Acid Rain Acid Deposition Acid Rain refers to









- Slides: 13

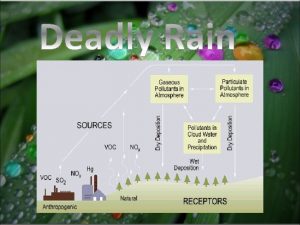
Acid Rain

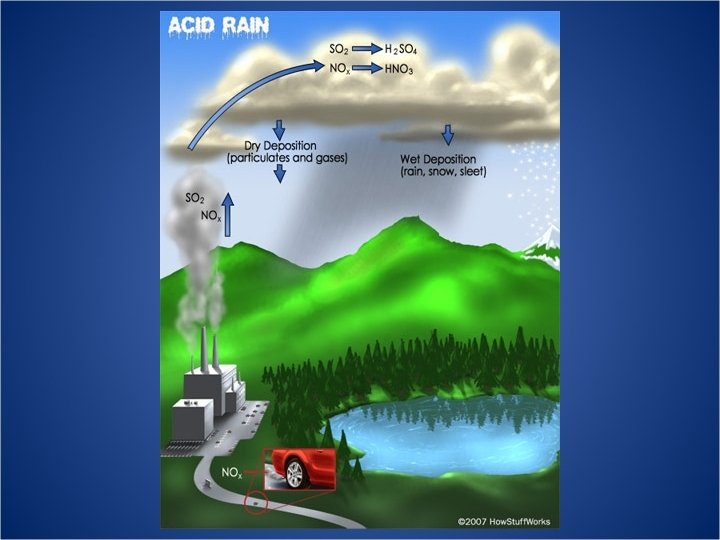
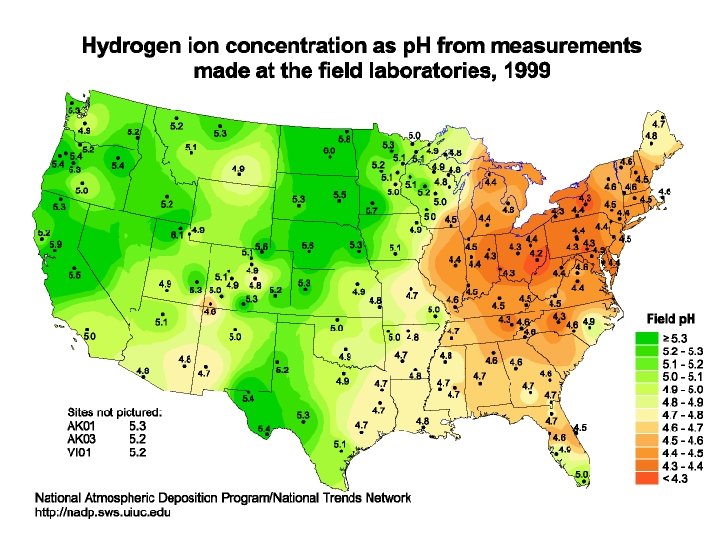
Acid Deposition • Acid Rain refers to the deposition of acidic components in either wet or dry forms • Defined by the p. H of the liquid. Less than 7 p. H is acidic, more than 7 is basic • Natural acid rain can be caused by volcanic emissions and biological processes • “Clean” rain has a natural acidity of about 5. 2 on the p. H scale due to water reacting with carbon dioxide in the air to form carbonic acid • H 2 O (l) + CO 2 (g) → H 2 CO 3 (aq) • 2 H 2 O (l) + H 2 CO 3 (aq) <–> CO 32− (aq) + 2 H 3 O+ (aq)


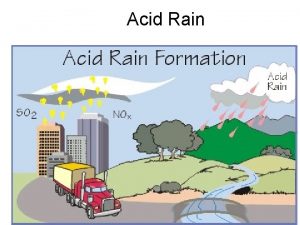
Human Attribution • Human emissions of sulfur dioxide and nitrogen oxides contribute to the acidification of rain • Emissions began during the industrial revolution, remaining unchecked until the 1970 s • Biggest contributor is the burning of coal • Annually 70 Tg (1012 g) of Sulfur emissions comes from fossil fuel burning, compared to 8 Tg from volcanoes and 2. 8 Tg from wildfires

The Coal Power Plant Problem • Burning coal is extremely cheap and efficient but dirty, releasing sulfur dioxide which becomes sulfuric acid in the atmosphere • Areas downwind of power plants receive heavy acid rain • Smoke stacks built to counteract direct deposition of sulfuric acid only spread the problem

Effects of Acid Rain • Not many things can grow in acidic conditions • Low p. H and high aluminum concentrations can damage or kill fish and aquatic populations • Soils can be damaged by the hydronium ion, which mobilizes aluminum and encourages leaching of minerals such as magnesium essential for plant life • Forests suffer from soil damage, however most food crops are unharmed because the nutrients lost are replaced in fertilizer


Other Effects • Monuments made of Calcium Carbonate (limestone and marble) will react with acid rain to form Gypsum • Increases the oxidation rate of metals such as copper and bronze

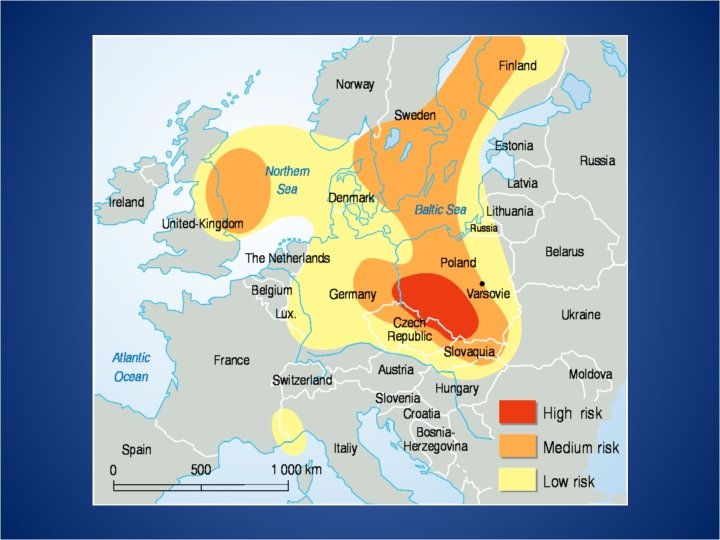
Areas of Highest Concern • Current problem areas are: • Eastern United States • South Western Canada • Eastern Europe • East Coast of China • Potential future problem areas: • Southern India • West Africa • Indonesia • Thailand



Prevention • Coal burning power plants use Flue gas desulfurization requiring a reaction tower that extracts the sulfuric acid by reacting it with lime or limestone slurry and removing the product with scrubbers • Reduction in automotive emissions cuts down on nitrogen oxides • Emissions trading put into practice to put economic incentive into cleaning industrial activities

Sources • • • http: //static. howstuffworks. com/gif/acid-rain-1 a. jpg http: //people. eku. edu/ritchisong/phfield. gif http: //nadp. sws. uiuc. edu http: //en. wikipedia. org/wiki/Acid_rain http: //www. epa. gov/acidrain/effects/surface_water. html http: //www. sfgate. com/cgibin/article. cgi? f=/c/a/2007/12/03/MNMMTJUS 1. DTL&hw= Cap+trade+Acid+Rain&sn=001&sc=1000
 What causes acid deposition
What causes acid deposition Acid deposition
Acid deposition Pollution management strategies for acid deposition
Pollution management strategies for acid deposition Rain, rain, go away by isaac asimov summary
Rain, rain, go away by isaac asimov summary Acid rain
Acid rain Acid rain weather rocks
Acid rain weather rocks Lord acid rain
Lord acid rain Acid rain chemical reaction
Acid rain chemical reaction Acid rain in europe
Acid rain in europe Acid rain causes
Acid rain causes Statue of liberty before and after acid rain
Statue of liberty before and after acid rain Acid vocabulary
Acid vocabulary Artificial rain chemical
Artificial rain chemical Which rock weathers most rapidly when exposed to acid rain?
Which rock weathers most rapidly when exposed to acid rain?