Complex Lipid Management Tara Dall MD FNLA Diplomate

























- Slides: 80

Complex Lipid Management Tara Dall, MD, FNLA Diplomate, American Board Clinical Lipidology President Advanced Lipidology Delafield, WI www. advlip. com www. lecturepad. org (ongoing clinician education)

Disclosures • Speakers Bureau 2012: Abbott, Liposcience, Santarus, HDL labs • 2011 Glaxo. Smith. Kleine • Research support: none • OFF LABEL DISCUSSION: none

Objectives • Review guidelines/consensus statements: NCEP ATP III, ADA/ACC, National Lipid Association Advanced Lipid testing and Biomarkers 2011, ATP IV (if available) • Discuss potential risk factors including triglycerides, HDLs • Identify successful strategies for the management of patients with complicated lipid disorders, high LDL, low HDL, high triglycerides, or combination disorders • Discuss recent combination therapy clinical trial data (ACCORD, AIM HIGH, SHARP)

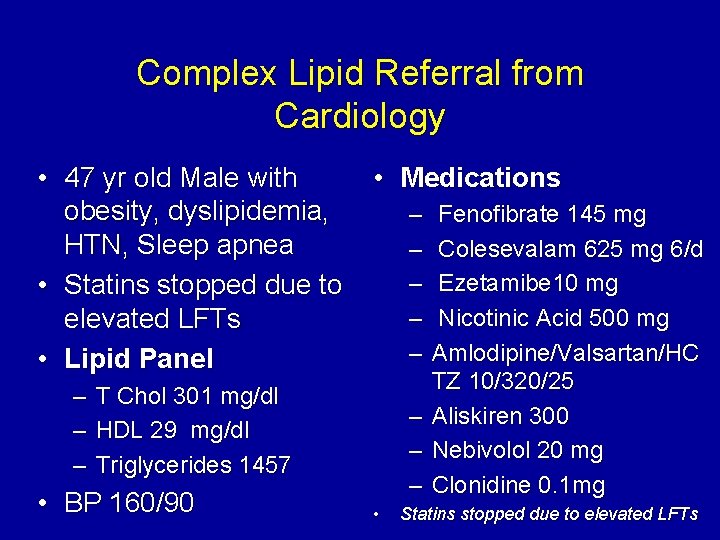
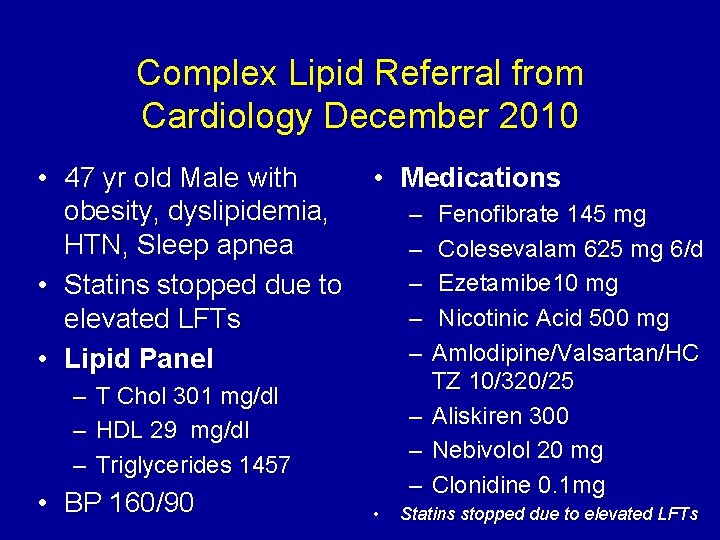
Complex Lipid Referral from Cardiology • 47 yr old Male with obesity, dyslipidemia, HTN, Sleep apnea • Statins stopped due to elevated LFTs • Lipid Panel – – – • Medications – – – T Chol 301 mg/dl HDL 29 mg/dl Triglycerides 1457 • BP 160/90 – – – • Fenofibrate 145 mg Colesevalam 625 mg 6/d Ezetamibe 10 mg Nicotinic Acid 500 mg Amlodipine/Valsartan/HC TZ 10/320/25 Aliskiren 300 Nebivolol 20 mg Clonidine 0. 1 mg Statins stopped due to elevated LFTs

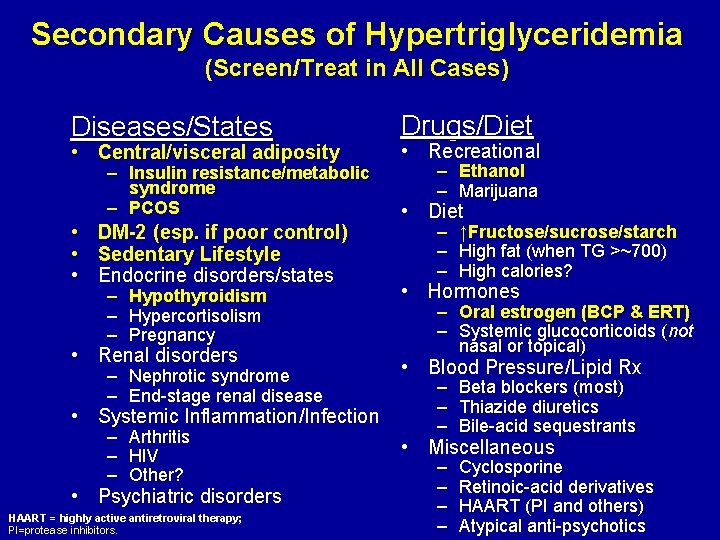
Secondary Causes of Hypertriglyceridemia (Screen/Treat in All Cases) Diseases/States • Central/visceral adiposity – Insulin resistance/metabolic syndrome – PCOS • DM-2 (esp. if poor control) • Sedentary Lifestyle • Endocrine disorders/states – Hypothyroidism – Hypercortisolism – Pregnancy • Renal disorders – Nephrotic syndrome – End-stage renal disease • Systemic Inflammation/Infection – Arthritis – HIV – Other? • Psychiatric disorders HAART = highly active antiretroviral therapy; PI=protease inhibitors. Drugs/Diet • Recreational – Ethanol – Marijuana • Diet – ↑Fructose/sucrose/starch – High fat (when TG >~700) – High calories? • Hormones – Oral estrogen (BCP & ERT) – Systemic glucocorticoids (not nasal or topical) • Blood Pressure/Lipid Rx – Beta blockers (most) – Thiazide diuretics – Bile-acid sequestrants • Miscellaneous – – Cyclosporine Retinoic-acid derivatives HAART (PI and others) Atypical anti-psychotics

Emerging Risk Markers • • hs. CRP Lp. PLA 2 Fibrinogen Homocysteine • LDL Particle concentration • Small dense LDL • Apo B • Lipoprotein (a)

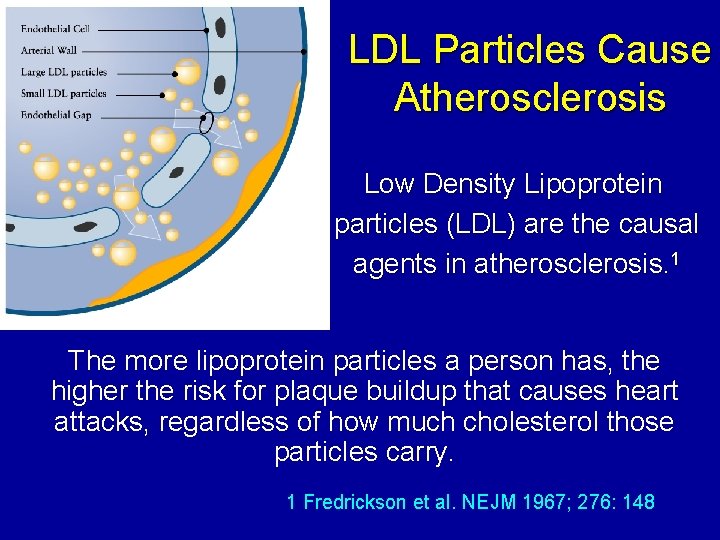
LDL Particles Cause Atherosclerosis Low Density Lipoprotein particles (LDL) are the causal agents in atherosclerosis. 1 The more lipoprotein particles a person has, the higher the risk for plaque buildup that causes heart attacks, regardless of how much cholesterol those particles carry. 1 Fredrickson et al. NEJM 1967; 276: 148

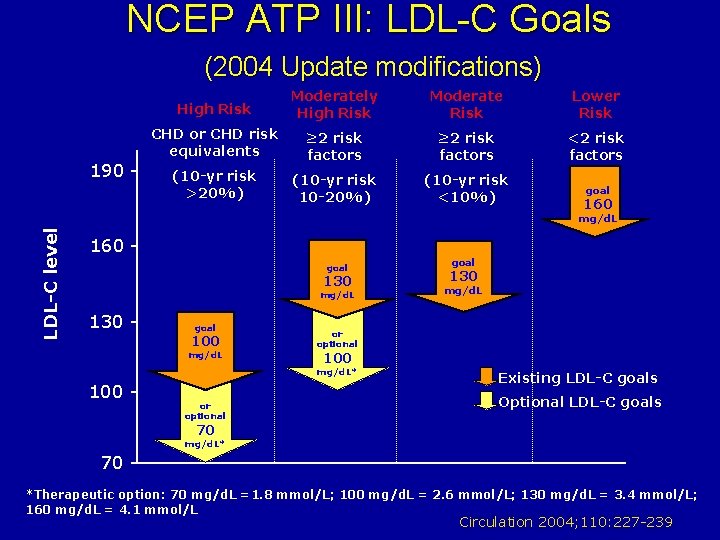
NCEP ATP III: LDL-C Goals (2004 Update modifications) 190 - High Risk Moderately High Risk Moderate Risk Lower Risk CHD or CHD risk equivalents ≥ 2 risk factors <2 risk factors (10 -yr risk >20%) (10 -yr risk 10 -20%) (10 -yr risk <10%) goal 160 LDL-C level mg/d. L 160 goal 130 mg/d. L 130 - goal 100 mg/d. L or optional 130 mg/d. L or optional 100 mg/d. L* 100 - goal Existing LDL-C goals Optional LDL-C goals 70 mg/d. L* 70 *Therapeutic option: 70 mg/d. L =1. 8 mmol/L; 100 mg/d. L = 2. 6 mmol/L; 130 mg/d. L = 3. 4 mmol/L; 160 mg/d. L = 4. 1 mmol/L Circulation 2004; 110: 227 -239

Canadian Targets for LDL-C (or apo. B): Nearly all clinical trials measure LDL-C as index of therapy. Recommendations: Æ Primary target is LDL-C decrease to < 2. 0 mmol/L or 50% relative reduction Æ We recommend apo. B < 0. 80 g/L as primary alternate target In high- and moderate-risk subjects

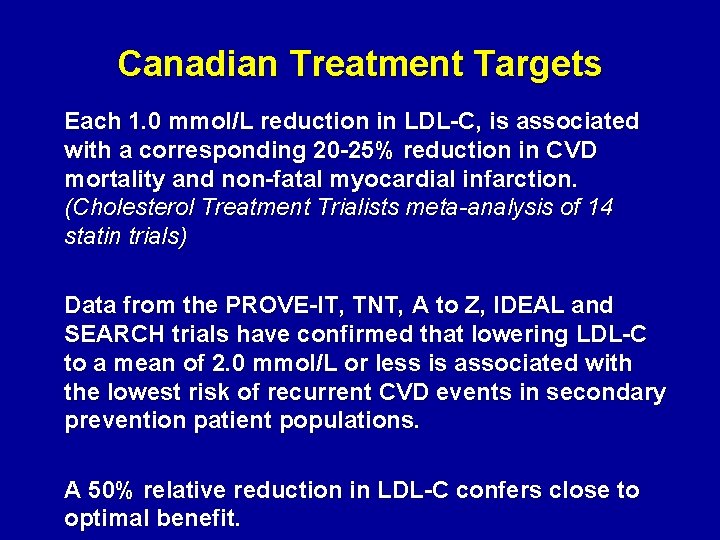
Canadian Treatment Targets Each 1. 0 mmol/L reduction in LDL-C, is associated with a corresponding 20 -25% reduction in CVD mortality and non-fatal myocardial infarction. (Cholesterol Treatment Trialists meta-analysis of 14 statin trials) Data from the PROVE-IT, TNT, A to Z, IDEAL and SEARCH trials have confirmed that lowering LDL-C to a mean of 2. 0 mmol/L or less is associated with the lowest risk of recurrent CVD events in secondary prevention patient populations. A 50% relative reduction in LDL-C confers close to optimal benefit.

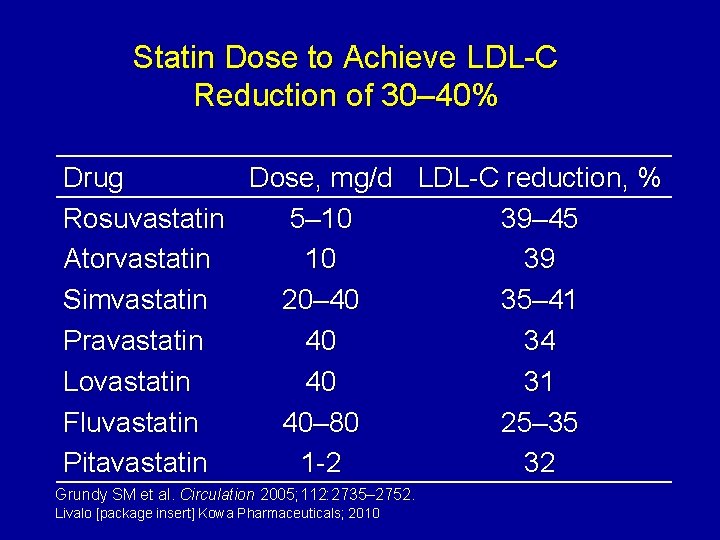
Statin Dose to Achieve LDL-C Reduction of 30– 40% Drug Dose, mg/d LDL-C reduction, % Rosuvastatin 5– 10 39– 45 Atorvastatin 10 39 Simvastatin 20– 40 35– 41 Pravastatin 40 34 Lovastatin 40 31 Fluvastatin 40– 80 25– 35 Pitavastatin 1 -2 32 Grundy SM et al. Circulation 2005; 112: 2735– 2752. Livalo [package insert] Kowa Pharmaceuticals; 2010

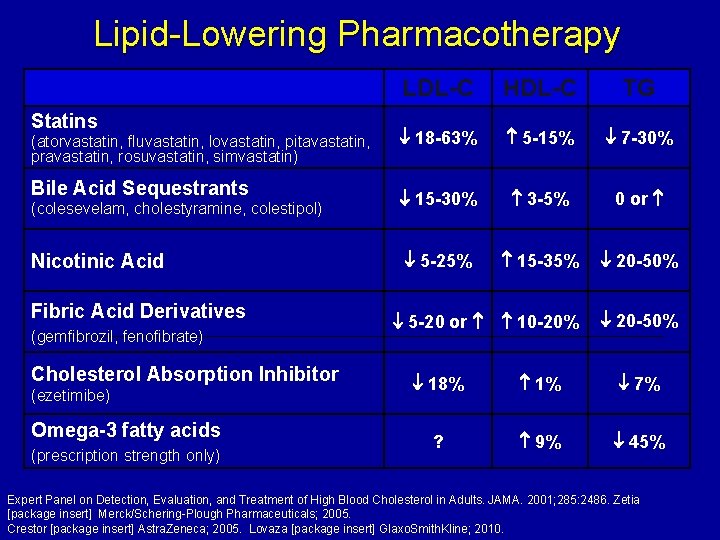
Lipid-Lowering Pharmacotherapy Statins (atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin) Bile Acid Sequestrants (colesevelam, cholestyramine, colestipol) Nicotinic Acid Fibric Acid Derivatives (gemfibrozil, fenofibrate) Cholesterol Absorption Inhibitor (ezetimibe) Omega-3 fatty acids (prescription strength only) LDL-C HDL-C TG 18 -63% 5 -15% 7 -30% 15 -30% 3 -5% 0 or 5 -25% 15 -35% 20 -50% 5 -20 or 10 -20% 20 -50% 18% 1% 7% ? 9% 45% Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486. Zetia [package insert] Merck/Schering-Plough Pharmaceuticals; 2005. Crestor [package insert] Astra. Zeneca; 2005. Lovaza [package insert] Glaxo. Smith. Kline; 2010.

Statins: Role in Therapy • The most effective agents to lower LDL-C, first-line therapy for most patients • Clinically proven to reduce mortality and recurrent cardiovascular events • Stabilizing plaques, reduces progression, partial regression • Most well tolerated

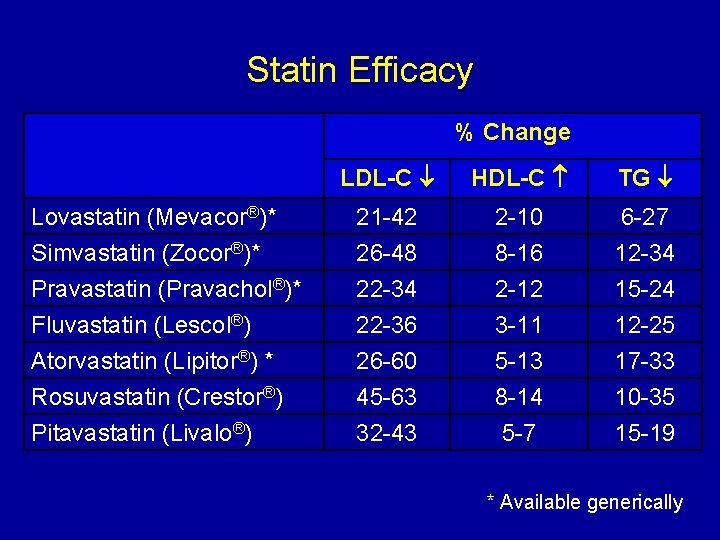
Statin Efficacy % Change LDL-C HDL-C TG Lovastatin (Mevacor®)* Simvastatin (Zocor®)* 21 -42 26 -48 2 -10 8 -16 6 -27 12 -34 Pravastatin (Pravachol®)* Fluvastatin (Lescol®) Atorvastatin (Lipitor®) * Rosuvastatin (Crestor®) Pitavastatin (Livalo®) 22 -34 22 -36 26 -60 45 -63 32 -43 2 -12 3 -11 5 -13 8 -14 5 -7 15 -24 12 -25 17 -33 10 -35 15 -19 * Available generically

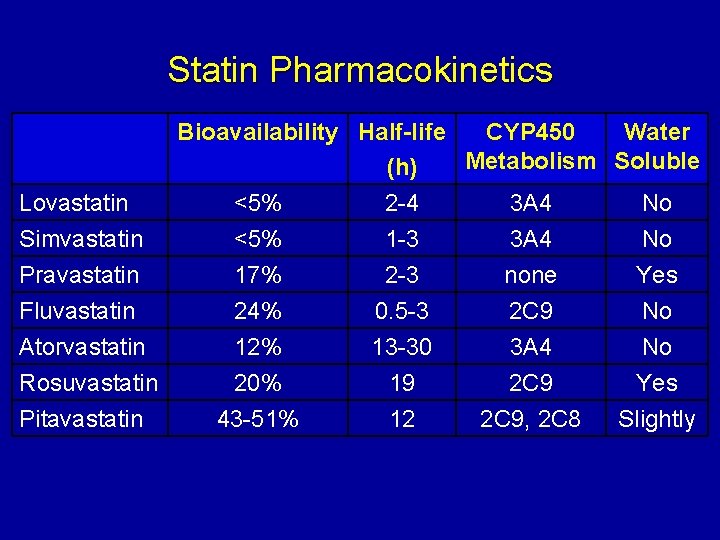
Statin Pharmacokinetics Bioavailability Half-life CYP 450 Water Metabolism Soluble (h) Lovastatin <5% 2 -4 3 A 4 No Simvastatin <5% 1 -3 3 A 4 No Pravastatin 17% 2 -3 none Yes Fluvastatin 24% 0. 5 -3 2 C 9 No Atorvastatin 12% 13 -30 3 A 4 No Rosuvastatin 20% 19 2 C 9 Yes Pitavastatin 43 -51% 12 2 C 9, 2 C 8 Slightly

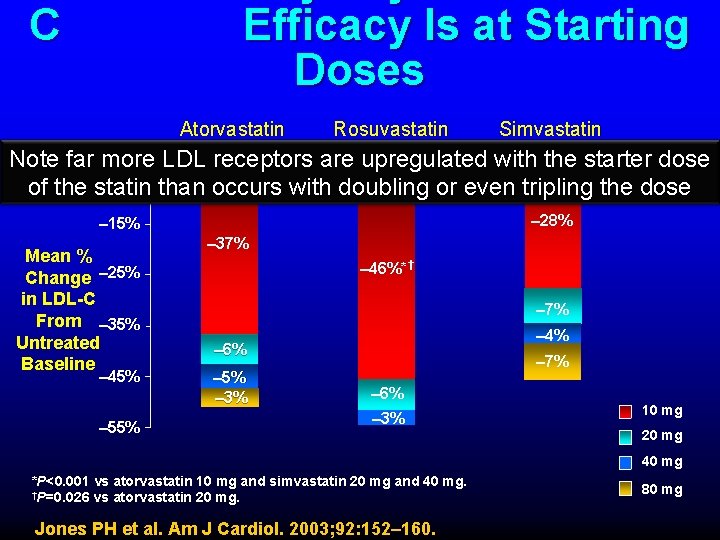
C Efficacy Is at Starting Doses Atorvastatin Rosuvastatin Simvastatin Note far more LDL receptors are upregulated with the starter dose – 5% of the statin than occurs with doubling or even tripling the dose – 28% – 15% Mean % Change – 25% in LDL-C From – 35% Untreated Baseline – 45% – 55% – 37% – 46%*† – 7% – 4% – 6% – 5% – 3% – 7% – 6% – 3% 10 mg 20 mg 40 mg *P<0. 001 vs atorvastatin 10 mg and simvastatin 20 mg and 40 mg. †P=0. 026 vs atorvastatin 20 mg. Jones PH et al. Am J Cardiol. 2003; 92: 152– 160. 80 mg

Candidates for Optional LDL-C Goal of <70 mg/d. L • Very high risk patients – Established atherosclerotic CVD Plus • multiple risk factors (esp. diabetes) • severe and poorly controlled risk factors (e. g. , cigarette smoking) • metabolic syndrome • acute coronary syndromes Grundy SM et al. Circulation 2004; 110: 227– 239.

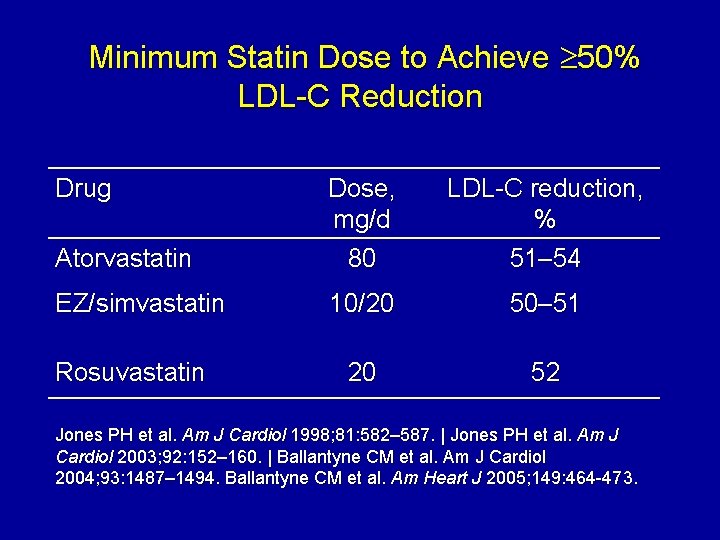
Minimum Statin Dose to Achieve 50% LDL-C Reduction Drug Atorvastatin Dose, mg/d 80 LDL-C reduction, % 51– 54 EZ/simvastatin 10/20 50– 51 20 52 Rosuvastatin Jones PH et al. Am J Cardiol 1998; 81: 582– 587. | Jones PH et al. Am J Cardiol 2003; 92: 152– 160. | Ballantyne CM et al. Am J Cardiol 2004; 93: 1487– 1494. Ballantyne CM et al. Am Heart J 2005; 149: 464 -473.

Combination Therapy to Reduce LDL-C • • • Statin + bile acid binding resin Statin + ezetimibe Statin + niacin Statin + BAR ± ezetimibe ± niacin Bile acid binding resin or ezetimibe + niacin Bile acid binding resin + ezetimibe

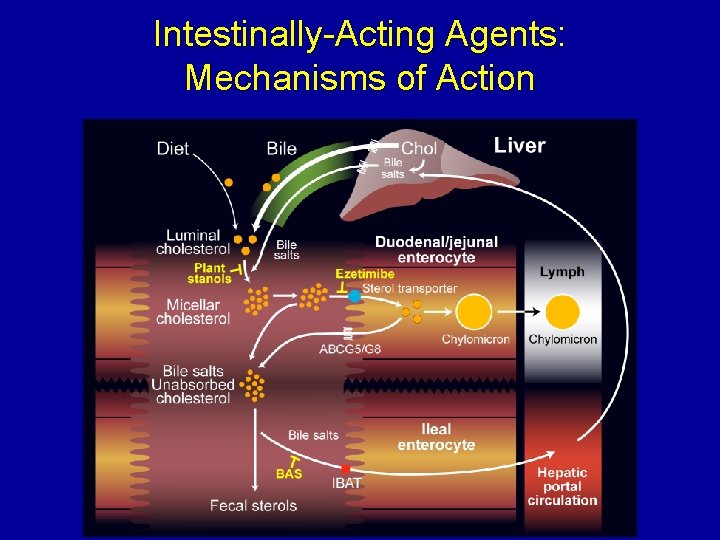
Intestinally-Acting Agents: Mechanisms of Action

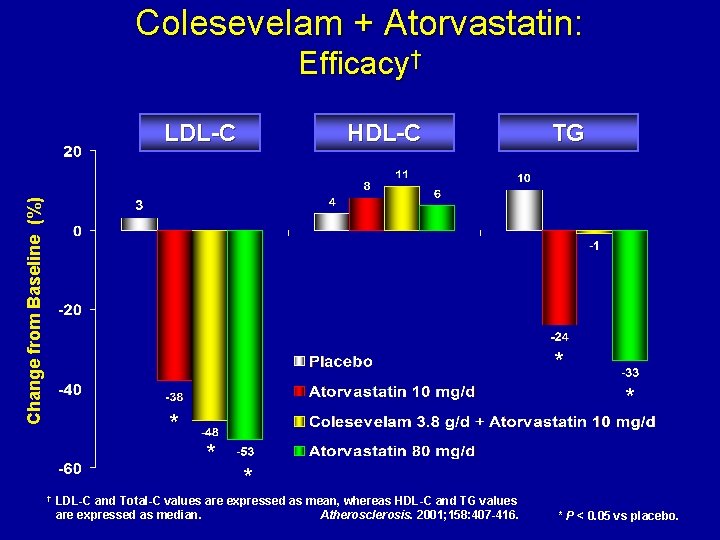
Colesevelam + Atorvastatin: Efficacy† Change from Baseline (%) LDL-C HDL-C TG * * † * LDL-C and Total-C values are expressed as mean, whereas HDL-C and TG values are expressed as median. Atherosclerosis. 2001; 158: 407 -416. * P < 0. 05 vs placebo.

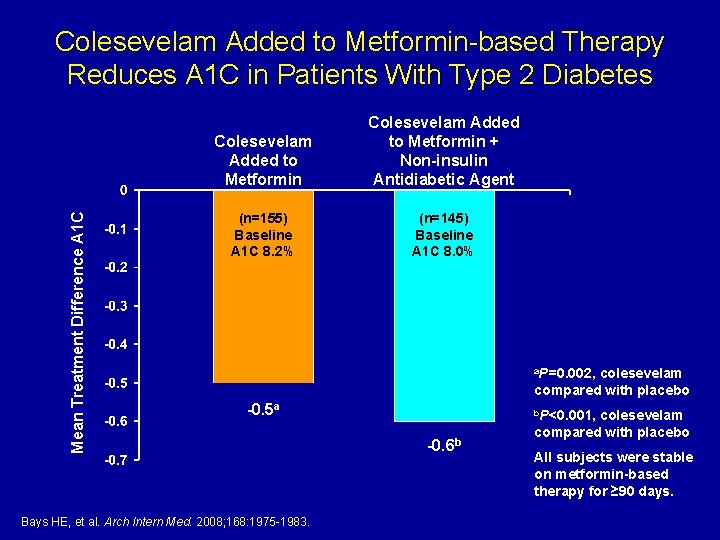
Mean Treatment Difference A 1 C Colesevelam Added to Metformin-based Therapy Reduces A 1 C in Patients With Type 2 Diabetes Colesevelam Added to Metformin + Non-insulin Antidiabetic Agent (n=155) Baseline A 1 C 8. 2% (n=145) Baseline A 1 C 8. 0% a. P=0. 002, colesevelam compared with placebo -0. 5 a Bays HE, et al. Arch Intern Med. 2008; 168: 1975 -1983. b. P<0. 001, -0. 6 b colesevelam compared with placebo All subjects were stable on metformin-based therapy for ≥ 90 days.

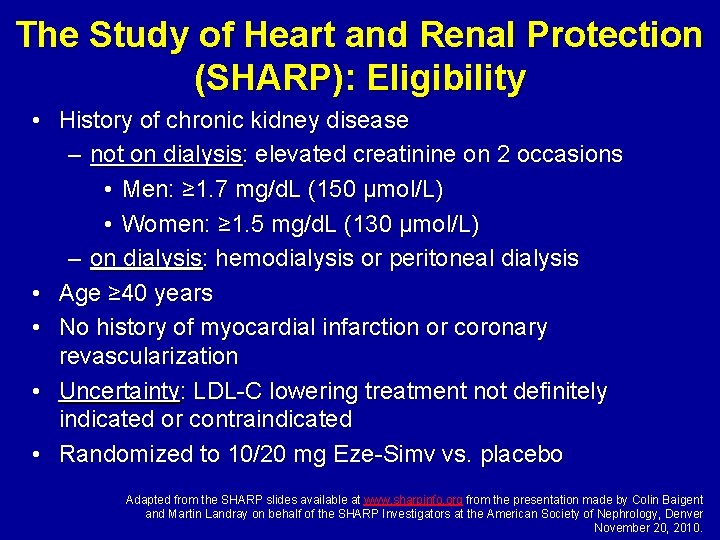
The Study of Heart and Renal Protection (SHARP): Eligibility • History of chronic kidney disease – not on dialysis: elevated creatinine on 2 occasions • Men: ≥ 1. 7 mg/d. L (150 µmol/L) • Women: ≥ 1. 5 mg/d. L (130 µmol/L) – on dialysis: hemodialysis or peritoneal dialysis • Age ≥ 40 years • No history of myocardial infarction or coronary revascularization • Uncertainty: LDL-C lowering treatment not definitely indicated or contraindicated • Randomized to 10/20 mg Eze-Simv vs. placebo Adapted from the SHARP slides available at www. sharpinfo. org from the presentation made by Colin Baigent and Martin Landray on behalf of the SHARP Investigators at the American Society of Nephrology, Denver November 20, 2010.

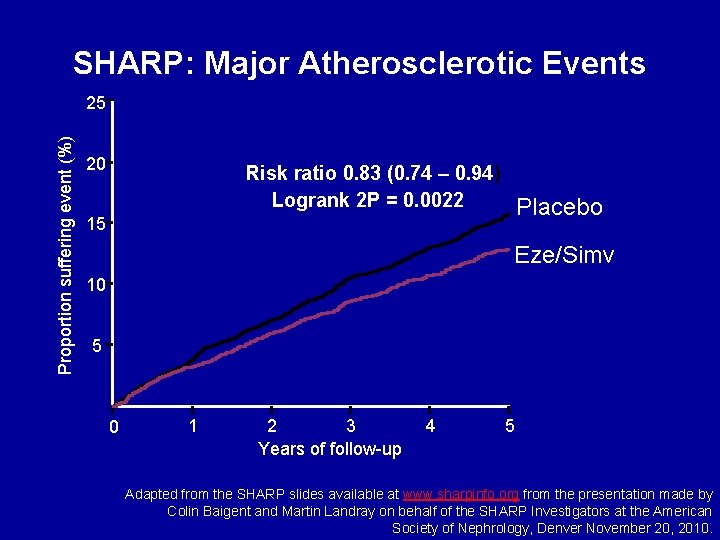
SHARP: Major Atherosclerotic Events Proportion suffering event (%) 25 20 Risk ratio 0. 83 (0. 74 – 0. 94) Logrank 2 P = 0. 0022 Placebo 15 Eze/Simv 10 5 0 1 2 3 Years of follow-up 4 5 Adapted from the SHARP slides available at www. sharpinfo. org from the presentation made by Colin Baigent and Martin Landray on behalf of the SHARP Investigators at the American Society of Nephrology, Denver November 20, 2010.

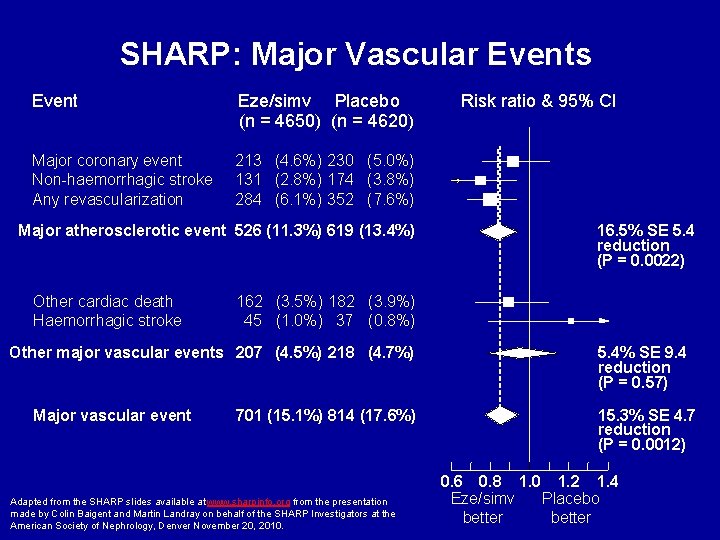
SHARP: Major Vascular Events Event Eze/simv Placebo (n = 4650) (n = 4620) Major coronary event Non-haemorrhagic stroke Any revascularization 213 (4. 6%) 230 (5. 0%) 131 (2. 8%) 174 (3. 8%) 284 (6. 1%) 352 (7. 6%) Major atherosclerotic event 526 (11. 3%) 619 (13. 4%) Other cardiac death Haemorrhagic stroke 16. 5% SE 5. 4 reduction (P = 0. 0022) 162 (3. 5%) 182 (3. 9%) 45 (1. 0%) 37 (0. 8%) Other major vascular events 207 (4. 5%) 218 (4. 7%) Major vascular event Risk ratio & 95% CI 701 (15. 1%) 814 (17. 6%) Adapted from the SHARP slides available at www. sharpinfo. org from the presentation made by Colin Baigent and Martin Landray on behalf of the SHARP Investigators at the American Society of Nephrology, Denver November 20, 2010. 5. 4% SE 9. 4 reduction (P = 0. 57) 15. 3% SE 4. 7 reduction (P = 0. 0012) 0. 6 0. 8 1. 0 1. 2 1. 4 Eze/simv Placebo better

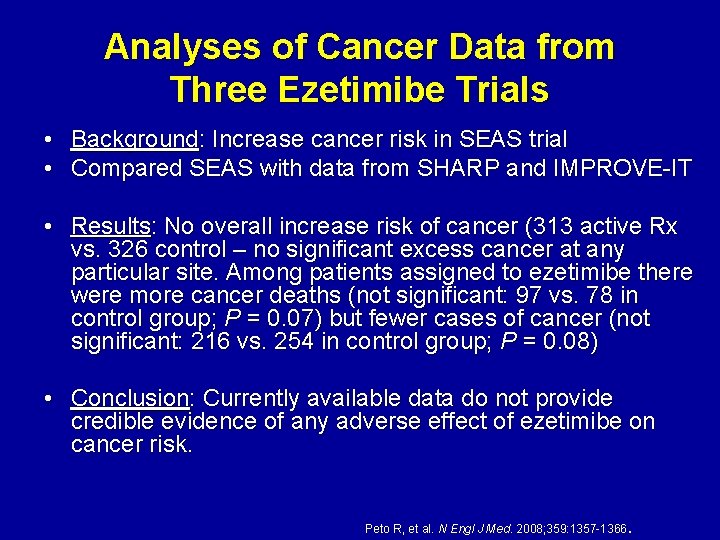
Analyses of Cancer Data from Three Ezetimibe Trials • • Background: Increase cancer risk in SEAS trial Compared SEAS with data from SHARP and IMPROVE-IT • Results: No overall increase risk of cancer (313 active Rx vs. 326 control – no significant excess cancer at any particular site. Among patients assigned to ezetimibe there were more cancer deaths (not significant: 97 vs. 78 in control group; P = 0. 07) but fewer cases of cancer (not significant: 216 vs. 254 in control group; P = 0. 08) • Conclusion: Currently available data do not provide credible evidence of any adverse effect of ezetimibe on cancer risk. . Peto R, et al. N Engl J Med. 2008; 359: 1357 -1366

Advanced Lipidology protocol for Statin Intolerance • Rule out and treat secondary causes (ex. hypothyroidism, Vit D deficiency) • Assess which statins trialed previously and dose • Consider adding Co Q 10 Ubiquinol 300600/day (no outcomes data to support this but in some patients it is effective) • Retrial with hydrophilic statin with less p 450 3 A 4 interactions (Pravastatin, Rosuvastatin, Pitavastatin) – Consider every other day Rosuvastatin 5 mg

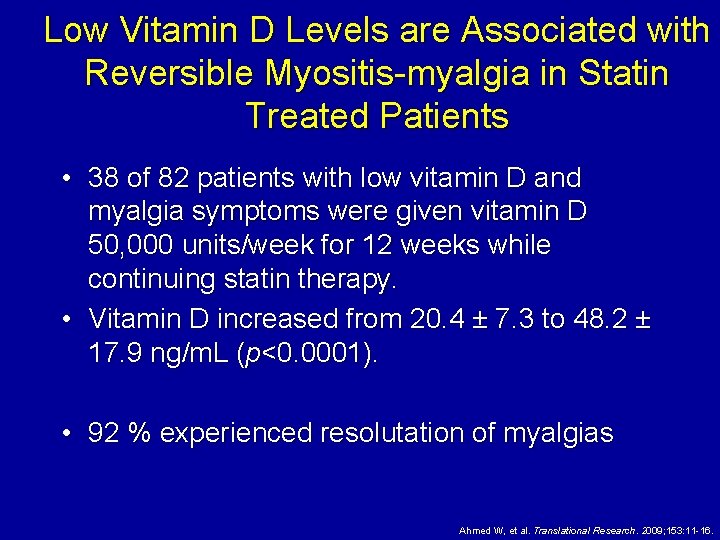
Low Vitamin D Levels are Associated with Reversible Myositis-myalgia in Statin Treated Patients • 38 of 82 patients with low vitamin D and myalgia symptoms were given vitamin D 50, 000 units/week for 12 weeks while continuing statin therapy. • Vitamin D increased from 20. 4 ± 7. 3 to 48. 2 ± 17. 9 ng/m. L (p<0. 0001). • 92 % experienced resolutation of myalgias Ahmed W, et al. Translational Research. 2009; 153: 11 -16.

Vitamin D deficiency has been associated with. . . • Increase in all CV events(F’ham Offspring, Wang, Circ 08) • Increase in MIs (Health Profs F/U Study, Giovannuci, AIM 08) • • Increase stroke risk (LURIC, Pitz, Stroke 08) Increased blood pressure (Foreman, HTN 07) Increased PVD by ABI (NHANES data, ATVB, 08) Increased CIMT (Targhan, Clin Endo 06)

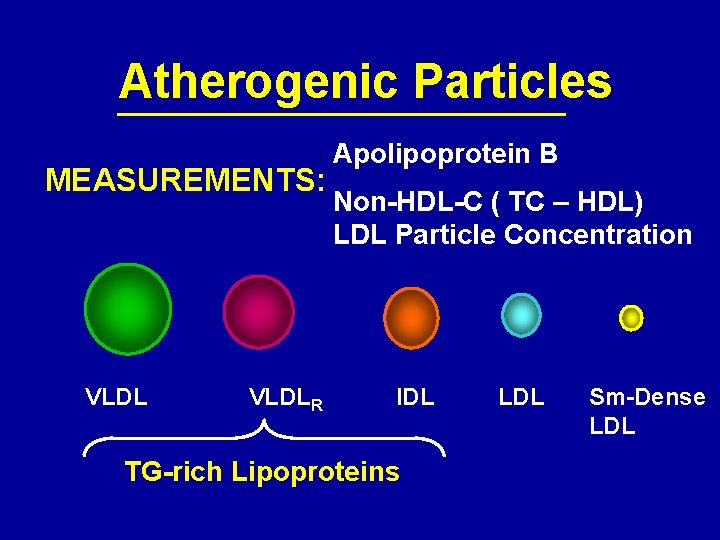
Atherogenic Particles MEASUREMENTS: VLDLR Apolipoprotein B Non-HDL-C ( TC – HDL) LDL Particle Concentration IDL TG-rich Lipoproteins LDL Sm-Dense LDL

Outcome Associations of LDL Particle Number (Apo. B) versus LDL Cholesterol (LDL-C) 1. Even in high TG patients, more than 90% of total plasma apo. B is associated with LDL particles, except in type III hyperlipidemia [1, 2] 2. LDL particle number (Apo. B) is a stronger indicator of CHD risk vs LDL-C in: • Prospective epidemiologic trials [3 -12] • In assessing residual risk on statin or fibrate therapy in intervention trials [1317]. 3. LDL particle number (Apo. B) is a stronger indicator of CHD risk vs non HDL-C in: • Prospective epidemiologic trials [8, 11, 18] • In assessing residual risk on statin or fibrate therapy in intervention trials [1315, ]. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. Atherosclerosis. 1991; 89: 109 -16. Clin Chem Acta. 1978; 82: 151 -60. Circulation. 1996; 94: 273 -8. Circulation. 1999; 99: 2517 -22. Lancet. 2001; 358: 2026 -33. Arterioscler Thromb Vasc Biol. 2002; 22: 1918 -23. AJC. 2005; 997 -1001. Diabetologica. 2006; 49: 937 -944. Arterioscler Thromb Vasc Biol. 2007; 27: 661 -670. JAMA. 2005; 294: 326 -33. J Lipid Res. 2007; 48: 2499 -2505. JAMA. 2007; 298: 776 -785. Circulation. 2000; 101: 477 -84. Circulation. 2002; 105: 1162 -9. Arterioscler Thromb Vasc Biol. 2000; 20: 2408 -2413. JACC. 1998; 32: 1648 -1656. Circulation. 2003; 107: 1733 -1737. Lancet. 2003; 361: 777 -780.

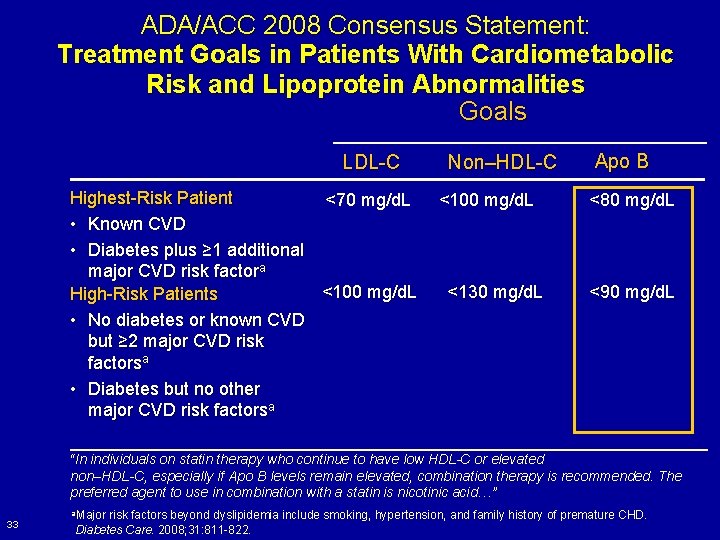
ADA/ACC 2008 Consensus Statement: Treatment Goals in Patients With Cardiometabolic Risk and Lipoprotein Abnormalities Goals LDL-C Highest-Risk Patient <70 mg/d. L • Known CVD • Diabetes plus ≥ 1 additional major CVD risk factora <100 mg/d. L High-Risk Patients • No diabetes or known CVD but ≥ 2 major CVD risk factorsa • Diabetes but no other major CVD risk factorsa Non–HDL-C <100 mg/d. L <130 mg/d. L Apo B <80 mg/d. L <90 mg/d. L “In individuals on statin therapy who continue to have low HDL-C or elevated non–HDL-C, especially if Apo B levels remain elevated, combination therapy is recommended. The preferred agent to use in combination with a statin is nicotinic acid…” 33 a. Major risk factors beyond dyslipidemia include smoking, hypertension, and family history of premature CHD. Diabetes Care. 2008; 31: 811 -822.

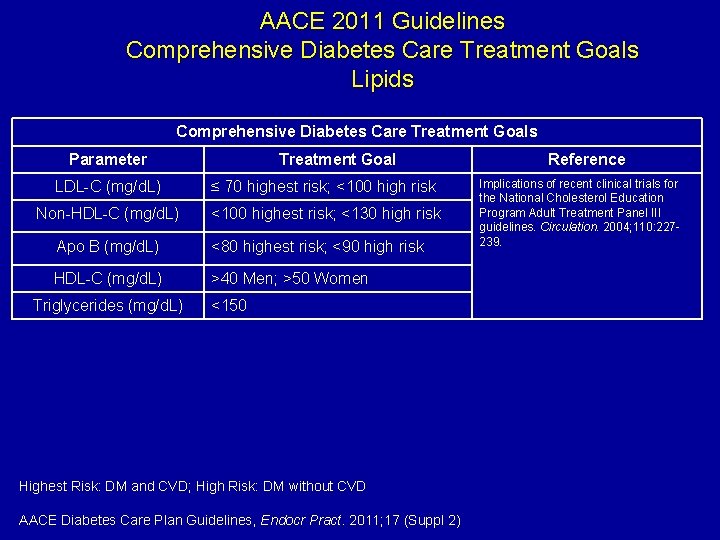
AACE 2011 Guidelines Comprehensive Diabetes Care Treatment Goals Lipids Comprehensive Diabetes Care Treatment Goals Parameter Treatment Goal LDL-C (mg/d. L) ≤ 70 highest risk; <100 high risk Non-HDL-C (mg/d. L) <100 highest risk; <130 high risk Apo B (mg/d. L) <80 highest risk; <90 high risk HDL-C (mg/d. L) >40 Men; >50 Women Triglycerides (mg/d. L) <150 Highest Risk: DM and CVD; High Risk: DM without CVD AACE Diabetes Care Plan Guidelines, Endocr Pract. 2011; 17 (Suppl 2) Reference Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004; 110: 227239.

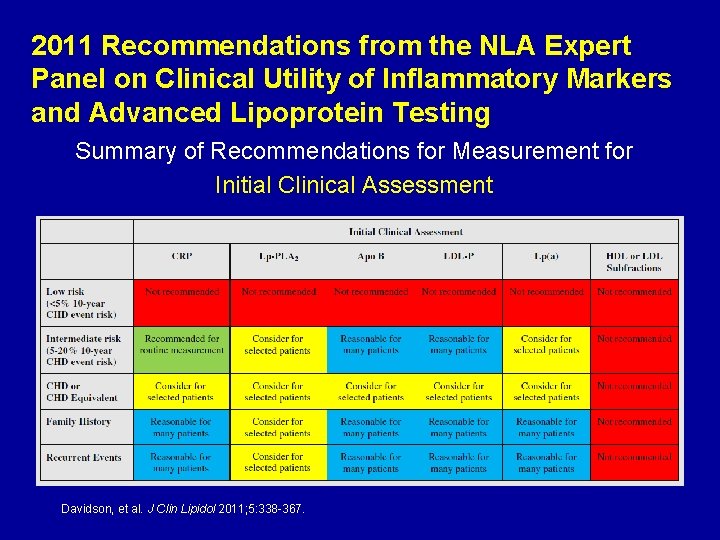
2011 Recommendations from the NLA Expert Panel on Clinical Utility of Inflammatory Markers and Advanced Lipoprotein Testing Summary of Recommendations for Measurement for Initial Clinical Assessment Davidson, et al. J Clin Lipidol 2011; 5: 338 -367.

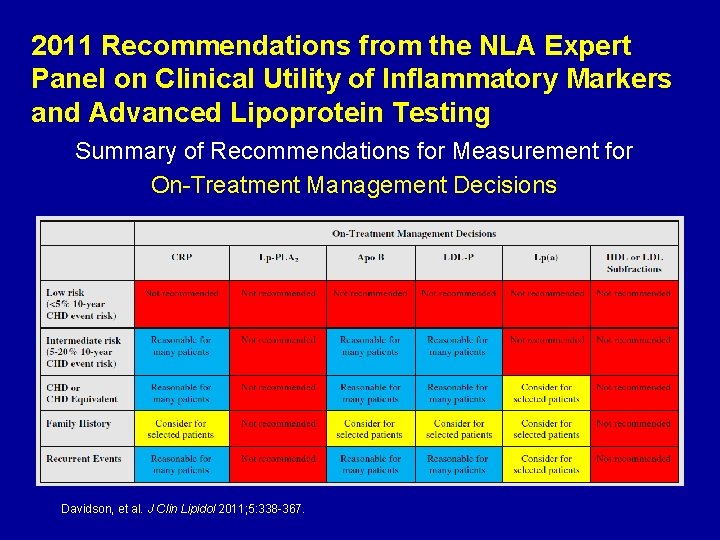
2011 Recommendations from the NLA Expert Panel on Clinical Utility of Inflammatory Markers and Advanced Lipoprotein Testing Summary of Recommendations for Measurement for On-Treatment Management Decisions Davidson, et al. J Clin Lipidol 2011; 5: 338 -367.

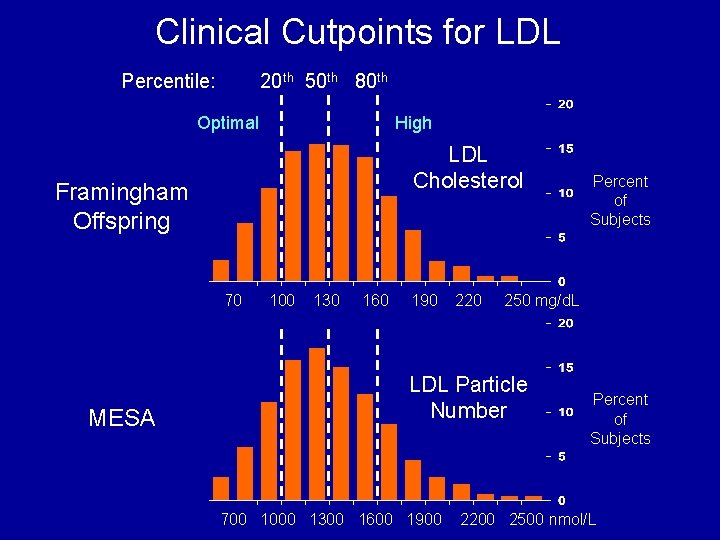
Clinical Cutpoints for LDL Percentile: 20 th 50 th 80 th Optimal High LDL Cholesterol Framingham Offspring 70 MESA 100 130 160 190 220 250 mg/d. L LDL Particle Number 700 1000 1300 1600 1900 Percent of Subjects 2200 2500 nmol/L

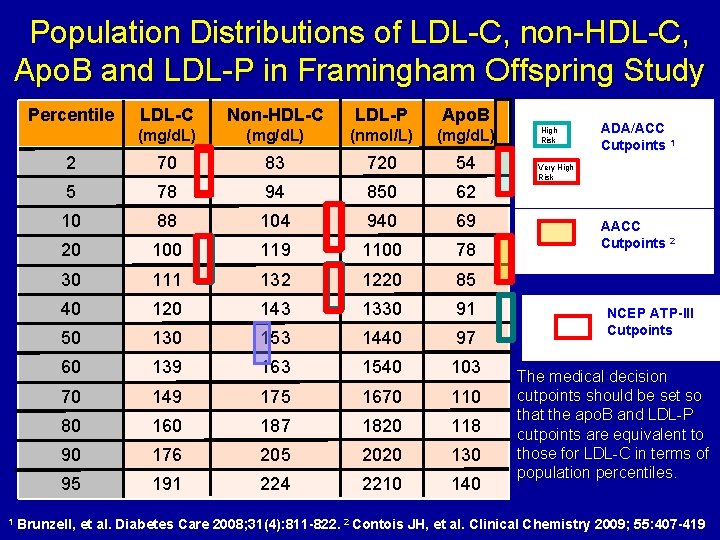
Population Distributions of LDL-C, non-HDL-C, Apo. B and LDL-P in Framingham Offspring Study Percentile 1 LDL-C Non-HDL-C LDL-P Apo. B (mg/d. L) (nmol/L) (mg/d. L) 2 70 83 720 54 5 78 94 850 62 10 88 104 940 69 20 100 119 1100 78 30 111 132 1220 85 40 120 143 1330 91 50 130 153 1440 97 60 139 163 1540 103 70 149 175 1670 110 80 160 187 1820 118 90 176 205 2020 130 95 191 224 2210 140 High Risk ADA/ACC Cutpoints 1 Very High Risk AACC Cutpoints 2 NCEP ATP-III Cutpoints The medical decision cutpoints should be set so that the apo. B and LDL-P cutpoints are equivalent to those for LDL-C in terms of population percentiles. Brunzell, et al. Diabetes Care 2008; 31(4): 811 -822. 2 Contois JH, et al. Clinical Chemistry 2009; 55: 407 -419

Beyond LDL HDL and Triglycerides

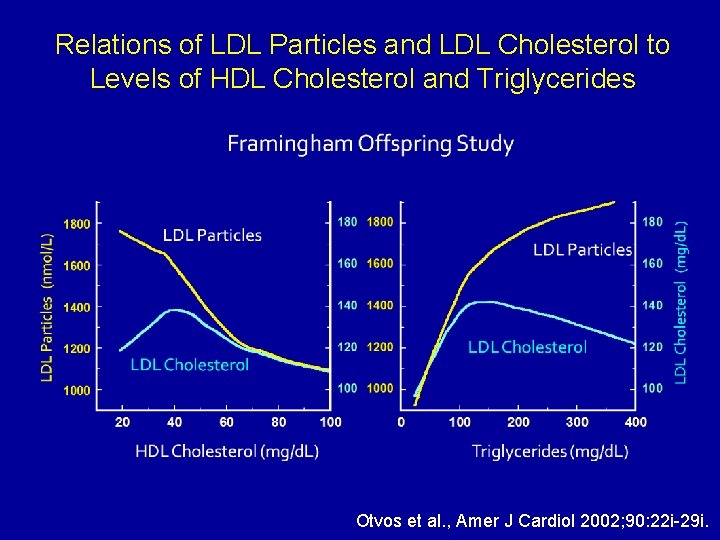
Relations of LDL Particles and LDL Cholesterol to Levels of HDL Cholesterol and Triglycerides Otvos et al. , Amer J Cardiol 2002; 90: 22 i-29 i.

Current HDL-C Cutpoints • NCEP ATP III: HDL Low if <40 mg/d. L • Metabolic syndrome criteria (ATP III, IDF) – Men <40 mg/d. L – Women <50 mg/d. L • AHA Women’s Cardiovascular Health Guidelines: 50 mg/d. L Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA 2001; 285: 2486 -2497. | Mosca L et al. Circulation 2011

AHA CVD Prevention Guidelines for Women 2011 Clinical Recommendations Major Risk Factor Interventions ► Lipids - Lipoproteins: (Class I, Level B) ► Optimal levels ► LDL-C < 100 mg/d. L ► HDL-C > 50 mg/d. L ► TG < 150 mg/d. L ► Non HDL-C < 130 mg/d. L • Pharmacotherapy for low HDL-C or high non–HDL-C* • Niacin or fibrate therapy (after LDL-C goal is reached) Mosca, L. et al. Circulation 2011; 123:

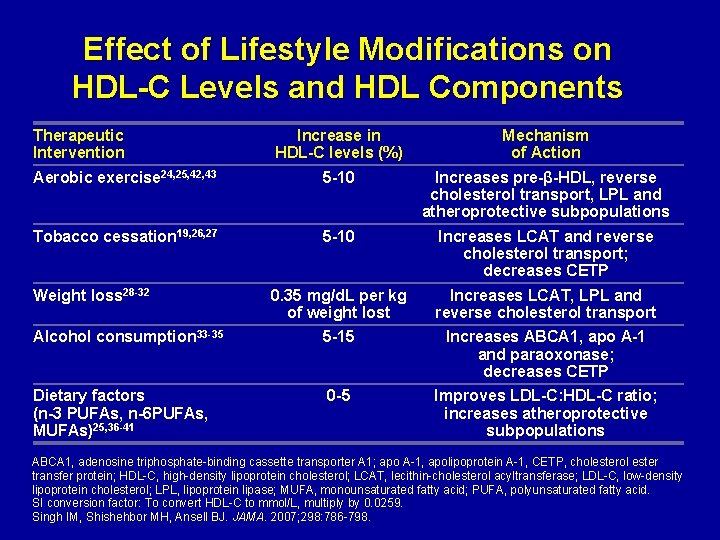
Effect of Lifestyle Modifications on HDL-C Levels and HDL Components Therapeutic Intervention Increase in HDL-C levels (%) Mechanism of Action Aerobic exercise 24, 25, 42, 43 5 -10 Increases pre-β-HDL, reverse cholesterol transport, LPL and atheroprotective subpopulations Tobacco cessation 19, 26, 27 5 -10 Increases LCAT and reverse cholesterol transport; decreases CETP 0. 35 mg/d. L per kg of weight lost Increases LCAT, LPL and reverse cholesterol transport Alcohol consumption 33 -35 5 -15 Increases ABCA 1, apo A-1 and paraoxonase; decreases CETP Dietary factors (n-3 PUFAs, n-6 PUFAs, MUFAs)25, 36 -41 0 -5 Improves LDL-C: HDL-C ratio; increases atheroprotective subpopulations Weight loss 28 -32 ABCA 1, adenosine triphosphate-binding cassette transporter A 1; apo A-1, apolipoprotein A-1, CETP, cholesterol ester transfer protein; HDL-C, high-density lipoprotein cholesterol; LCAT, lecithin-cholesterol acyltransferase; LDL-C, low-density lipoprotein cholesterol; LPL, lipoprotein lipase; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid. SI conversion factor: To convert HDL-C to mmol/L, multiply by 0. 0259. Singh IM, Shishehbor MH, Ansell BJ. JAMA. 2007; 298: 786 -798.

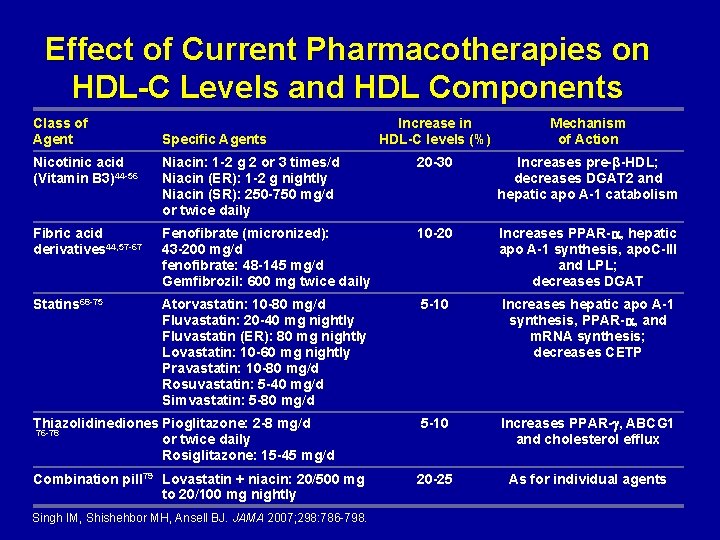
Effect of Current Pharmacotherapies on HDL-C Levels and HDL Components Class of Agent Specific Agents Increase in HDL-C levels (%) Mechanism of Action Nicotinic acid (Vitamin B 3)44 -56 Niacin: 1 -2 g 2 or 3 times/d Niacin (ER): 1 -2 g nightly Niacin (SR): 250 -750 mg/d or twice daily 20 -30 Increases pre-β-HDL; decreases DGAT 2 and hepatic apo A-1 catabolism Fibric acid derivatives 44, 57 -67 Fenofibrate (micronized): 43 -200 mg/d fenofibrate: 48 -145 mg/d Gemfibrozil: 600 mg twice daily 10 -20 Increases PPAR- , hepatic apo A-1 synthesis, apo. C-III and LPL; decreases DGAT Statins 68 -75 Atorvastatin: 10 -80 mg/d Fluvastatin: 20 -40 mg nightly Fluvastatin (ER): 80 mg nightly Lovastatin: 10 -60 mg nightly Pravastatin: 10 -80 mg/d Rosuvastatin: 5 -40 mg/d Simvastatin: 5 -80 mg/d 5 -10 Increases hepatic apo A-1 synthesis, PPAR- , and m. RNA synthesis; decreases CETP Thiazolidinediones Pioglitazone: 2 -8 mg/d 76 -78 or twice daily Rosiglitazone: 15 -45 mg/d 5 -10 Increases PPAR- , ABCG 1 and cholesterol efflux Combination pill 79 Lovastatin + niacin: 20/500 mg to 20/100 mg nightly 20 -25 As for individual agents Singh IM, Shishehbor MH, Ansell BJ. JAMA 2007; 298: 786 -798.

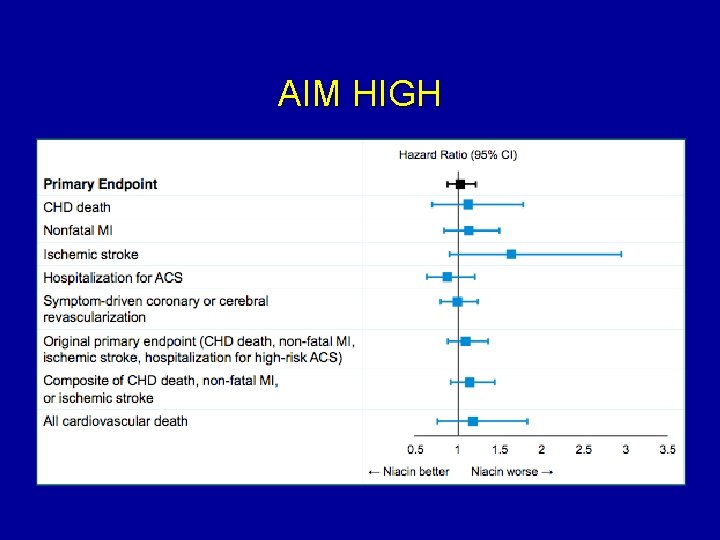
AIM HIGH

What to do about Niacin post AIMHIGH? • Having an “LDL-Particle(NMR)” /”Apo B” vs “cholesterol” lens changes our perspective on studies like AIM HIGH • Baseline NORMAL apo B in AIM HIGH (not a clinical scenario lipidologists would add niacin) • Niacin is a powerful agent for LDL –P/Apo B reduction, this effect known and may be more important clinically then HDL cholesterol increase with niacin • Bottom line: continue using niacin

Otvos comments regarding Niacin and AIM HIGH study • Niacin treatment has different effects on the cholesterol and particle measures of LDL and HDL. Niacin significantly raises HDL-C, but without increasing HDL particle number (HDL-P), and modestly reduces LDL-C but usually lowers LDL particle number (LDL-P) much more. Many clinicians invested in managing the particle abnormalities of their patients use niacin more for LDL-lowering than HDL-raising. The AIM-HIGH trial assessed the efficacy of increasing HDLC and correcting other non-LDL lipid abnormalities with niacin in CHD patients with optimally-treated LDL-C, but the trial design did not allow the potential benefit of LDL-P lowering to be assessed. The lack of clinical benefit of niacin treatment in AIM-HIGH was generally considered a surprise, but was not surprising given the known effects of niacin on lipoprotein particles and lipoprotein-mediated cardiovascular risk Otvos, J clin lipidology Vol 5: 5, 368 -370. Sept 2011

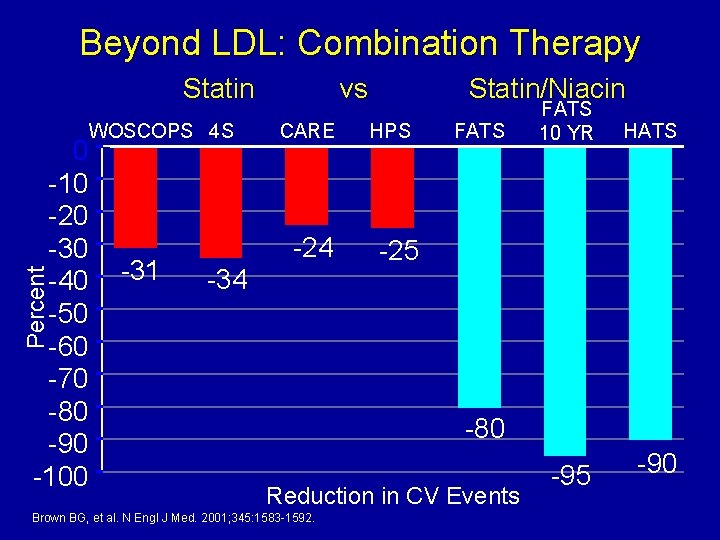
Beyond LDL: Combination Therapy Statin Percent 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 WOSCOPS 4 S -31 vs CARE -24 -34 Statin/Niacin HPS FATS 10 YR HATS -25 -80 Reduction in CV Events Brown BG, et al. N Engl J Med. 2001; 345: 1583 -1592. -95 -90

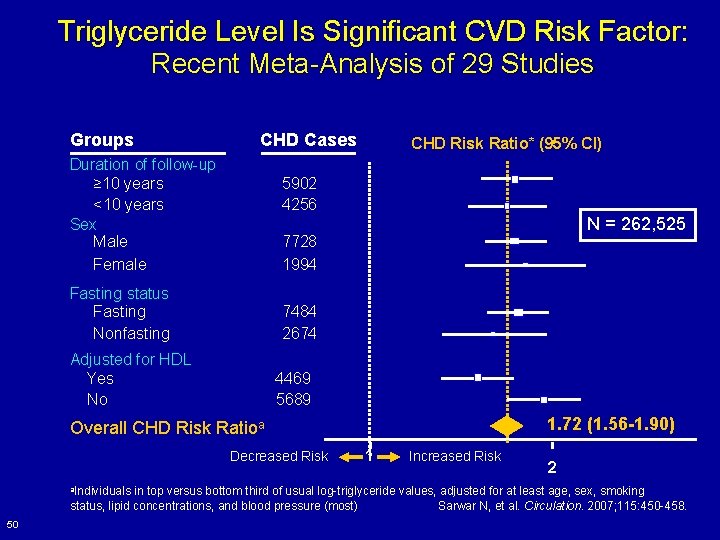
Triglyceride Level Is Significant CVD Risk Factor: Recent Meta-Analysis of 29 Studies Groups CHD Cases Duration of follow-up ≥ 10 years <10 years Sex Male Female CHD Risk Ratio* (95% CI) 5902 4256 N = 262, 525 7728 1994 Fasting status Fasting Nonfasting 7484 2674 Adjusted for HDL Yes No 4469 5689 1. 72 (1. 56 -1. 90) Overall CHD Risk Ratioa Decreased Risk a. Individuals 1 Increased Risk 2 in top versus bottom third of usual log-triglyceride values, adjusted for at least age, sex, smoking status, lipid concentrations, and blood pressure (most) Sarwar N, et al. Circulation. 2007; 115: 450 -458. 50

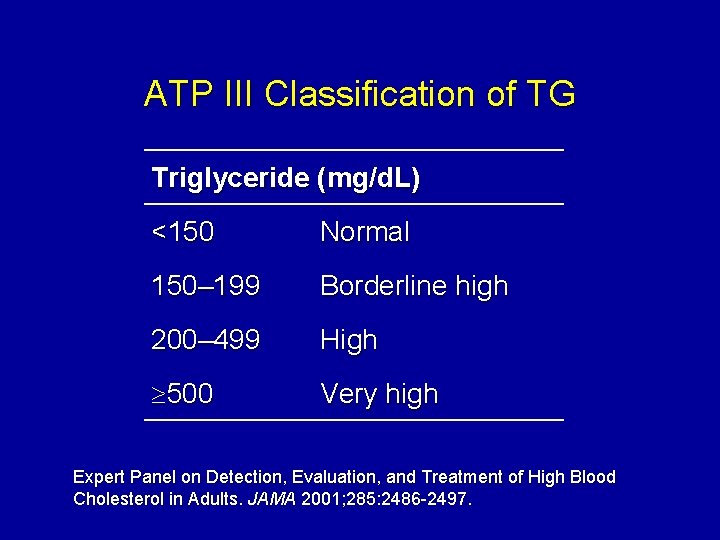
ATP III Classification of TG Triglyceride (mg/d. L) <150 Normal 150– 199 Borderline high 200– 499 High 500 Very high Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA 2001; 285: 2486 -2497.

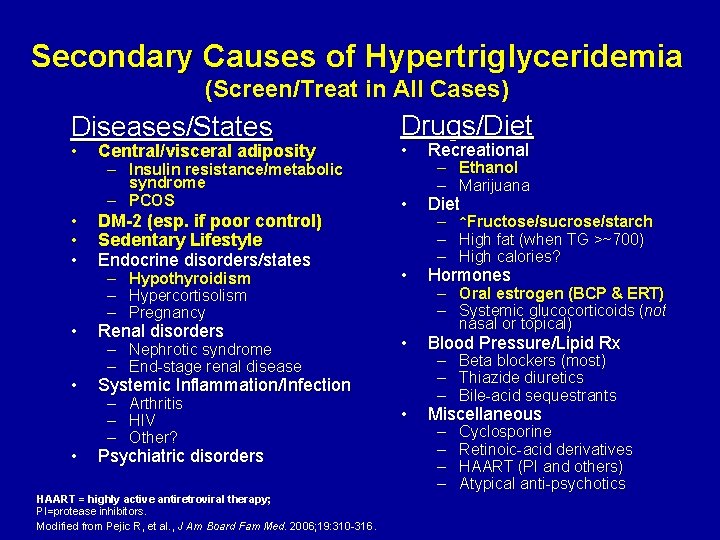
Secondary Causes of Hypertriglyceridemia (Screen/Treat in All Cases) Diseases/States • • Central/visceral adiposity – Insulin resistance/metabolic syndrome – PCOS DM-2 (esp. if poor control) Sedentary Lifestyle Endocrine disorders/states – – – Hypothyroidism Hypercortisolism Pregnancy Renal disorders – Nephrotic syndrome – End-stage renal disease Drugs/Diet • Recreational • Diet • Hormones • Blood Pressure/Lipid Rx • Miscellaneous Systemic Inflammation/Infection – – – Arthritis HIV Other? Psychiatric disorders HAART = highly active antiretroviral therapy; PI=protease inhibitors. Modified from Pejic R, et al. , J Am Board Fam Med. 2006; 19: 310 -316. – Ethanol – Marijuana – – – ↑Fructose/sucrose/starch High fat (when TG >~700) High calories? – Oral estrogen (BCP & ERT) – Systemic glucocorticoids (not nasal or topical) – – – Beta blockers (most) Thiazide diuretics Bile-acid sequestrants – – Cyclosporine Retinoic-acid derivatives HAART (PI and others) Atypical anti-psychotics

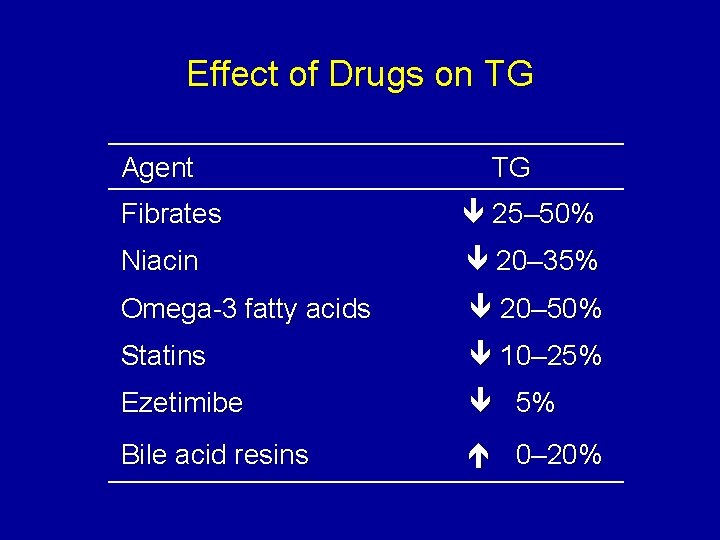
Effect of Drugs on TG Agent TG Fibrates 25– 50% Niacin 20– 35% Omega-3 fatty acids 20– 50% Statins 10– 25% Ezetimibe 5% Bile acid resins 0– 20%

Approach to the Patient with High TG History and Physical • Family history of elevated TG • History of Alcohol intake • History of other drugs (oral estrogen, betablockers, diuretics, isotretinoin, protease inhibitors) • Lifestyle: diet and exercise pattern, BMI, waist circumference • History of rashes (eruptive xanthomas), abdominal pain (pancreatitis)

Approach to the Patient with High TG THINK DIABETES Lab tests to evaluate secondary causes • Think Diabetes!: glucose, hemoglobin A 1 C • Renal disease: BUN/creatinine, urinalysis to check for proteinuria • Thyroid disease: TSH • Liver disease: ALT, AST (frequently have hepatic steatosis)

General Approach to the Patient with Hypertriglyceridemia (Type IV, V) • • Intensive diabetes control Lifestyle modification – low-fat diet initially if >1000 then key is to reduce carbohydrates LOW CARB DIET, stop ETOH – exercise (30 min walking) daily – weight loss • Set goals with patient and explain relationship between TG, abdominal obesity, and glucose • If TG > 500 mg/d. L, begin drug and lifestyle therapy concomitantly

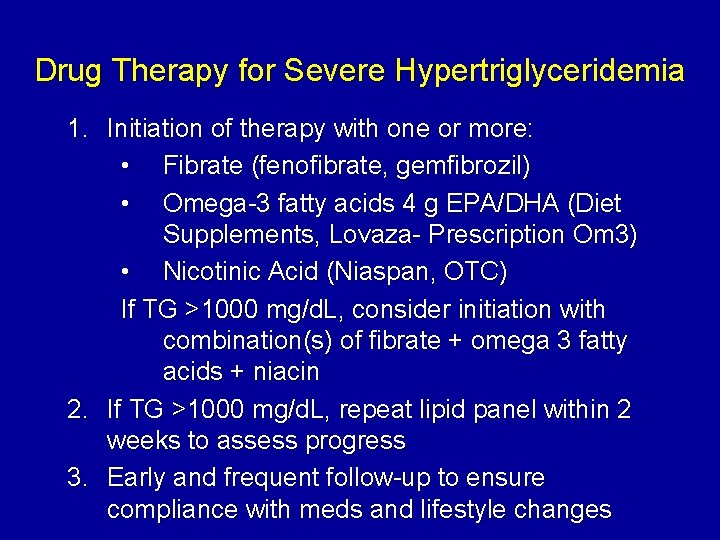
Drug Therapy for Severe Hypertriglyceridemia 1. Initiation of therapy with one or more: • Fibrate (fenofibrate, gemfibrozil) • Omega-3 fatty acids 4 g EPA/DHA (Diet Supplements, Lovaza- Prescription Om 3) • Nicotinic Acid (Niaspan, OTC) If TG >1000 mg/d. L, consider initiation with combination(s) of fibrate + omega 3 fatty acids + niacin 2. If TG >1000 mg/d. L, repeat lipid panel within 2 weeks to assess progress 3. Early and frequent follow-up to ensure compliance with meds and lifestyle changes

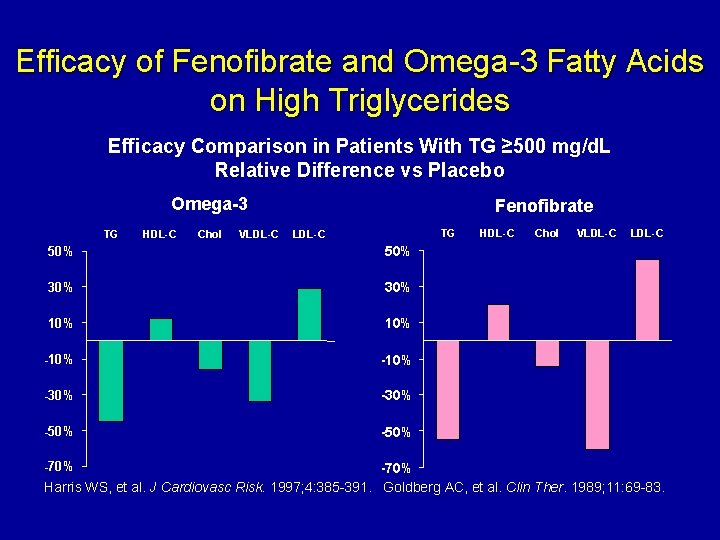
Efficacy of Fenofibrate and Omega-3 Fatty Acids on High Triglycerides Efficacy Comparison in Patients With TG ≥ 500 mg/d. L Relative Difference vs Placebo Omega-3 TG HDL-C Chol VLDL-C Fenofibrate TG LDL-C 50% 30% 10% -10% -30% -50% -70% HDL-C Chol VLDL-C -70% Harris WS, et al. J Cardiovasc Risk. 1997; 4: 385 -391. Goldberg AC, et al. Clin Ther. 1989; 11: 69 -83.

Treatment of Severe HTG: Unanswered Questions 1. No randomized trials to show that treatment of severe HTG reduces pancreatitis 2. No trials that have enrolled patients based on high TGs to examine whether lipid-modifying therapy reduces Cardiovascular Disease

Approach to the Patient with Mixed Dyslipidemia (High Cholesterol + High TG 150– 500 mg/d. L) 1. 2. Lifestyle modifications (low carb, exercise) Statins are preferred initial choice if both LDL-C and non-HDL-C meet criteria for drug therapy 3. Obtain follow-up labs after 6– 8 weeks and reassess need for combination therapy

Additional Agents in Combination with a Statin for Mixed Hyperlipidemia • Statin + niacin ER (1000 mg-1500) • Statin + fibrate (fenofibrate preferred due to dru interactions with Gemfibrozil or Gemfibrozil with Fluvastatin XL which has no interaction) • Statin + omega-3 fatty acids (4 gm EPA/DHA) • Statin + fibrate + niacin ± omega 3 • Statin + ezetimibe + fibrate, niacin and/or omega 3

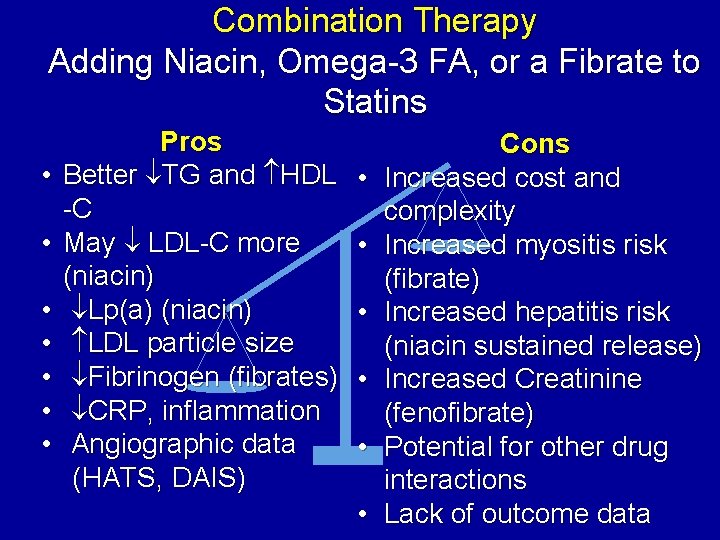
Combination Therapy Adding Niacin, Omega-3 FA, or a Fibrate to Statins • • Pros Better TG and HDL -C May LDL-C more (niacin) Lp(a) (niacin) LDL particle size Fibrinogen (fibrates) CRP, inflammation Angiographic data (HATS, DAIS) • • • Cons Increased cost and complexity Increased myositis risk (fibrate) Increased hepatitis risk (niacin sustained release) Increased Creatinine (fenofibrate) Potential for other drug interactions Lack of outcome data

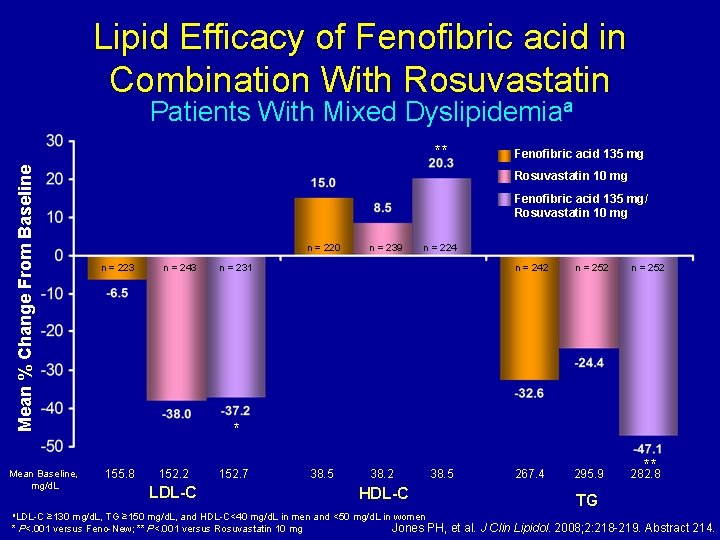
Lipid Efficacy of Fenofibric acid in Combination With Rosuvastatin Patients With Mixed Dyslipidemiaa Mean % Change From Baseline ** Mean Baseline, mg/d. L a. LDL-C Fenofibric acid 135 mg Rosuvastatin 10 mg Fenofibric acid 135 mg/ Rosuvastatin 10 mg n = 220 n = 223 n = 243 n = 239 n = 224 n = 231 n = 242 n = 252 267. 4 295. 9 282. 8 * 155. 8 152. 2 LDL-C 152. 7 38. 5 38. 2 38. 5 HDL-C ≥ 130 mg/d. L, TG ≥ 150 mg/d. L, and HDL-C<40 mg/d. L in men and <50 mg/d. L in women Jones PH, * P<. 001 versus Feno-New; ** P<. 001 versus Rosuvastatin 10 mg ** TG et al. J Clin Lipidol. 2008; 2: 218 -219. Abstract 214.

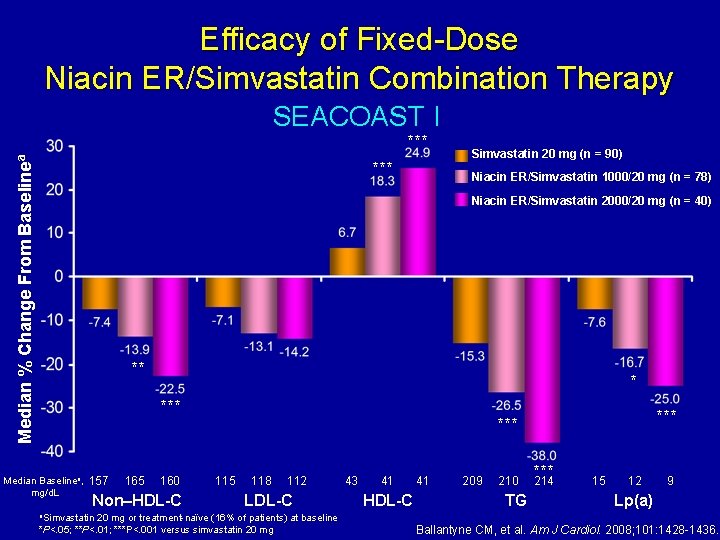
Efficacy of Fixed-Dose Niacin ER/Simvastatin Combination Therapy SEACOAST I Median % Change From Baselinea *** Simvastatin 20 mg (n = 90) Niacin ER/Simvastatin 1000/20 mg (n = 78) Niacin ER/Simvastatin 2000/20 mg (n = 40) ** * *** Median Baselinea, mg/d. L 157 165 160 Non–HDL-C a. Simvastatin *** 115 118 112 LDL-C 20 mg or treatment-naïve (16% of patients) at baseline *P<. 05; **P<. 01; ***P<. 001 versus simvastatin 20 mg 43 41 HDL-C 41 209 210 TG *** 214 15 12 9 Lp(a) Ballantyne CM, et al. Am J Cardiol. 2008; 101: 1428 -1436.

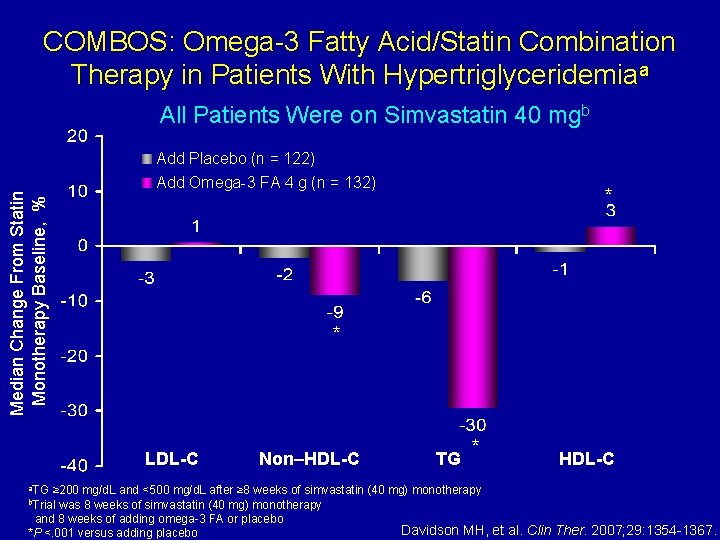
COMBOS: Omega-3 Fatty Acid/Statin Combination Therapy in Patients With Hypertriglyceridemiaa Median Change From Statin Monotherapy Baseline, % All Patients Were on Simvastatin 40 mgb Add Placebo (n = 122) Add Omega-3 FA 4 g (n = 132) * LDL-C a. TG * Non–HDL-C TG * HDL-C ≥ 200 mg/d. L and <500 mg/d. L after ≥ 8 weeks of simvastatin (40 mg) monotherapy was 8 weeks of simvastatin (40 mg) monotherapy and 8 weeks of adding omega-3 FA or placebo Davidson MH, et al. Clin Ther. 2007; 29: 1354 -1367. *P <. 001 versus adding placebo b. Trial

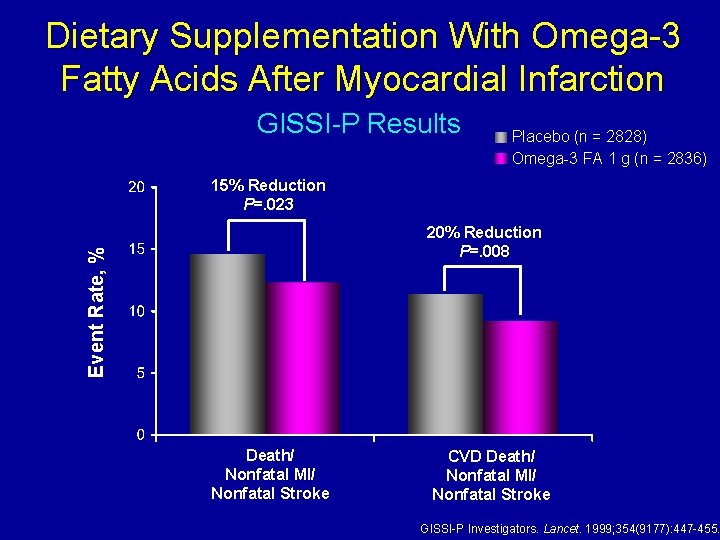
Dietary Supplementation With Omega-3 Fatty Acids After Myocardial Infarction GISSI-P Results Placebo (n = 2828) Omega-3 FA 1 g (n = 2836) 15% Reduction P=. 023 Event Rate, % 20% Reduction P=. 008 Death/ Nonfatal MI/ Nonfatal Stroke CVD Death/ Nonfatal MI/ Nonfatal Stroke GISSI-P Investigators. Lancet. 1999; 354(9177): 447 -455.

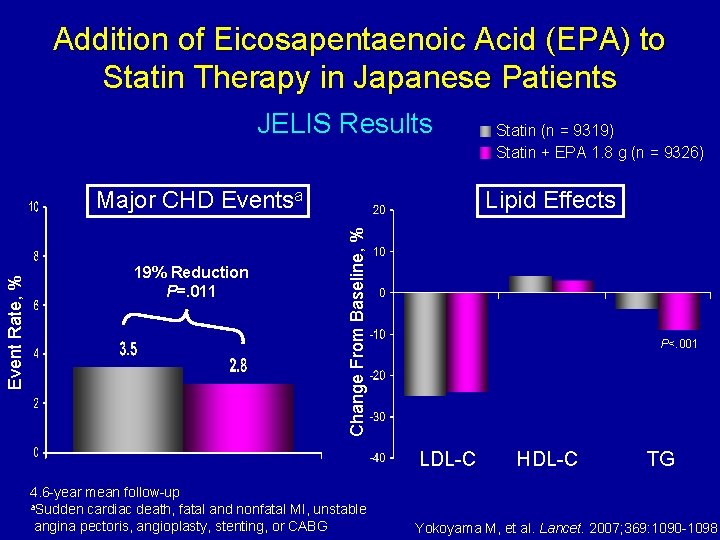
Addition of Eicosapentaenoic Acid (EPA) to Statin Therapy in Japanese Patients JELIS Results 19% Reduction P=. 011 Lipid Effects Change From Baseline, % Event Rate, % Major CHD Eventsa Statin (n = 9319) Statin + EPA 1. 8 g (n = 9326) P<. 001 LDL-C 4. 6 -year mean follow-up a. Sudden cardiac death, fatal and nonfatal MI, unstable angina pectoris, angioplasty, stenting, or CABG HDL-C TG Yokoyama M, et al. Lancet. 2007; 369: 1090 -1098.

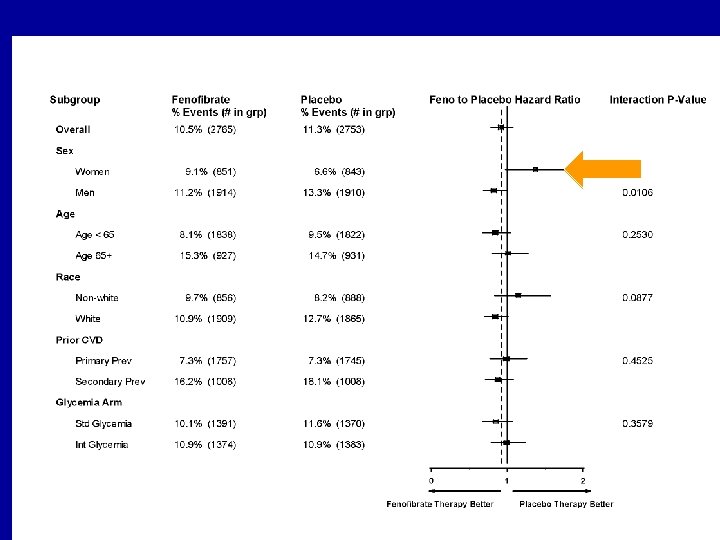
Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study • Question: Would statin plus a fibrate as compared with statin monotherapy reduce CVD in diabetics at high risk for CVD? • 5518 patients with type 2 DM treated with open label simvastatin were treated with either masked fenofibrate or placebo • Primary outcome measure: nonfatal MI, nonfatal stroke, or death from CV causes • Follow-up: 4. 7 years ACCORD Study Group. N Engl J Med. 2010; 362: 1563 -1574.

Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study • Results: Annual primary outcome was 2. 2% in fenofibrate group and 2. 4% in placebo group (NS) • Annual rate of death was 1. 5% in fenofibrate group and 1. 6% in placebo group (NS) • Prespecified subgroup analysis suggested heterogeneity in treatment according to sex, with a benefit for men and possible harm for women (P = 0. 01 for interaction) • Possible benefit seen in fenofibrate group if baseline triglycerides high (>204 mg/d. L) and HDL-C low (<34 mg/d. L): primary outcome rate 12. 4% in fenofibrate group vs. 17. 3% in placebo (P = 0. 057 for interaction) ACCORD Study Group. N Engl J Med. 2010; 362: 1563 -1574.

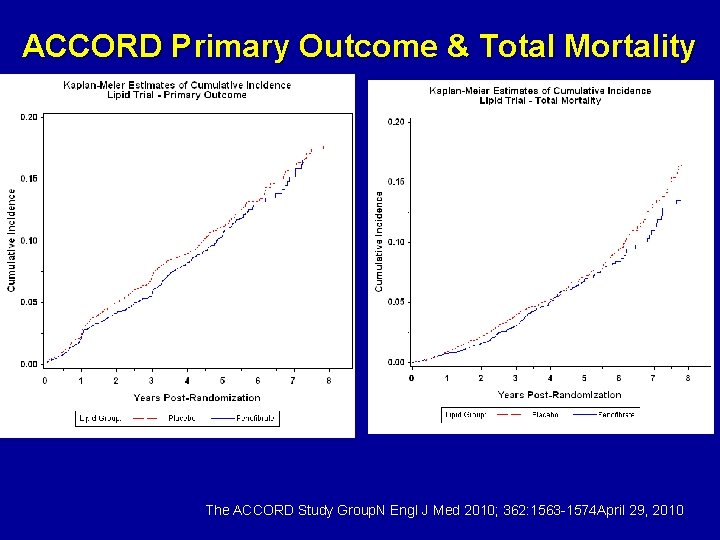
ACCORD Primary Outcome & Total Mortality The ACCORD Study Group. N Engl J Med 2010; 362: 1563 -1574 April 29, 2010

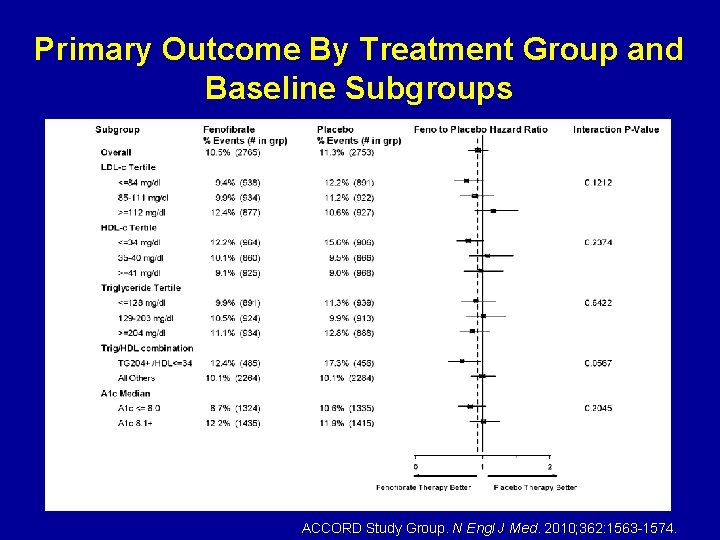
Primary Outcome By Treatment Group and Baseline Subgroups ACCORD Study Group. N Engl J Med. 2010; 362: 1563 -1574.

Primary Outcome By Treatment Group and Baseline Subgroups

ACCORD results The combination of fenofibrate and simvastatin did not reduce the rate of fatal cardiovascular events, nonfatal myocardial infarction, or nonfatal stroke, as compared with simvastatin alone. These results do not support the routine use of combination therapy with fenofibrate and simvastatin to reduce cardiovascular risk in the majority of high-risk patients with type 2 diabetes

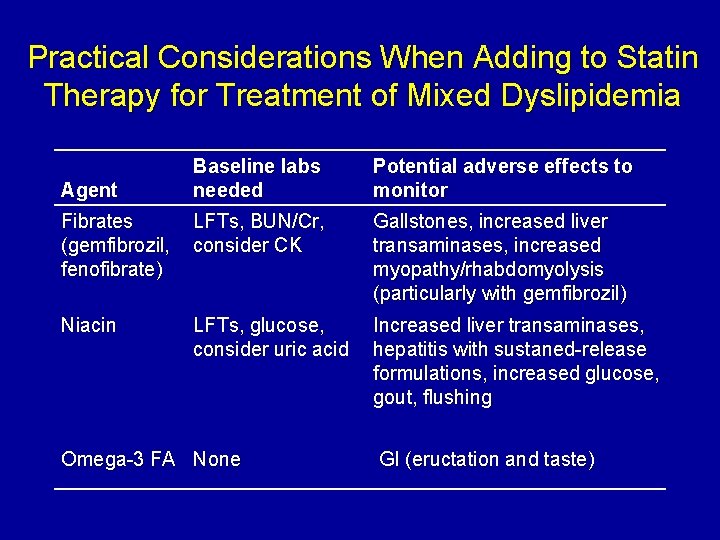
Practical Considerations When Adding to Statin Therapy for Treatment of Mixed Dyslipidemia Baseline labs needed Potential adverse effects to monitor Fibrates (gemfibrozil, fenofibrate) LFTs, BUN/Cr, consider CK Gallstones, increased liver transaminases, increased myopathy/rhabdomyolysis (particularly with gemfibrozil) Niacin LFTs, glucose, consider uric acid Increased liver transaminases, hepatitis with sustaned-release formulations, increased glucose, gout, flushing Agent Omega-3 FA None GI (eructation and taste)

Major Trials In Progress • AIM-HIGH: Additional Data pending including Lipoprotein and Lp(a) Sub-Analyses • IMPROVE-IT: Simvastatin 40 mg vs ezetimibe 10 mg/simvastatin 40 mg in patients enrolled following acute coronary syndrome. Endpoint of major CV events. • HPS 2 -Thrive: Simvastatin ER niacin/laropiprant CVD Endpoint, 20, 000 patients 2 o prevention 7000 with DM • HOPE-3: 2 x 2 factorial trial, rosuvastatin 10 mg vs placebo, candesartan/HCTZ vs placebo. Enrolling people at average risk, without CHD. Endpoint of major CV events.

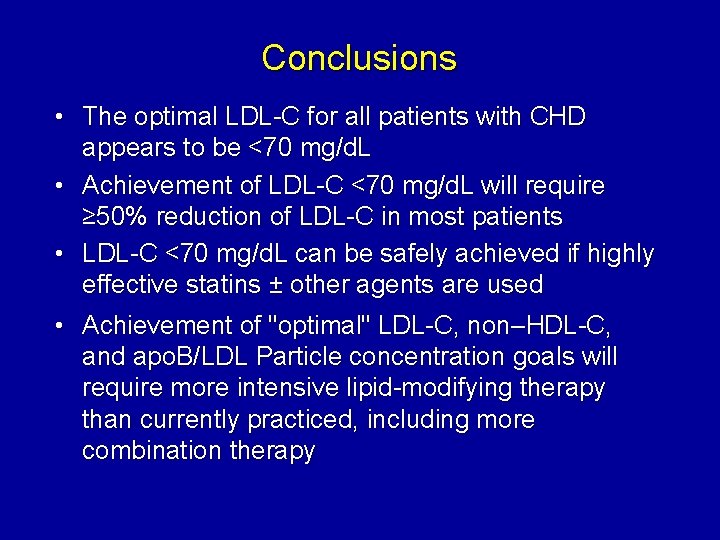
Conclusions • The optimal LDL-C for all patients with CHD appears to be <70 mg/d. L • Achievement of LDL-C <70 mg/d. L will require ≥ 50% reduction of LDL-C in most patients • LDL-C <70 mg/d. L can be safely achieved if highly effective statins ± other agents are used • Achievement of "optimal" LDL-C, non–HDL-C, and apo. B/LDL Particle concentration goals will require more intensive lipid-modifying therapy than currently practiced, including more combination therapy

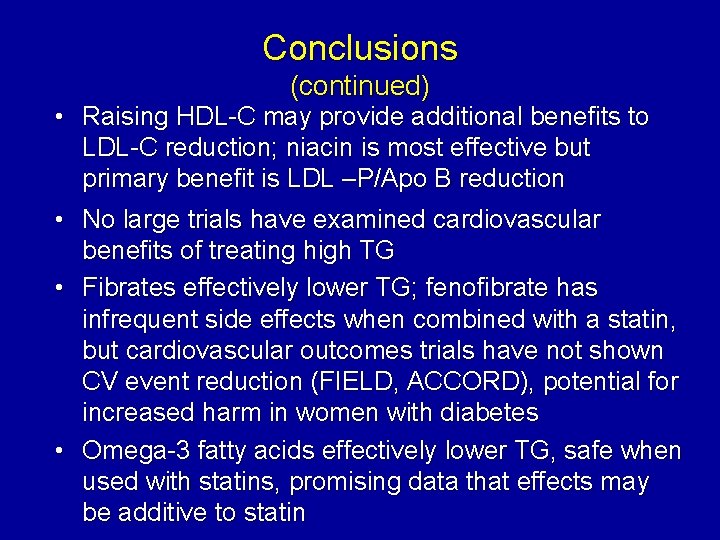
Conclusions (continued) • Raising HDL-C may provide additional benefits to LDL-C reduction; niacin is most effective but primary benefit is LDL –P/Apo B reduction • No large trials have examined cardiovascular benefits of treating high TG • Fibrates effectively lower TG; fenofibrate has infrequent side effects when combined with a statin, but cardiovascular outcomes trials have not shown CV event reduction (FIELD, ACCORD), potential for increased harm in women with diabetes • Omega-3 fatty acids effectively lower TG, safe when used with statins, promising data that effects may be additive to statin

Complex Lipid Referral from Cardiology December 2010 • 47 yr old Male with obesity, dyslipidemia, HTN, Sleep apnea • Statins stopped due to elevated LFTs • Lipid Panel – – – • Medications – – – T Chol 301 mg/dl HDL 29 mg/dl Triglycerides 1457 • BP 160/90 – – – • Fenofibrate 145 mg Colesevalam 625 mg 6/d Ezetamibe 10 mg Nicotinic Acid 500 mg Amlodipine/Valsartan/HC TZ 10/320/25 Aliskiren 300 Nebivolol 20 mg Clonidine 0. 1 mg Statins stopped due to elevated LFTs

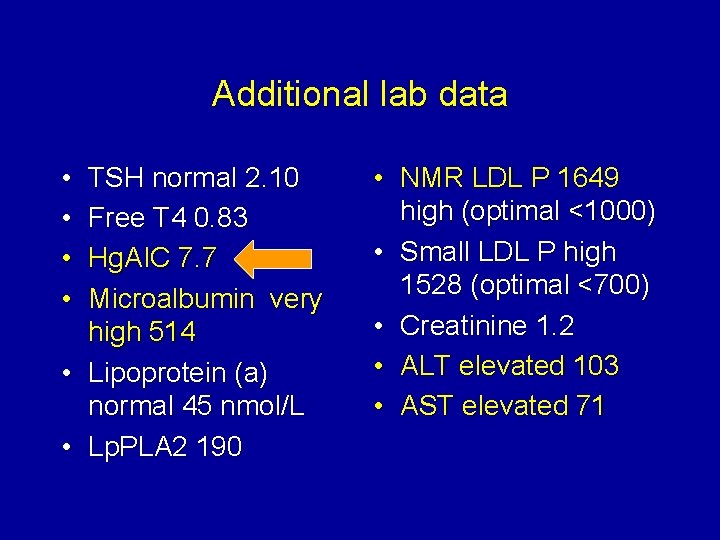
Additional lab data • • TSH normal 2. 10 Free T 4 0. 83 Hg. AIC 7. 7 Microalbumin very high 514 • Lipoprotein (a) normal 45 nmol/L • Lp. PLA 2 190 • NMR LDL P 1649 high (optimal <1000) • Small LDL P high 1528 (optimal <700) • Creatinine 1. 2 • ALT elevated 103 • AST elevated 71

Case high risk with severe hypertriglyceridemia • • • 1) Stop Colesevalam (increases trigs) 2) Stop Fenofibrate (not effective) 3) Stop Ezetamibe 4) Start Rosuvastatin 40 mg 5) Start prescription omega 3(Lovaza) 4 g/day for triglyceride lowering • 6) Start Metformin ER titrate to 1500/d

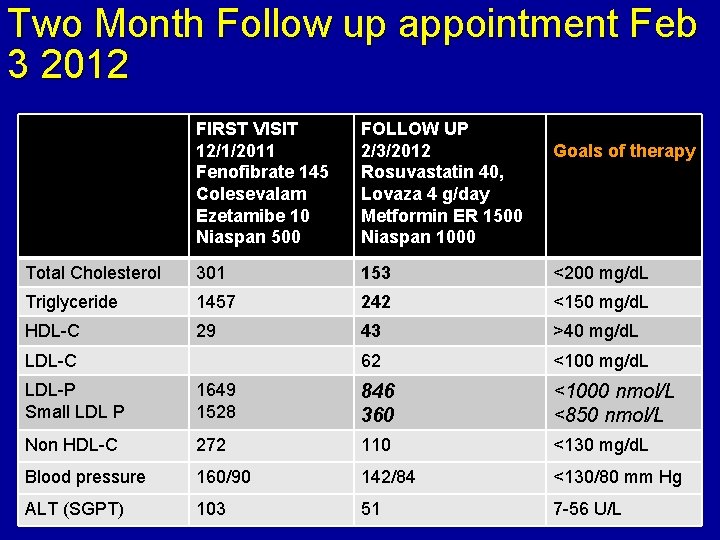
Two Month Follow up appointment Feb 3 2012 FIRST VISIT 12/1/2011 Fenofibrate 145 Colesevalam Ezetamibe 10 Niaspan 500 FOLLOW UP 2/3/2012 Rosuvastatin 40, Lovaza 4 g/day Metformin ER 1500 Niaspan 1000 Total Cholesterol 301 153 <200 mg/d. L Triglyceride 1457 242 <150 mg/d. L HDL-C 29 43 >40 mg/d. L 62 <100 mg/d. L LDL-C Goals of therapy LDL-P Small LDL P 1649 1528 846 360 <1000 nmol/L <850 nmol/L Non HDL-C 272 110 <130 mg/d. L Blood pressure 160/90 142/84 <130/80 mm Hg ALT (SGPT) 103 51 7 -56 U/L

Questions? taradall@lecturepad. org www. advlip. com (Advanced Lipidology clinic website) www. lecturepad. org (free website for ongoing education)