Acid Rain What is Acid Rain Acid rain










- Slides: 14

Acid Rain

What is Acid Rain? • Acid rain is rain that has been acidic by pollutants in the made _______ air • There are three major pollutants: – Carbon dioxide (CO 2) – Nitrogen oxides (NOx) – Sulfur oxides (SOx)

Carbon Dioxide acidic • Normal rain is usually slightly ______, with a p. H of ___ 5. 6 – CO 2 dissolves in moisture in the atmosphere to form dilute carbonic acid ________ CO 2(g) + H 2 O(l) H 2 CO 3(aq)

Carbon dioxide • CO 2 occurs naturally in our atmosphere – Breathed out in _______ respiration – Produced from forest fires and _____ volcanic eruptions

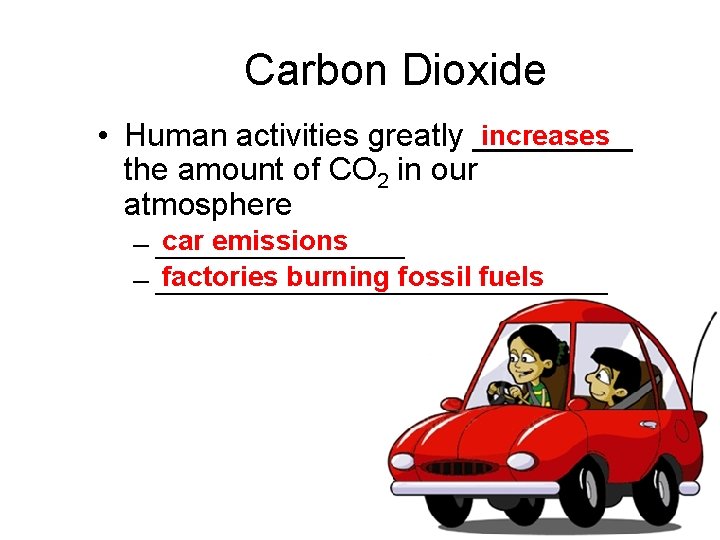
Carbon Dioxide increases • Human activities greatly _____ the amount of CO 2 in our atmosphere car emissions – ________ factories burning fossil fuels – _______________

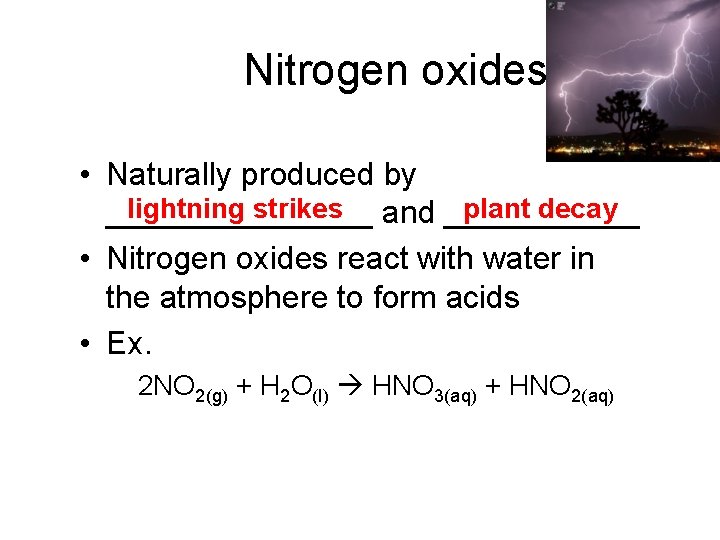
Nitrogen oxides • Naturally produced by plant decay lightning strikes and _______________ • Nitrogen oxides react with water in the atmosphere to form acids • Ex. 2 NO 2(g) + H 2 O(l) HNO 3(aq) + HNO 2(aq)

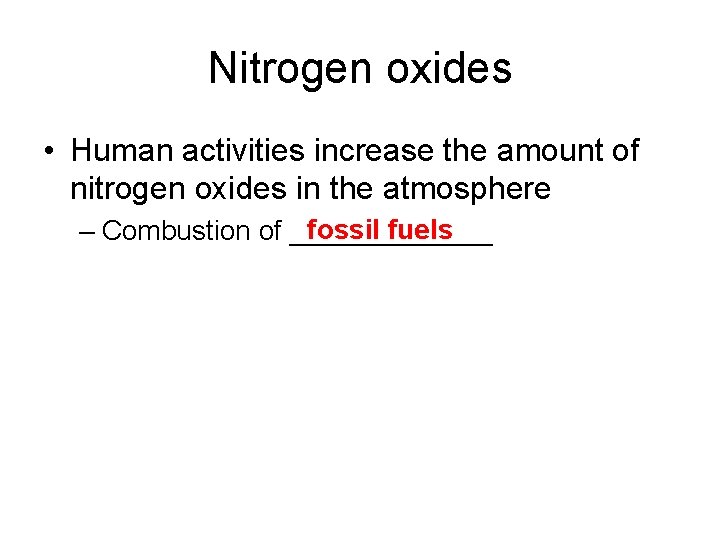
Nitrogen oxides • Human activities increase the amount of nitrogen oxides in the atmosphere fossil fuels – Combustion of _______

Sulfur oxides • Naturally exists in our atmosphere volcanic eruptions – Produced in __________

Sulfur oxides • Reacts with water in the atmosphere to produce acids • Ex. SO 2(g) +H 2 O(l) H 2 SO 3(aq) • Human activity increases levels of sulfur oxides – Combustion of coal and oil (SO 2) Smelting of ores in factories – _____

Why is Acid Rain bad? • Lakes aquatic life – Cannot support _______ toxic metals that can accumulate – Increases levels of ________ in fish • Forests – Damage to wildlife and plants • Humans Erosion of buildings – ______ – Buildup of toxic metals in fish these fish are consumed by humans ________

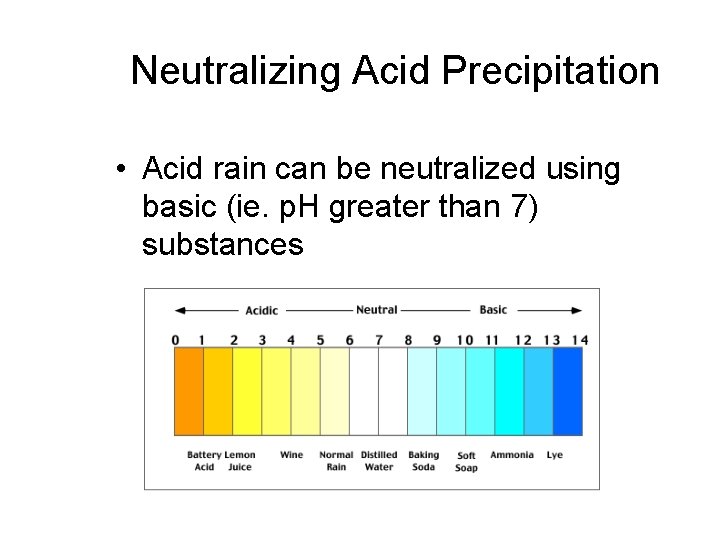
Neutralizing Acid Precipitation • Acid rain can be neutralized using basic (ie. p. H greater than 7) substances

Liming limestone (basic substance) • Addition of _____ neutralize acidic lake water and to _____ surrounding soil – Varying degrees of success around the Canadian Shield

Liming • Pros: easy to distribute – Inexpensive and __________ – Dissolves easily in water Short term effects improved water – __________: clarity and restoration of habitat • Cons: Needs to be repeated – ____________

Other Solutions • Most solutions aim to reduce the emission of pollutants __________ – Reduced automobile use (carpooling, etc) – Retrofit older homes to eliminate air leaks – Plant trees (they take in CO 2 during photosynthesis) – Adding “scrubbers” to smokestacks on factories that reduce pollutants
 Rain, rain, go away by isaac asimov summary
Rain, rain, go away by isaac asimov summary Acid rain
Acid rain Acid rain map
Acid rain map Before and after acid rain
Before and after acid rain Acid rain chernobyl
Acid rain chernobyl Acid rain
Acid rain Acid rain
Acid rain Which rock weathers most rapidly when exposed to acid rain?
Which rock weathers most rapidly when exposed to acid rain? Acid rain pathway
Acid rain pathway Acid rain
Acid rain Acid rain reaction
Acid rain reaction Objectives of acid rain
Objectives of acid rain Acid rain osu
Acid rain osu :https://m.youtube.com/watch?v=iqnhllwd61g
:https://m.youtube.com/watch?v=iqnhllwd61g Brainpop air pollution
Brainpop air pollution