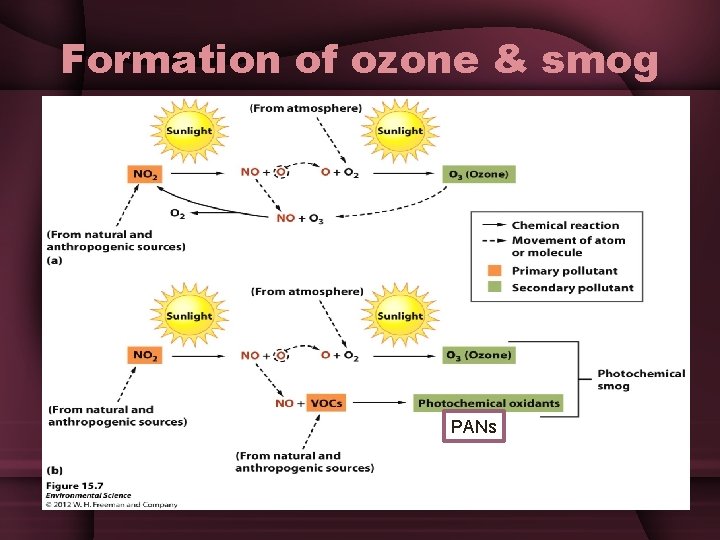
Formation of ozone & smog • Part I: Takes place during the day, in the presence of sunlight –NO 2 + sunlight= NO + O 3 (ozone) • When VOCs are limited, the cycle of ozone formation & destruction generally takes place on a daily basis w/relatively small amounts of photochemical smog formation • Part II: When VOCs are present, they combine with nitrogen oxide to form photochemical oxidants • This causes a larger amount of ozone to accumulate because nitrogen oxide is no longer available to break down the ozone

Formation of ozone & smog PANs

Photochemical Oxidants • PANs • Peroxyacyl nitrates • Cause eyes to burn and damage vegetation

Atmospheric Temperatures affect formation of photochemical smog • Emissions of VOCs increase as temperature increases (evaporation) • NOx emissions increase as airconditioning demands for electricity on the hottest days • Ozone formation increases at higher temperatures
 Wet wet wet
Wet wet wet Rain rain go away isaac asimov summary
Rain rain go away isaac asimov summary Cloudy sprinkler rain wet grass
Cloudy sprinkler rain wet grass Conditionals 0 1 2 3
Conditionals 0 1 2 3 Dry corrosion and wet corrosion
Dry corrosion and wet corrosion Isotropic material examples
Isotropic material examples Forbes method of emulsion preparation is also known as
Forbes method of emulsion preparation is also known as Wet bonding vs dry bonding
Wet bonding vs dry bonding Dry oxidation vs wet oxidation
Dry oxidation vs wet oxidation Semi pressure lubrication system
Semi pressure lubrication system Difference between alpha and beta hemihydrate
Difference between alpha and beta hemihydrate What are the 3 types of nursery
What are the 3 types of nursery Difference between wet corrosion and dry corrosion
Difference between wet corrosion and dry corrosion Process of salting fish
Process of salting fish Dry heat definition cooking
Dry heat definition cooking Rowenta pro 1500w wet and dry
Rowenta pro 1500w wet and dry Adiabatic lapse rate
Adiabatic lapse rate English method emulsion
English method emulsion Superadded putrefaction
Superadded putrefaction Wetland preparation
Wetland preparation Water seal chamber
Water seal chamber Dry cell vs wet cell
Dry cell vs wet cell Avg wetgeving
Avg wetgeving Bonding agent generations
Bonding agent generations Modern bundh
Modern bundh Slug dry granulation
Slug dry granulation Dry gum method of emulsion preparation
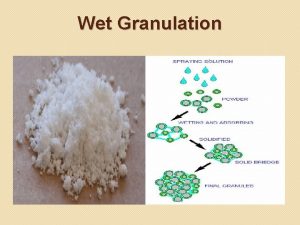
Dry gum method of emulsion preparation Advantages of wet granulation
Advantages of wet granulation Wet bonding vs dry bonding
Wet bonding vs dry bonding What is season
What is season Wet etching vs dry etching
Wet etching vs dry etching Cell injury and inflammation
Cell injury and inflammation Pohlman whistle
Pohlman whistle