Clinical Care of Renal Transplant Recipients An Internists
























- Slides: 64

Clinical Care of Renal Transplant Recipients: An Internist’s Guide Matthew R. Weir, M. D. Professor and Director Division of Nephrology University of Maryland School of Medicine

Overview • Short-term risks • Long-term risks – Erosion of graft function – Cardiovascular disease – malignancy • Drug – Drug interactions • Future opportunities

Overview • Short-term risks • Long-term risks – Erosion of graft function – Cardiovascular disease – malignancy • Drug – Drug interactions • Future opportunities

What are the short-term risks? • Rejection • Donor factors leading to poorer outcome • Recipient death – Operative complications – Infection – Malignancy – Cardiovascular disease • Complications of immunosuppressive therapy

Short-Term Risks: Infection • First 6 weeks: standard post-surgical issues including UTI, line infection, thrombophlebitis, pneumonia/atelectasis, wound infection, thrush • After 6 weeks: opportunistic infection including CMV, EBV, PCP, listeria, aspergillus, etc. • Chemoprophylaxis: clotrimazole, TMS, valganciclovir

Overview • Short-term risks • Long-term risks – Erosion of graft function – Cardiovascular disease – malignancy • Drug – Drug interactions • Future opportunities

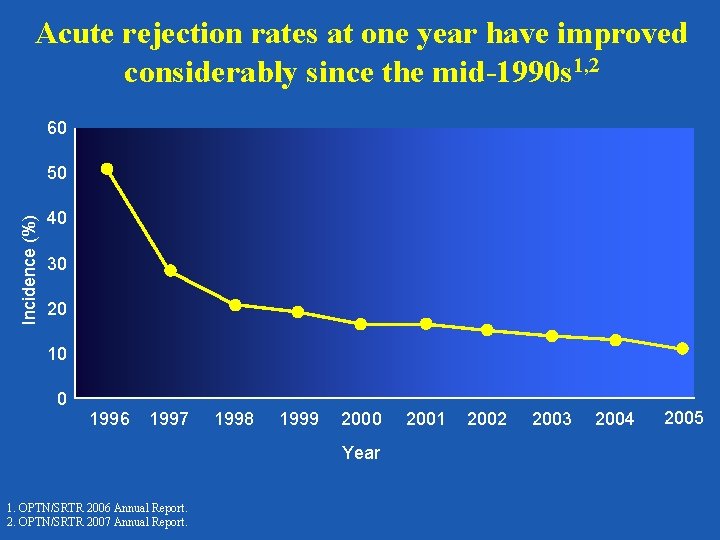
Acute rejection rates at one year have improved considerably since the mid-1990 s 1, 2 60 Incidence (%) 50 40 30 20 10 0 1996 1997 1998 1999 2000 Year 1. OPTN/SRTR 2006 Annual Report. 2. OPTN/SRTR 2007 Annual Report. 2001 2002 2003 2004 2005

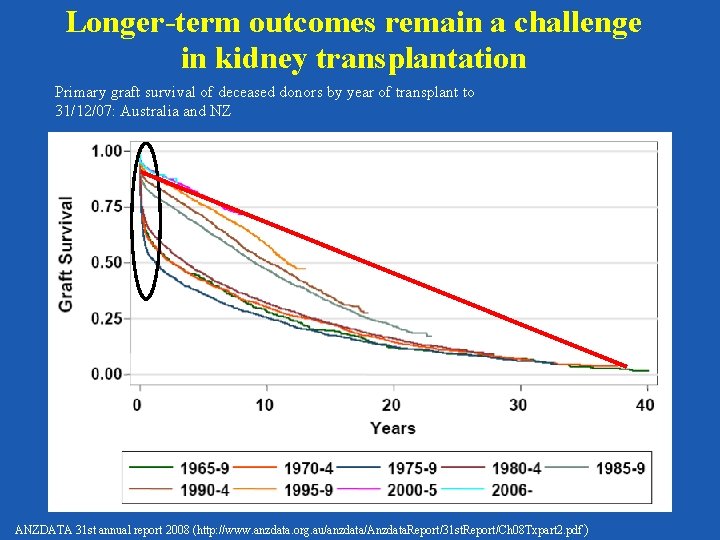
Longer-term outcomes remain a challenge in kidney transplantation Primary graft survival of deceased donors by year of transplant to 31/12/07: Australia and NZ ANZDATA 31 st annual report 2008 (http: //www. anzdata. org. au/anzdata/Anzdata. Report/31 st. Report/Ch 08 Txpart 2. pdf )

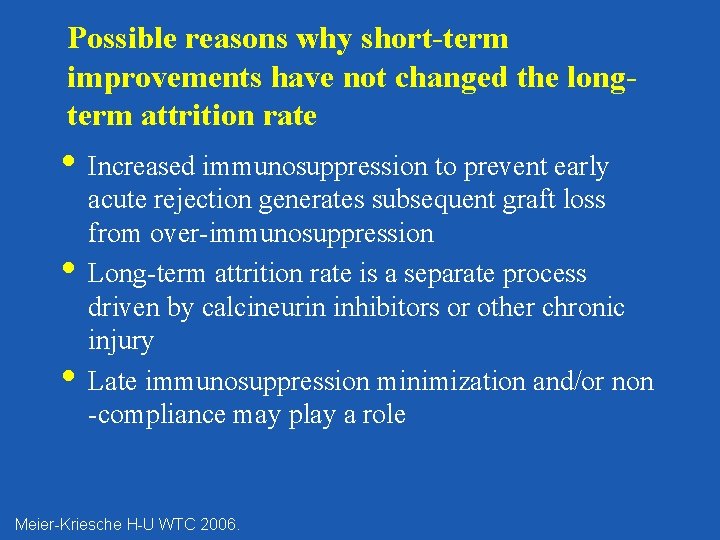
Possible reasons why short-term improvements have not changed the longterm attrition rate • Increased immunosuppression to prevent early • • acute rejection generates subsequent graft loss from over-immunosuppression Long-term attrition rate is a separate process driven by calcineurin inhibitors or other chronic injury Late immunosuppression minimization and/or non -compliance may play a role Meier-Kriesche H-U WTC 2006.

Why hasn't improved early graft survival resulted in better late graft survival? • Immunosuppression may have early benefits but late adverse effects on graft survival • Late graft failure may occur via mechanisms unrelated to immune injury • Immunosuppression may be inadequate late because of nonadherence BK nephropathy, other late infections, malignancies, CVD CNI nephrotoxicity, recurrent disease, senescence Multiple and/or late acute rejection episodes, subclinical rejection, AMR

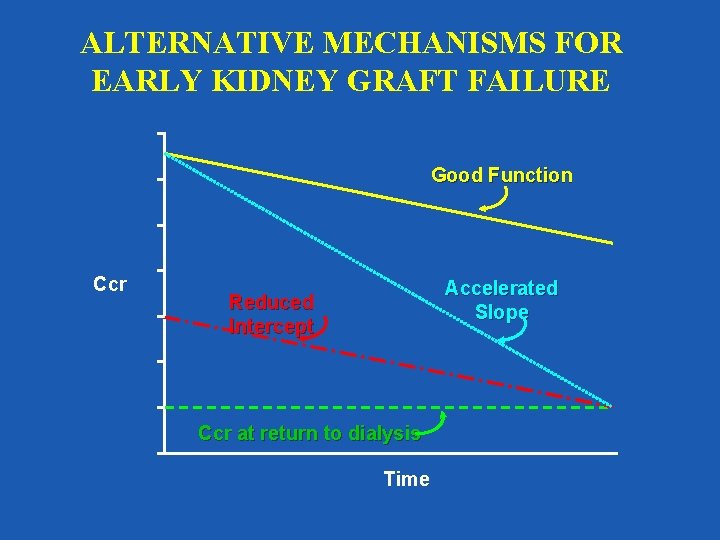
ALTERNATIVE MECHANISMS FOR EARLY KIDNEY GRAFT FAILURE Good Function Ccr Accelerated Slope Reduced Intercept Ccr at return to dialysis Time

Disease and medical trends: Keys to long-term success • Graft survival –Acute rejection –One year survival renal function 1 long-term survival 2 • Patient survival –Cardiovascular disease 3 –Post-transplant malignancy 4 1. Meier-Kreische HU, et al. Am J Transplant. 2004; 4: 378– 383. 2. Hariharan S, et al. Kidney Intl. 2002; 62: 311– 318. 3. Meier-Kreische HU, et al. Transplantation. 2003; 75: 1291– 1295. 4. Campistol J, et al. JASN. 2006; 17: 581– 589.

What are the important late risks? • Donor factors leading to poorer outcome • Recipient death – Cardiovascular disease – Malignancy – Infection • Late graft loss – Chronic allograft nephropathy – Subclinical ACR or AMR – Infection

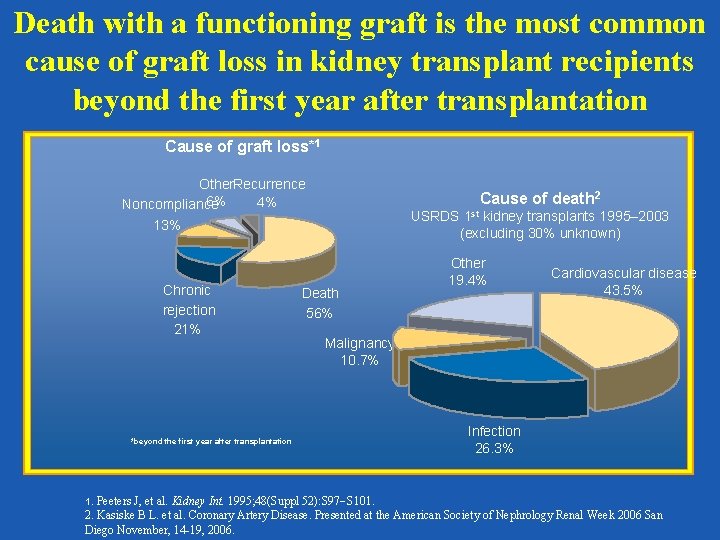
Death with a functioning graft is the most common cause of graft loss in kidney transplant recipients beyond the first year after transplantation Cause of graft loss*1 Other. Recurrence 6% 4% Noncompliance Cause of death 2 USRDS 1 st kidney transplants 1995– 2003 (excluding 30% unknown) 13% Chronic rejection 21% *beyond the first year after transplantation 1. Death 56% Other 19. 4% Cardiovascular disease 43. 5% Malignancy 10. 7% Infection 26. 3% Peeters J, et al. Kidney Int. 1995; 48(Suppl 52): S 97 S 101. 2. Kasiske B L. et al. Coronary Artery Disease. Presented at the American Society of Nephrology Renal Week 2006 San Diego November, 14 -19, 2006.

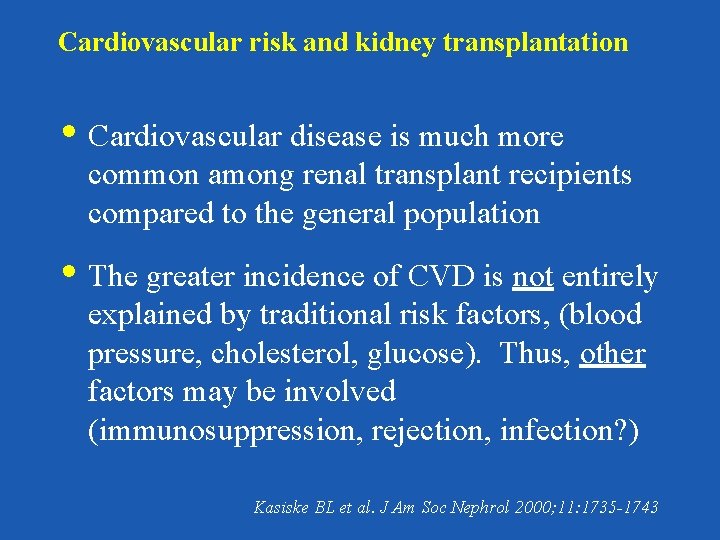
Cardiovascular risk and kidney transplantation • Cardiovascular disease is much more common among renal transplant recipients compared to the general population • The greater incidence of CVD is not entirely explained by traditional risk factors, (blood pressure, cholesterol, glucose). Thus, other factors may be involved (immunosuppression, rejection, infection? ) Kasiske BL et al. J Am Soc Nephrol 2000; 11: 1735 -1743

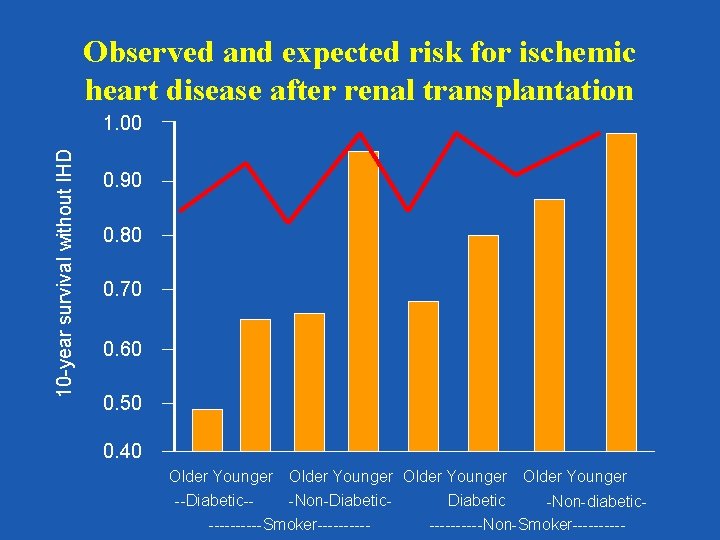
Observed and expected risk for ischemic heart disease after renal transplantation 10 -year survival without IHD 1. 00 0. 90 0. 80 0. 70 0. 60 0. 50 0. 40 Older Younger --Diabetic--Non-Diabetic -Non-diabetic-----Smoker----------Non-Smoker-----

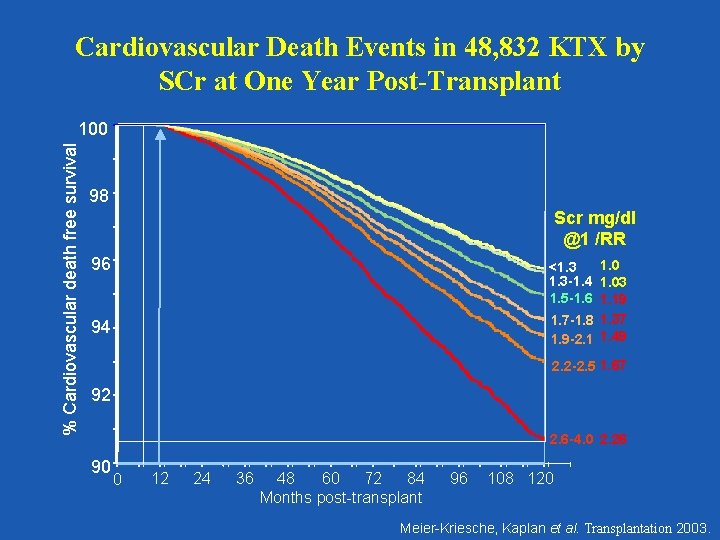
Cardiovascular Death Events in 48, 832 KTX by SCr at One Year Post-Transplant % Cardiovascular death free survival 100 98 Scr mg/dl @1 /RR 96 1. 0 <1. 3 -1. 4 1. 03 1. 5 -1. 6 1. 19 1. 7 -1. 8 1. 37 1. 9 -2. 1 1. 49 94 2. 2 -2. 5 1. 67 92 2. 6 -4. 0 2. 26 90 0 12 24 36 48 60 72 84 Months post-transplant 96 108 120 Meier-Kriesche, Kaplan et al. Transplantation 2003.

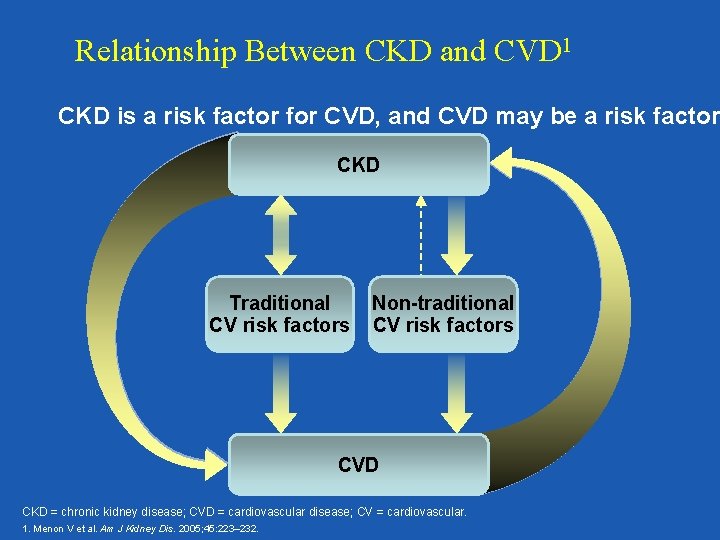
Relationship Between CKD and CVD 1 CKD is a risk factor for CVD, and CVD may be a risk factor CKD Traditional CV risk factors Non-traditional CV risk factors CVD CKD = chronic kidney disease; CVD = cardiovascular disease; CV = cardiovascular. 1. Menon V et al. Am J Kidney Dis. 2005; 45: 223– 232.

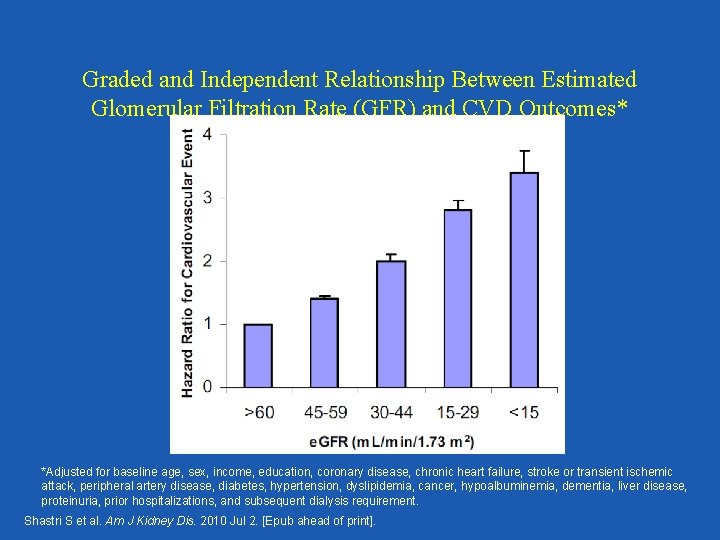
Graded and Independent Relationship Between Estimated Glomerular Filtration Rate (GFR) and CVD Outcomes* *Adjusted for baseline age, sex, income, education, coronary disease, chronic heart failure, stroke or transient ischemic attack, peripheral artery disease, diabetes, hypertension, dyslipidemia, cancer, hypoalbuminemia, dementia, liver disease, proteinuria, prior hospitalizations, and subsequent dialysis requirement. Shastri S et al. Am J Kidney Dis. 2010 Jul 2. [Epub ahead of print].

The key understanding is that patients with CKD benefit as much as non-CKD patients with appropriate medications and therapies, if not more, because of their increased risk!

Decreased GFR has consistently been found to be an independent risk factor for CVD outcomes and all cause mortality!

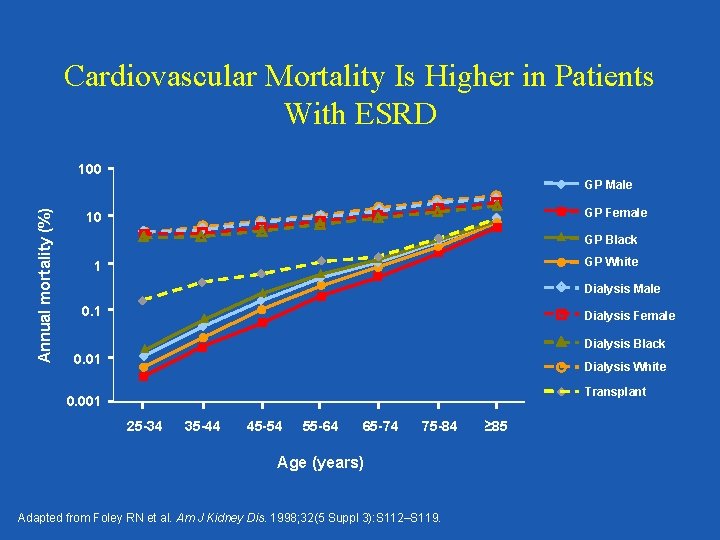
Cardiovascular Mortality Is Higher in Patients With ESRD 100 Annual mortality (%) GP Male GP Female 10 GP Black GP White 1 Dialysis Male 0. 1 Dialysis Female Dialysis Black 0. 01 Dialysis White Transplant 0. 001 25 -34 35 -44 45 -54 55 -64 65 -74 75 -84 Age (years) Adapted from Foley RN et al. Am J Kidney Dis. 1998; 32(5 Suppl 3): S 112–S 119. ≥ 85

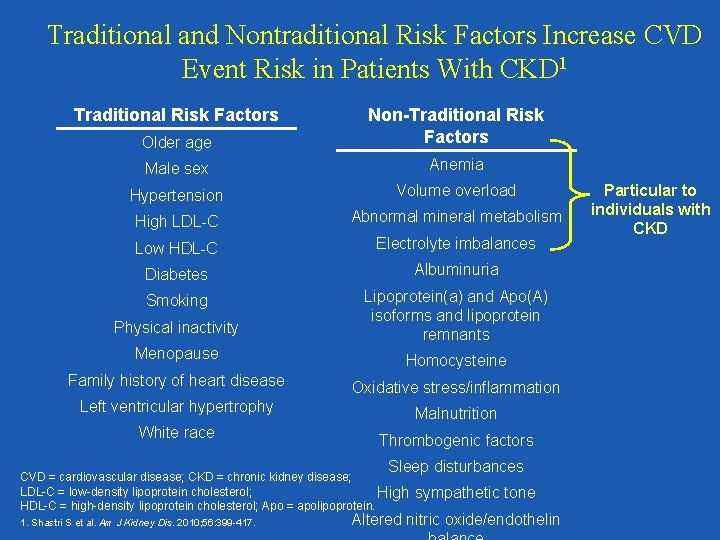
Traditional and Nontraditional Risk Factors Increase CVD Event Risk in Patients With CKD 1 Traditional Risk Factors Older age Non-Traditional Risk Factors Male sex Anemia Hypertension Volume overload High LDL-C Abnormal mineral metabolism Low HDL-C Electrolyte imbalances Diabetes Albuminuria Smoking Physical inactivity Lipoprotein(a) and Apo(A) isoforms and lipoprotein remnants Menopause Homocysteine Family history of heart disease Oxidative stress/inflammation Left ventricular hypertrophy Malnutrition White race Thrombogenic factors Sleep disturbances CVD = cardiovascular disease; CKD = chronic kidney disease; LDL-C = low-density lipoprotein cholesterol; High HDL-C = high-density lipoprotein cholesterol; Apo = apolipoprotein. 1. Shastri S et al. Am J Kidney Dis. 2010; 56: 399 -417. sympathetic tone Altered nitric oxide/endothelin Particular to individuals with CKD

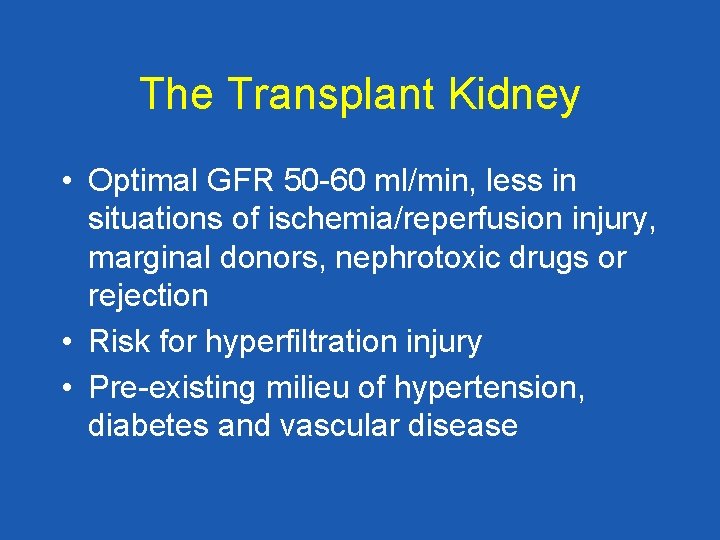
The Transplant Kidney • Optimal GFR 50 -60 ml/min, less in situations of ischemia/reperfusion injury, marginal donors, nephrotoxic drugs or rejection • Risk for hyperfiltration injury • Pre-existing milieu of hypertension, diabetes and vascular disease

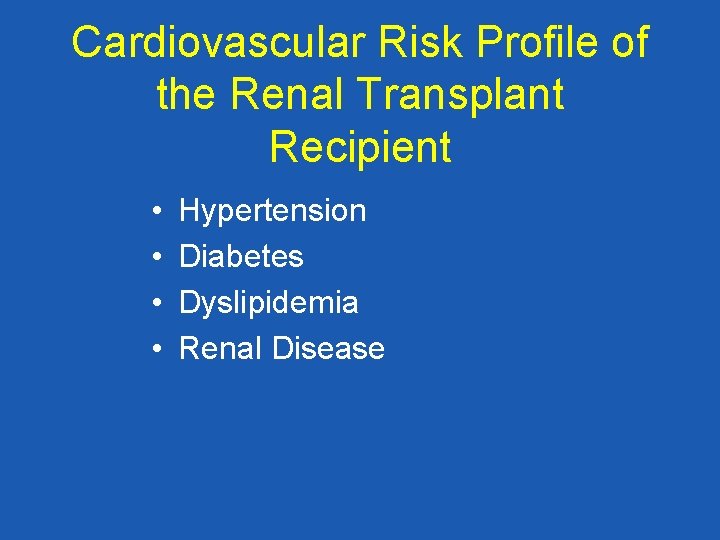
Cardiovascular Risk Profile of the Renal Transplant Recipient • • Hypertension Diabetes Dyslipidemia Renal Disease

Appreciate that we have no prospective randomized controlled trials to evaluate optimal treatment regimens and goals in patients with kidney transplants!

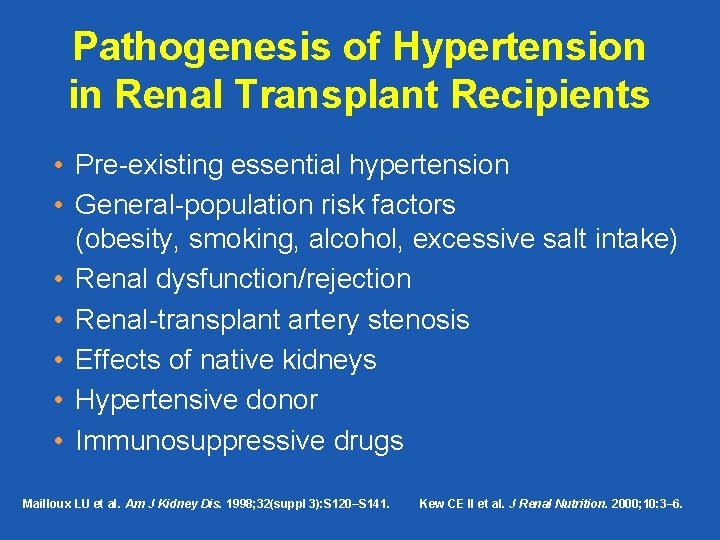
Pathogenesis of Hypertension in Renal Transplant Recipients • Pre-existing essential hypertension • General-population risk factors (obesity, smoking, alcohol, excessive salt intake) • Renal dysfunction/rejection • Renal-transplant artery stenosis • Effects of native kidneys • Hypertensive donor • Immunosuppressive drugs Mailloux LU et al. Am J Kidney Dis. 1998; 32(suppl 3): S 120–S 141. Kew CE II et al. J Renal Nutrition. 2000; 10: 3– 6.

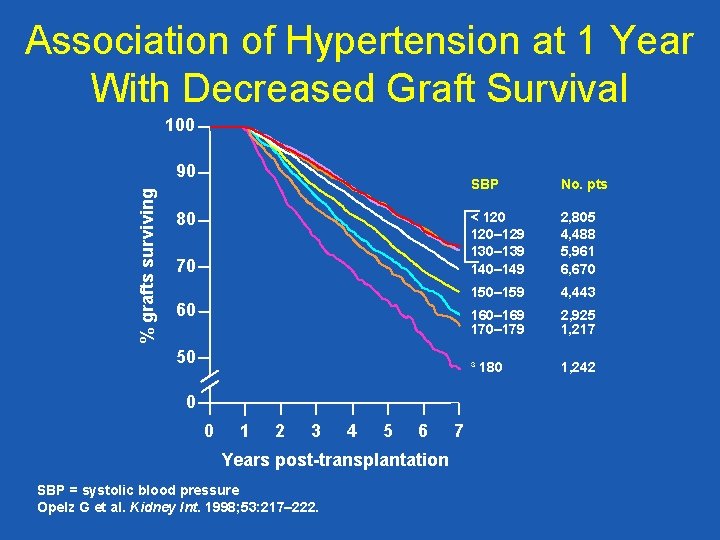
Association of Hypertension at 1 Year With Decreased Graft Survival 100 % grafts surviving 90 80 70 60 50 0 0 1 2 3 4 5 6 Years post-transplantation SBP = systolic blood pressure Opelz G et al. Kidney Int. 1998; 53: 217– 222. 7 SBP No. pts < 120– 129 130– 139 140– 149 2, 805 4, 488 5, 961 6, 670 150– 159 4, 443 160– 169 170– 179 2, 925 1, 217 ³ 180 1, 242

Does treatment of high blood pressure in renal transplant recipients reduce graft loss and patient death?

Probably! • No outcome studies have been performed • It is likely that these high risk patients will derive benefit for the heart, brain and transplant kidney

Drug Therapy: Treatment Diuretics: As needed to control volume: more studies need to focus on the advantages of thiazides vs loop diuretics Beta-blockers: Heart rate control, CAD Alpha blockers: BPH, outflow obstruction

ACEI and ARB • Preferred treatment strategies antihypertensive antiproteinuric antiproliferative

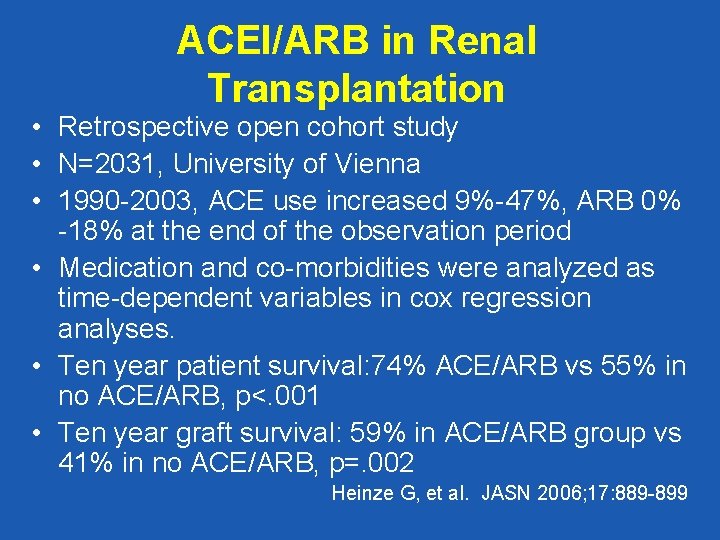
ACEI/ARB in Renal Transplantation • Retrospective open cohort study • N=2031, University of Vienna • 1990 -2003, ACE use increased 9%-47%, ARB 0% -18% at the end of the observation period • Medication and co-morbidities were analyzed as time-dependent variables in cox regression analyses. • Ten year patient survival: 74% ACE/ARB vs 55% in no ACE/ARB, p<. 001 • Ten year graft survival: 59% in ACE/ARB group vs 41% in no ACE/ARB, p=. 002 Heinze G, et al. JASN 2006; 17: 889 -899

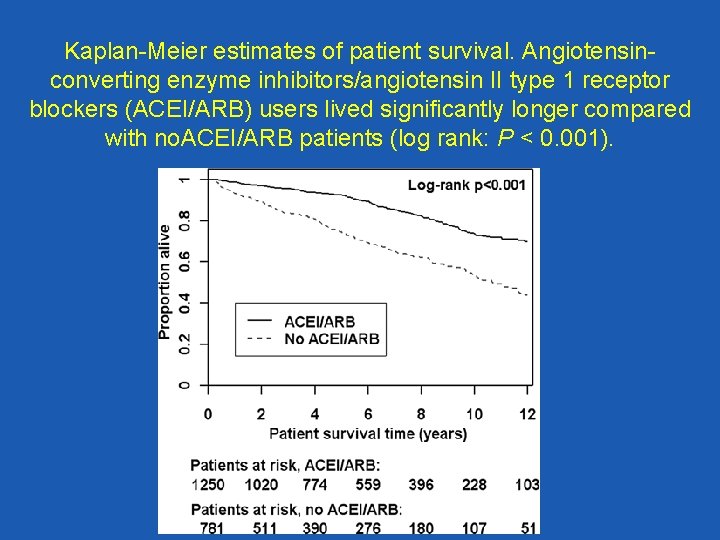
Kaplan-Meier estimates of patient survival. Angiotensinconverting enzyme inhibitors/angiotensin II type 1 receptor blockers (ACEI/ARB) users lived significantly longer compared with no. ACEI/ARB patients (log rank: P < 0. 001).

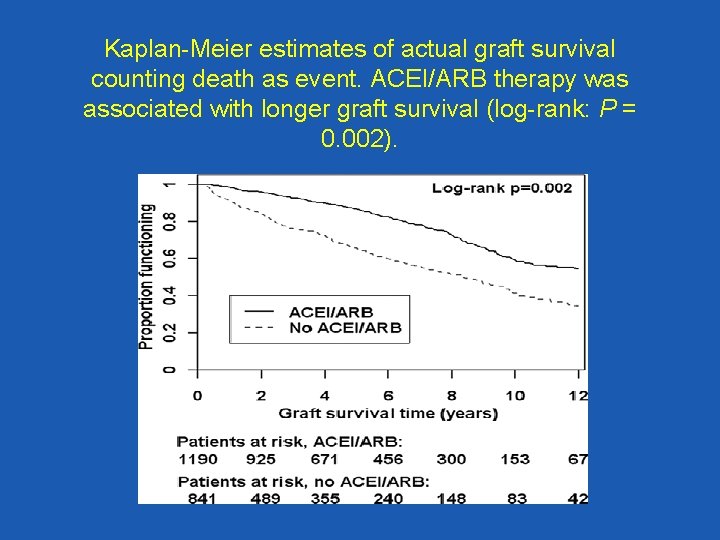
Kaplan-Meier estimates of actual graft survival counting death as event. ACEI/ARB therapy was associated with longer graft survival (log-rank: P = 0. 002).

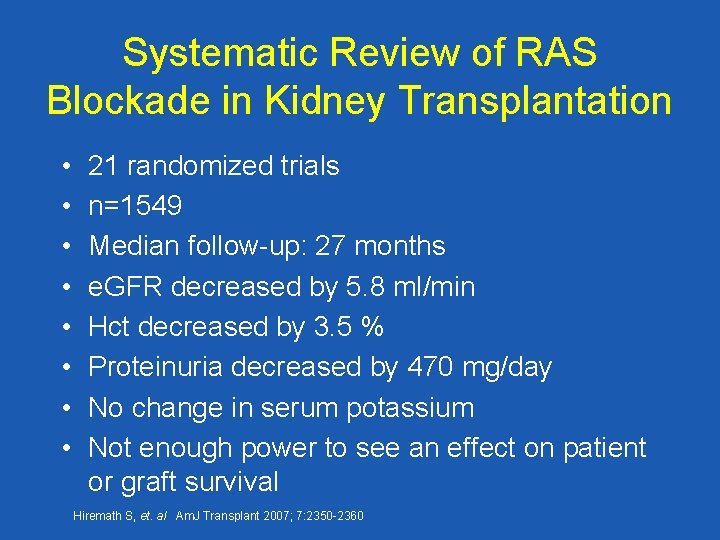
Systematic Review of RAS Blockade in Kidney Transplantation • • 21 randomized trials n=1549 Median follow-up: 27 months e. GFR decreased by 5. 8 ml/min Hct decreased by 3. 5 % Proteinuria decreased by 470 mg/day No change in serum potassium Not enough power to see an effect on patient or graft survival Hiremath S, et. al Am. J Transplant 2007; 7: 2350 -2360

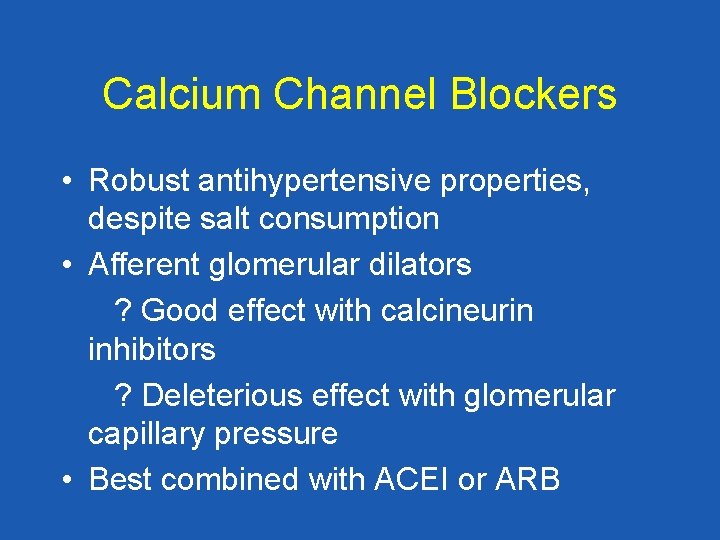
Calcium Channel Blockers • Robust antihypertensive properties, despite salt consumption • Afferent glomerular dilators ? Good effect with calcineurin inhibitors ? Deleterious effect with glomerular capillary pressure • Best combined with ACEI or ARB

Diabetes in Kidney Transplant Patients • Associated with reduced patient survival • Associated with reduced graft survival Histologic appearance of diabetic kidney disease within 5 years.

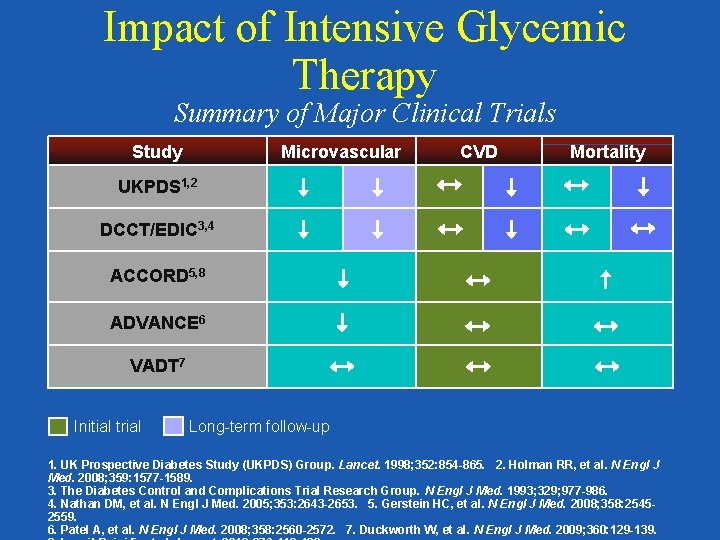
Impact of Intensive Glycemic Therapy Summary of Major Clinical Trials Study Microvascular CVD Mortality UKPDS 1, 2 DCCT/EDIC 3, 4 ACCORD 5, 8 ADVANCE 6 VADT 7 Initial trial Long-term follow-up 1. UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 854 -865. 2. Holman RR, et al. N Engl J Med. 2008; 359: 1577 -1589. 3. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329; 977 -986. 4. Nathan DM, et al. N Engl J Med. 2005; 353: 2643 -2653. 5. Gerstein HC, et al. N Engl J Med. 2008; 358: 25452559. 6. Patel A, et al. N Engl J Med. 2008; 358: 2560 -2572. 7. Duckworth W, et al. N Engl J Med. 2009; 360: 129 -139.

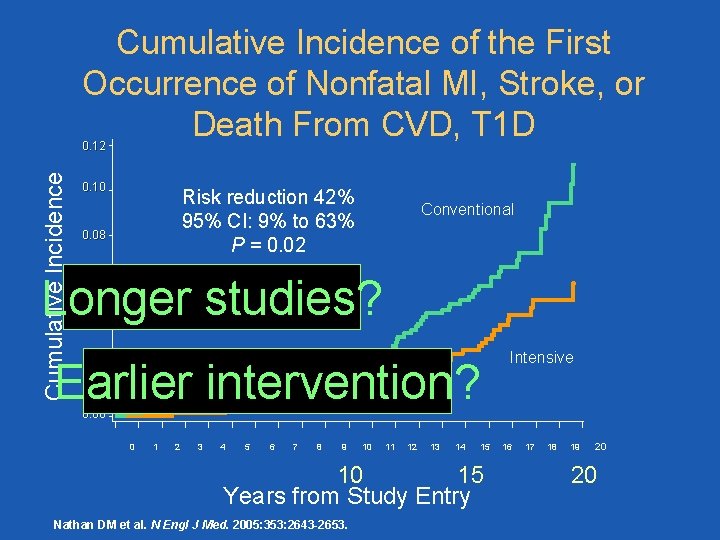
Cumulative Incidence of the First Occurrence of Nonfatal MI, Stroke, or Death From CVD, T 1 D Cumulative Incidence 0. 12 0. 10 Risk reduction 42% 95% CI: 9% to 63% P = 0. 02 0. 08 Conventional Longer studies? 0. 06 0. 04 Earlier intervention? 0. 02 Intensive 0. 00 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 15 10 Years from Study Entry Nathan DM et al. N Engl J Med. 2005: 353: 2643 -2653. 16 17 18 19 20 20

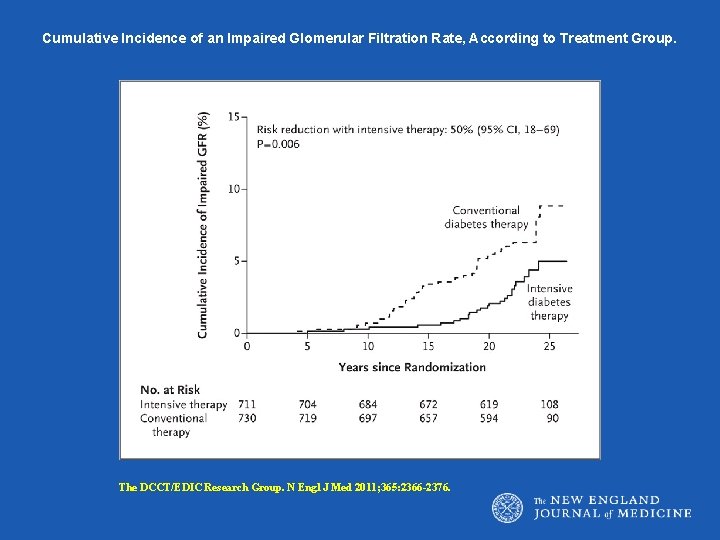
Cumulative Incidence of an Impaired Glomerular Filtration Rate, According to Treatment Group. The DCCT/EDIC Research Group. N Engl J Med 2011; 365: 2366 -2376.

Rationale for Lipid Lowering Clinical Trials in the CKD Population • CKD and ESRD patients are at increased risk of cardiovascular complications • CKD and ESRD patients have abnormal lipid profiles • Secondary analyses of lipid lowering studies indicated statin treatment improved CV outcomes in CKD patients • Secondary analyses of these studies also demonstrated slowing of CKD progression • Need for randomized placebo-controlled statin trials in CKD and ESRD patients 1. Scandinavian Simvastatin Survival Study (4 S). Lancet. 1994; 344(8934): 1383– 1389. 2. Shepherd J et al. N Engl J Med. 1995; 333(20): 1301– 1307. 3. Heart Protection Study Collaborative Group. Lancet. 2002; 360(9326): 7– 22. 4. Seliger SL et al. Kidney Int. 2002; 61(1): 297– 304. 5. Liao JK. Am J Cardiol. 2005; 96(5 A): 24 F– 33 F. 6. Fellström BC et al. Kidney Int. 2003; 63(Suppl 84): S 204–S 206.

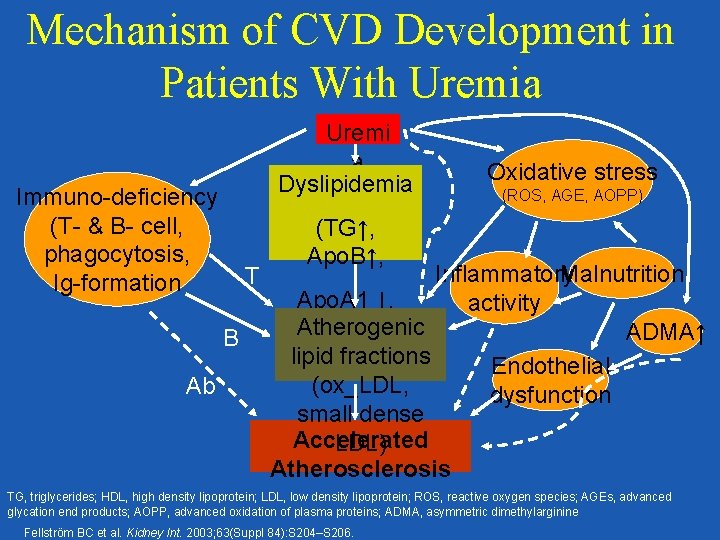
Mechanism of CVD Development in Patients With Uremia Uremi a Dyslipidemia Immuno-deficiency (T- & B- cell, phagocytosis, Ig-formation T B Ab Oxidative stress (ROS, AGE, AOPP) (TG↑, Apo. B↑, Malnutrition Inflammatory Apo. A 1 ↓, activity Atherogenic HDL↓) ADMA↑ lipid fractions Endothelial (ox_LDL, dysfunction small dense Accelerated LDL) Atherosclerosis TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; ROS, reactive oxygen species; AGEs, advanced glycation end products; AOPP, advanced oxidation of plasma proteins; ADMA, asymmetric dimethylarginine Fellström BC et al. Kidney Int. 2003; 63(Suppl 84): S 204–S 206.

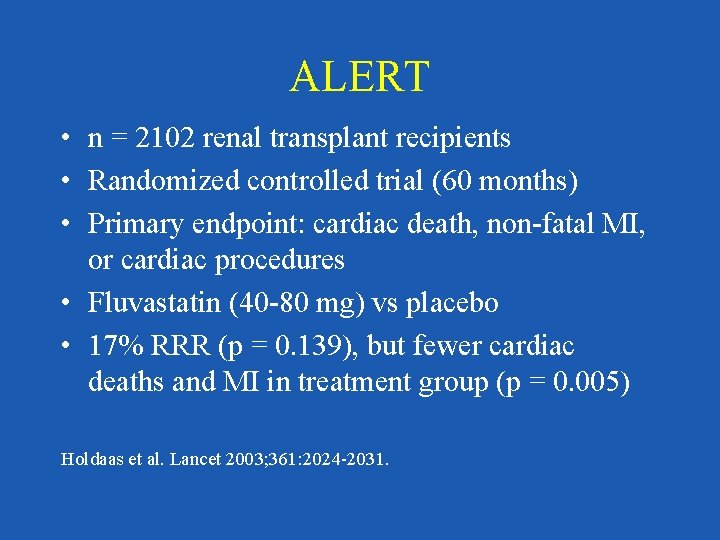
ALERT • n = 2102 renal transplant recipients • Randomized controlled trial (60 months) • Primary endpoint: cardiac death, non-fatal MI, or cardiac procedures • Fluvastatin (40 -80 mg) vs placebo • 17% RRR (p = 0. 139), but fewer cardiac deaths and MI in treatment group (p = 0. 005) Holdaas et al. Lancet 2003; 361: 2024 -2031.

SHARP: Eligibility • History of chronic kidney disease – not on dialysis: elevated creatinine on 2 occasions • Men: ≥ 1. 7 mg/d. L (150 µmol/L) • Women: ≥ 1. 5 mg/d. L (130 µmol/L) – on dialysis: haemodialysis or peritoneal dialysis • Age ≥ 40 years • No history of myocardial infarction or coronary revascularisation • Uncertainty: LDL-lowering treatment not definitely indicated or contraindicated

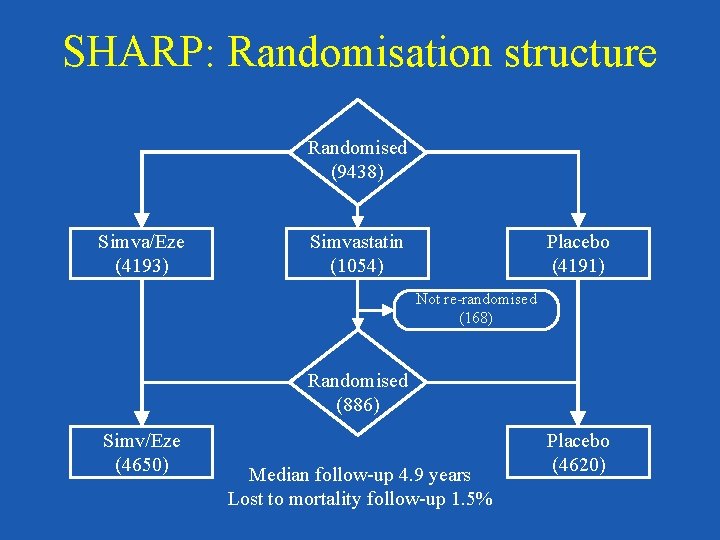
SHARP: Randomisation structure Randomised (9438) Simva/Eze (4193) Simvastatin (1054) Placebo (4191) Not re-randomised (168) Randomised (886) Simv/Eze (4650) Median follow-up 4. 9 years Lost to mortality follow-up 1. 5% Placebo (4620)

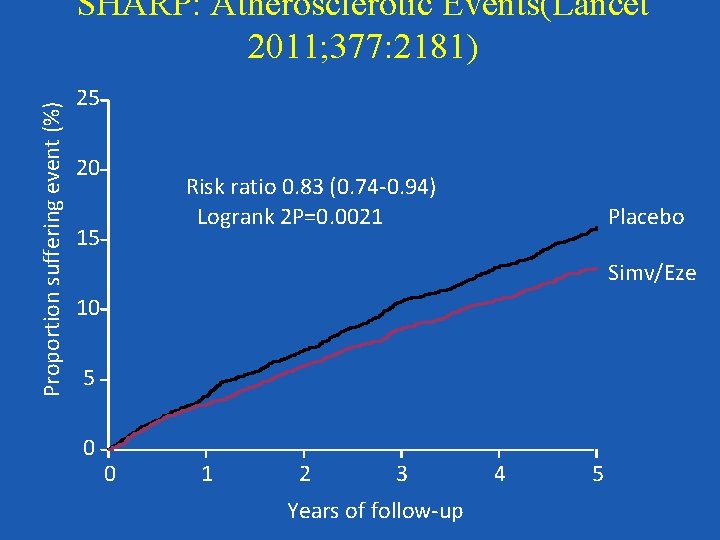
Proportion suffering event (%) SHARP: Atherosclerotic Events(Lancet 2011; 377: 2181) 25 20 Risk ratio 0. 83 (0. 74 -0. 94) Logrank 2 P=0. 0021 15 Placebo Simv/Eze 10 5 0 0 1 2 3 Years of follow-up 4 5

Summary Nevertheless, given the data from the HPS and the SHARP study, we feel that there is a strong argument to abandon a threshold-based algorithm for treating hyperlipidemia. Rather it may be advisable to treat those with high risk for atherosclerotic cardiac events regardless of initial LDL level, and to treat with a potent dose of a statin alone or in combination with a second line drug to achieve a marked (at least 40%) reduction in LDL, at least to ATP-III LDL goal levels.

CKD Resets the Focus on CV Risk Reduction Strategies • BP <130/80 mm. Hg? • Evaluate and treat lipids • Extinguish microalbuminuria/proteinuria? • Reduction in dietary salt/saturated fat • Intensify glycemic control • Control anemia • Control calcium / phosphorus balance • Anti-platelet therapy

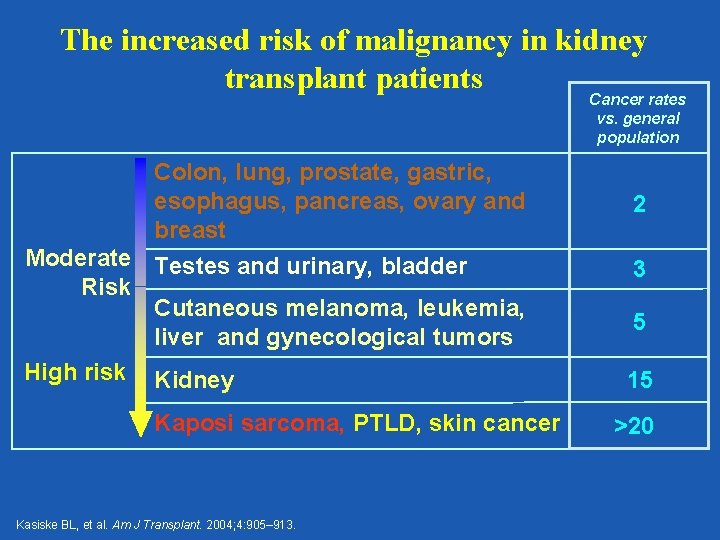
The increased risk of malignancy in kidney transplant patients Cancer rates vs. general population Colon, lung, prostate, gastric, esophagus, pancreas, ovary and breast Moderate Testes and urinary, bladder Risk Cutaneous melanoma, leukemia, liver and gynecological tumors High risk Kidney Kaposi sarcoma, PTLD, skin cancer Kasiske BL, et al. Am J Transplant. 2004; 4: 905– 913. 2 3 5 15 >20

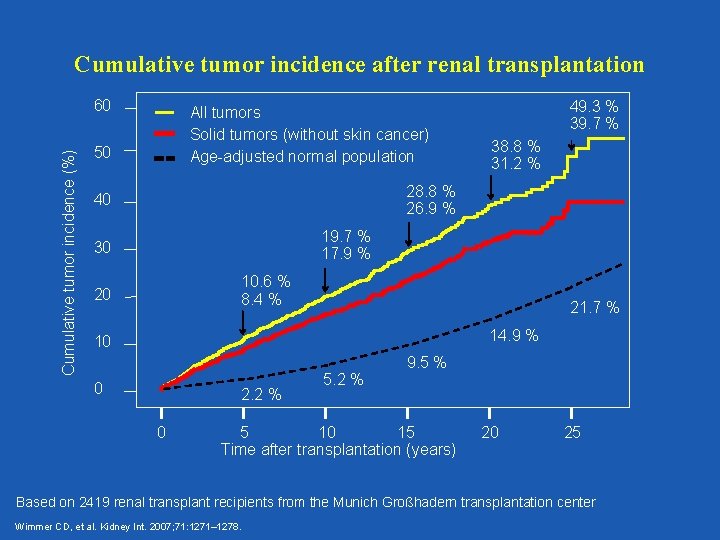
Cumulative tumor incidence after renal transplantation Cumulative tumor incidence (%) 60 All tumors Solid tumors (without skin cancer) Age-adjusted normal population 50 49. 3 % 39. 7 % 38. 8 % 31. 2 % 28. 8 % 26. 9 % 40 19. 7 % 17. 9 % 30 10. 6 % 8. 4 % 20 21. 7 % 14. 9 % 10 0 2. 2 % 0 5. 2 % 9. 5 % 5 10 15 Time after transplantation (years) 20 25 Based on 2419 renal transplant recipients from the Munich Großhadern transplantation center Wimmer CD, et al. Kidney Int. 2007; 71: 1271– 1278.

Overview • Short-term risks • Long-term risks – Erosion of graft function – Cardiovascular disease – malignancy • Drug – Drug interactions • Future opportunities

Drug – Drug Infections A Major Concern!

Reality Check FK MMF Prednisone Val GCV Bactrim Mycelex Lipitor Fe. SO 4 Prevacid Actos Glipizide ASA Norvasc Atenolol Lisinopril Lasix Viagra ?

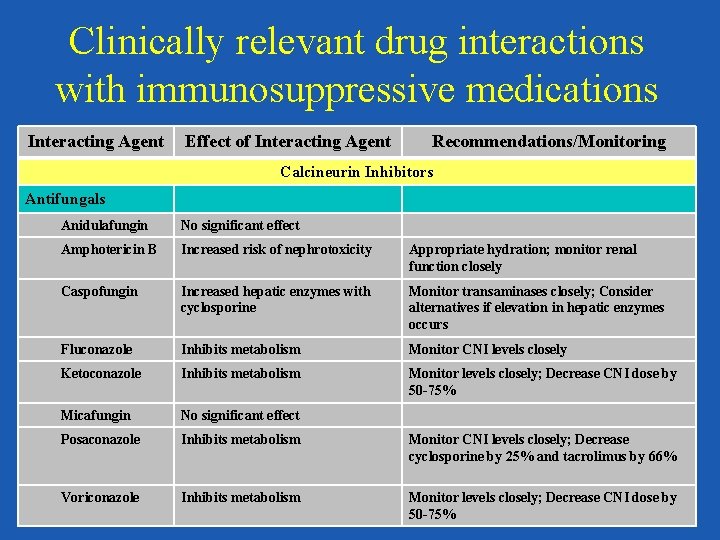
Clinically relevant drug interactions with immunosuppressive medications Interacting Agent Effect of Interacting Agent Recommendations/Monitoring Calcineurin Inhibitors Antifungals Anidulafungin No significant effect Amphotericin B Increased risk of nephrotoxicity Appropriate hydration; monitor renal function closely Caspofungin Increased hepatic enzymes with cyclosporine Monitor transaminases closely; Consider alternatives if elevation in hepatic enzymes occurs Fluconazole Inhibits metabolism Monitor CNI levels closely Ketoconazole Inhibits metabolism Monitor levels closely; Decrease CNI dose by 50 -75% Micafungin No significant effect Posaconazole Inhibits metabolism Monitor CNI levels closely; Decrease cyclosporine by 25% and tacrolimus by 66% Voriconazole Inhibits metabolism Monitor levels closely; Decrease CNI dose by 50 -75%

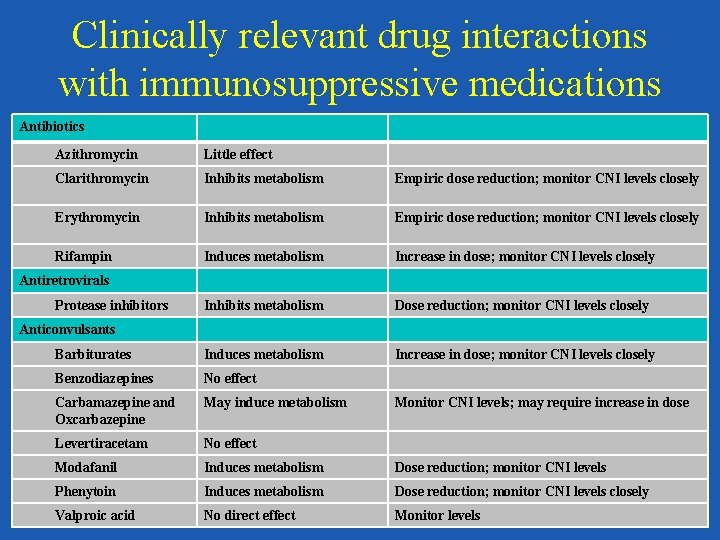
Clinically relevant drug interactions with immunosuppressive medications Antibiotics Azithromycin Little effect Clarithromycin Inhibits metabolism Empiric dose reduction; monitor CNI levels closely Erythromycin Inhibits metabolism Empiric dose reduction; monitor CNI levels closely Rifampin Induces metabolism Increase in dose; monitor CNI levels closely Inhibits metabolism Dose reduction; monitor CNI levels closely Barbiturates Induces metabolism Increase in dose; monitor CNI levels closely Benzodiazepines No effect Carbamazepine and Oxcarbazepine May induce metabolism Levertiracetam No effect Modafanil Induces metabolism Dose reduction; monitor CNI levels Phenytoin Induces metabolism Dose reduction; monitor CNI levels closely Valproic acid No direct effect Monitor levels Antiretrovirals Protease inhibitors Anticonvulsants Monitor CNI levels; may require increase in dose

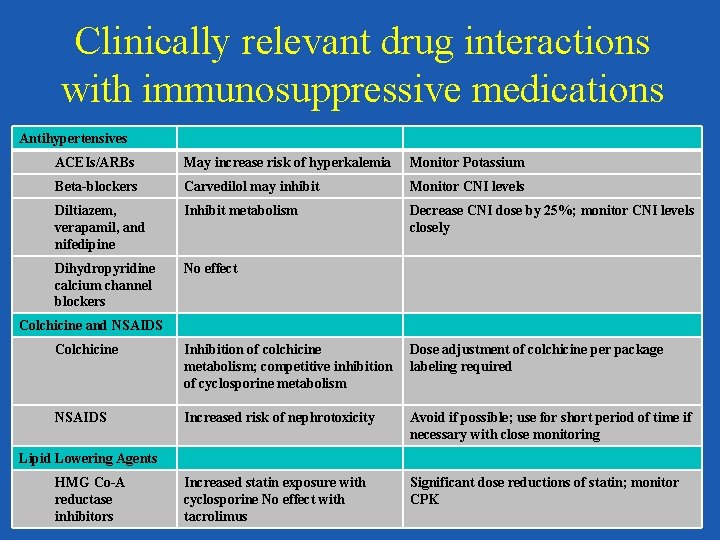
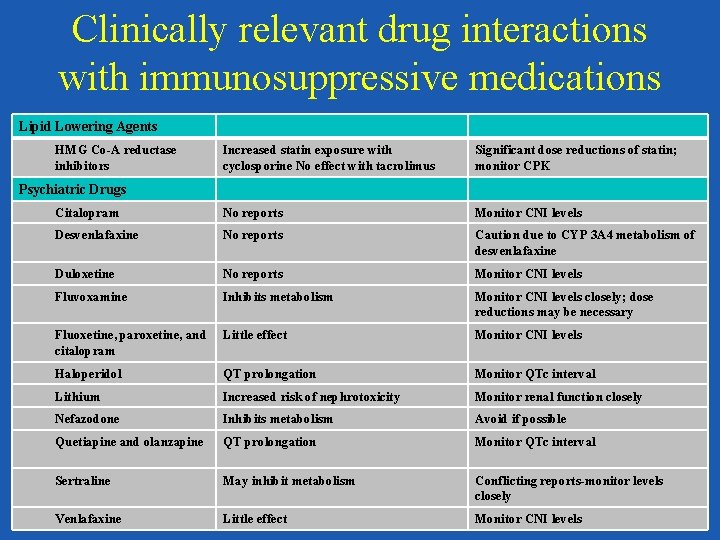
Clinically relevant drug interactions with immunosuppressive medications Antihypertensives ACEIs/ARBs May increase risk of hyperkalemia Monitor Potassium Beta-blockers Carvedilol may inhibit Monitor CNI levels Diltiazem, verapamil, and nifedipine Inhibit metabolism Decrease CNI dose by 25%; monitor CNI levels closely Dihydropyridine calcium channel blockers No effect Colchicine and NSAIDS Colchicine Inhibition of colchicine metabolism; competitive inhibition of cyclosporine metabolism Dose adjustment of colchicine per package labeling required NSAIDS Increased risk of nephrotoxicity Avoid if possible; use for short period of time if necessary with close monitoring Increased statin exposure with cyclosporine No effect with tacrolimus Significant dose reductions of statin; monitor CPK Lipid Lowering Agents HMG Co-A reductase inhibitors

Clinically relevant drug interactions with immunosuppressive medications Lipid Lowering Agents HMG Co-A reductase inhibitors Increased statin exposure with cyclosporine No effect with tacrolimus Significant dose reductions of statin; monitor CPK Citalopram No reports Monitor CNI levels Desvenlafaxine No reports Caution due to CYP 3 A 4 metabolism of desvenlafaxine Duloxetine No reports Monitor CNI levels Fluvoxamine Inhibits metabolism Monitor CNI levels closely; dose reductions may be necessary Fluoxetine, paroxetine, and citalopram Little effect Monitor CNI levels Haloperidol QT prolongation Monitor QTc interval Lithium Increased risk of nephrotoxicity Monitor renal function closely Nefazodone Inhibits metabolism Avoid if possible Quetiapine and olanzapine QT prolongation Monitor QTc interval Sertraline May inhibit metabolism Conflicting reports-monitor levels closely Venlafaxine Little effect Monitor CNI levels Psychiatric Drugs

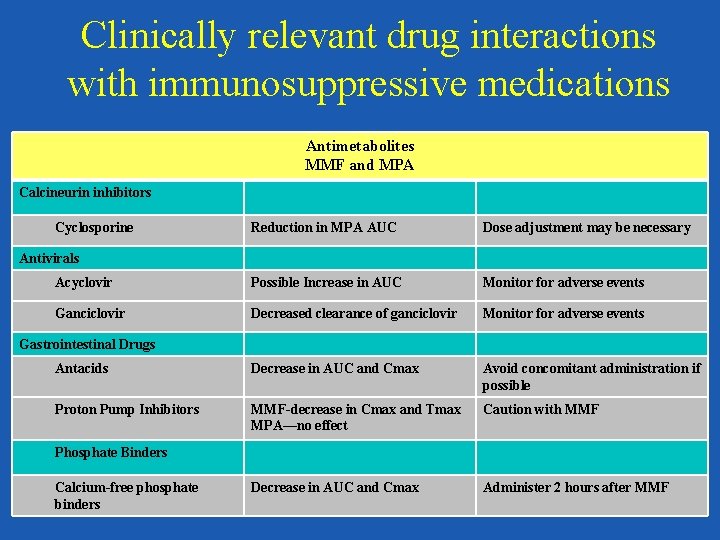
Clinically relevant drug interactions with immunosuppressive medications Antimetabolites MMF and MPA Calcineurin inhibitors Cyclosporine Reduction in MPA AUC Dose adjustment may be necessary Acyclovir Possible Increase in AUC Monitor for adverse events Ganciclovir Decreased clearance of ganciclovir Monitor for adverse events Antacids Decrease in AUC and Cmax Avoid concomitant administration if possible Proton Pump Inhibitors MMF-decrease in Cmax and Tmax MPA—no effect Caution with MMF Decrease in AUC and Cmax Administer 2 hours after MMF Antivirals Gastrointestinal Drugs Phosphate Binders Calcium-free phosphate binders

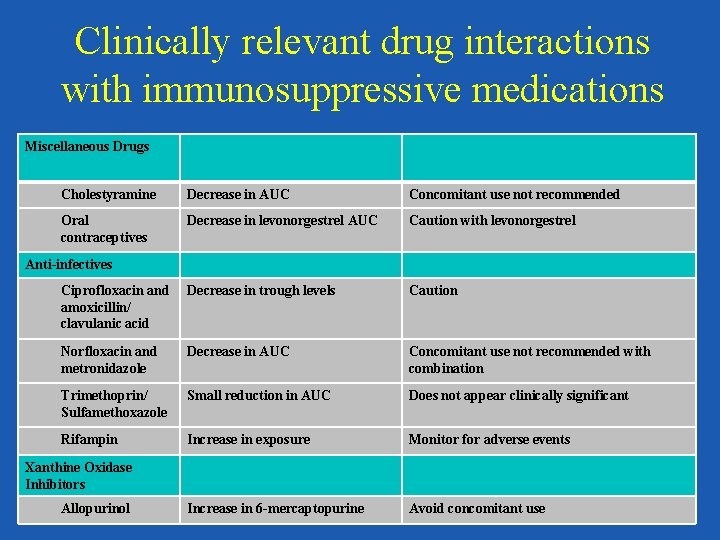
Clinically relevant drug interactions with immunosuppressive medications Miscellaneous Drugs Cholestyramine Decrease in AUC Concomitant use not recommended Oral contraceptives Decrease in levonorgestrel AUC Caution with levonorgestrel Ciprofloxacin and amoxicillin/ clavulanic acid Decrease in trough levels Caution Norfloxacin and metronidazole Decrease in AUC Concomitant use not recommended with combination Trimethoprin/ Sulfamethoxazole Small reduction in AUC Does not appear clinically significant Rifampin Increase in exposure Monitor for adverse events Increase in 6 -mercaptopurine Avoid concomitant use Anti-infectives Xanthine Oxidase Inhibitors Allopurinol

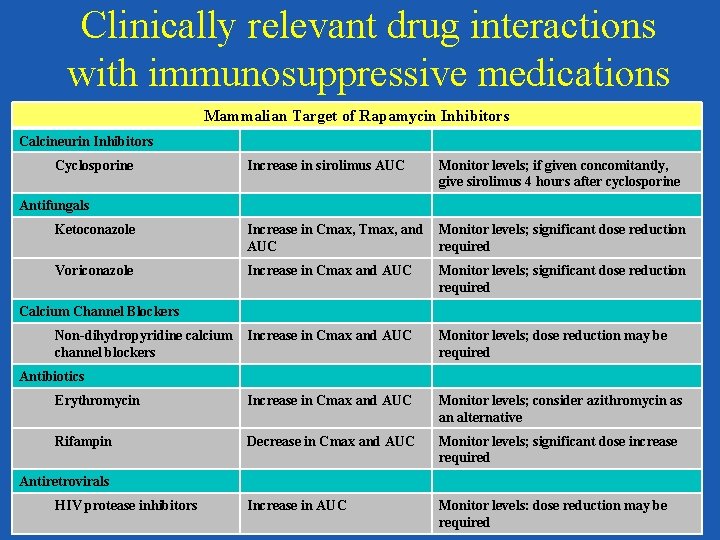
Clinically relevant drug interactions with immunosuppressive medications Mammalian Target of Rapamycin Inhibitors Calcineurin Inhibitors Cyclosporine Increase in sirolimus AUC Monitor levels; if given concomitantly, give sirolimus 4 hours after cyclosporine Ketoconazole Increase in Cmax, Tmax, and AUC Monitor levels; significant dose reduction required Voriconazole Increase in Cmax and AUC Monitor levels; significant dose reduction required Increase in Cmax and AUC Monitor levels; dose reduction may be required Erythromycin Increase in Cmax and AUC Monitor levels; consider azithromycin as an alternative Rifampin Decrease in Cmax and AUC Monitor levels; significant dose increase required Increase in AUC Monitor levels: dose reduction may be required Antifungals Calcium Channel Blockers Non-dihydropyridine calcium channel blockers Antibiotics Antiretrovirals HIV protease inhibitors

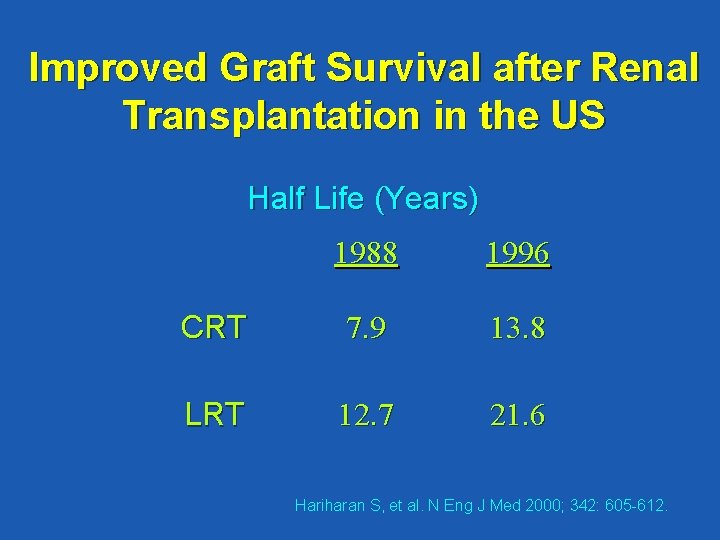
Improved Graft Survival after Renal Transplantation in the US Half Life (Years) 1988 1996 CRT 7. 9 13. 8 LRT 12. 7 21. 6 Hariharan S, et al. N Eng J Med 2000; 342: 605 -612.

Perspective The old consideration that control of rejection changes short-term outcomes but not long-term outcomes is not correct. Kidney transplants should serve their owners for their life expectancy. Perhaps the biggest problem is: INADEQUATE FOLLOW-UP AND SURVEILLANCE and LACK OF RESPONSE TO CHANGE IN FUNCTION!

Conclusions • Many kidney transplant patients in general medical practice • Always work with a transplant center and a nephrologist who is well trained in immunosuppression management • Many new immunosuppression drugs • When in doubt, ask for help!
 Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Teoria do nefron intacto
Teoria do nefron intacto Vasa recta vs peritubular capillaries
Vasa recta vs peritubular capillaries Traer informal command
Traer informal command What does the ppp loan application look like
What does the ppp loan application look like Hip fracture clinical care standard
Hip fracture clinical care standard Primary secondary and tertiary health care
Primary secondary and tertiary health care Bone marrow transplant diet
Bone marrow transplant diet Kidney donor transplant
Kidney donor transplant Transplant management forum
Transplant management forum Next
Next Face transplant
Face transplant Embryo transplant gcse
Embryo transplant gcse Neograft hair transplant in miami
Neograft hair transplant in miami Lll leukemia
Lll leukemia Disadvantage of kidney transplant
Disadvantage of kidney transplant Transplant de ficat costuri
Transplant de ficat costuri Face transplant
Face transplant Heterotopic liver transplant meaning
Heterotopic liver transplant meaning Transplant
Transplant Slk eligibility criteria
Slk eligibility criteria Intraosseous
Intraosseous Oncogens
Oncogens National transplant registry malaysia
National transplant registry malaysia Face transplant
Face transplant Clasificarea mamiferelor dupa modul de hranire
Clasificarea mamiferelor dupa modul de hranire Health and social care values unit 2
Health and social care values unit 2 Duty of care outcome care certificate
Duty of care outcome care certificate Care sunt simturile prin care sunt evocate
Care sunt simturile prin care sunt evocate Polii magnetici de acelasi nume se
Polii magnetici de acelasi nume se Standard 13 health and safety
Standard 13 health and safety Palliative care versus hospice care
Palliative care versus hospice care Carcinoma renal de células claras fuhrman
Carcinoma renal de células claras fuhrman Respiratory alkalosis renal compensation
Respiratory alkalosis renal compensation Rrt aeiou
Rrt aeiou Insuficiencia renal aguda
Insuficiencia renal aguda Braquiencefalica
Braquiencefalica Clasificacion kdigo
Clasificacion kdigo Renal failure
Renal failure Renal portal system
Renal portal system Distal tubule
Distal tubule Infarto
Infarto Ajkd atlas of renal pathology
Ajkd atlas of renal pathology Sistoraji
Sistoraji Renal dysplasia
Renal dysplasia Renal hücreli karsinom
Renal hücreli karsinom Lesion renal aguda
Lesion renal aguda Panchajani
Panchajani Urinary system histology
Urinary system histology Renal
Renal Why does efferent arteriole constriction increased gfr
Why does efferent arteriole constriction increased gfr Oncocitoma renal
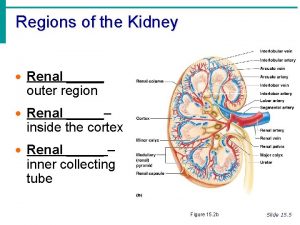
Oncocitoma renal Outer region of the kidney
Outer region of the kidney Parietal layer of bowman's capsule
Parietal layer of bowman's capsule Usually
Usually Cortical nephron
Cortical nephron Bladder male
Bladder male Respiratory alkalosis renal compensation
Respiratory alkalosis renal compensation Dapar
Dapar Kidney function test
Kidney function test Cociente albumina creatinina en orina valores normales
Cociente albumina creatinina en orina valores normales Lovenox renal dosing
Lovenox renal dosing Renal function
Renal function Composition of normal urine
Composition of normal urine Urethral sphincter
Urethral sphincter