RENAL TRANSPLANTATION INTO HIGH RISK HIGHLY SENSITIZED RECIPIENTS

















- Slides: 44

RENAL TRANSPLANTATION INTO HIGH RISK, HIGHLY SENSITIZED RECIPIENTS: A SINGLE CENTER EXPERIENCE Randy Hennigar Ph. D, MD Director, Nephropathology and Electron Microscopy Emory University Hospital Atlanta , GA

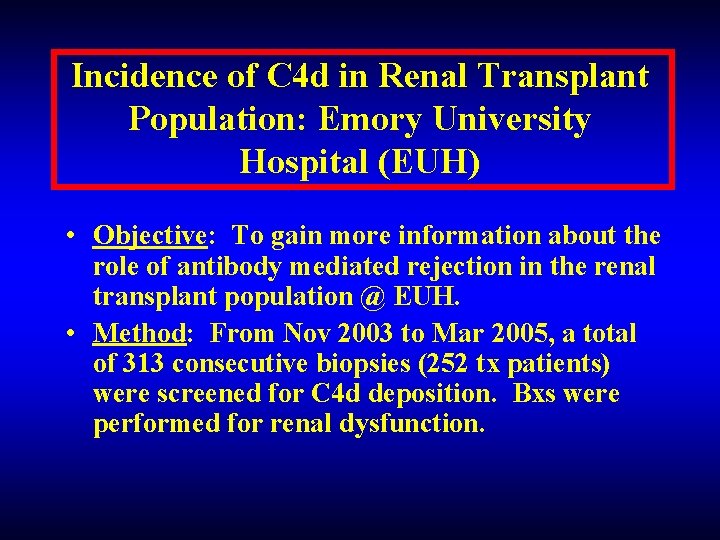
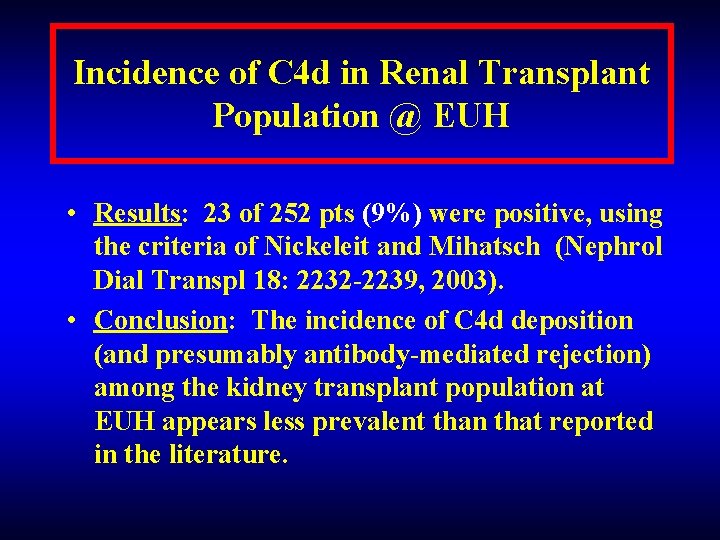
Incidence of C 4 d in Renal Transplant Population: Emory University Hospital (EUH) • Objective: To gain more information about the role of antibody mediated rejection in the renal transplant population @ EUH. • Method: From Nov 2003 to Mar 2005, a total of 313 consecutive biopsies (252 tx patients) were screened for C 4 d deposition. Bxs were performed for renal dysfunction.

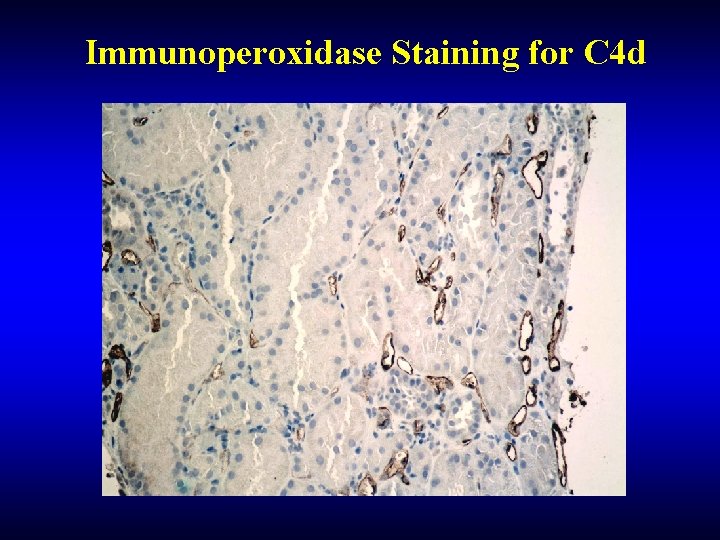
Immunoperoxidase Staining for C 4 d

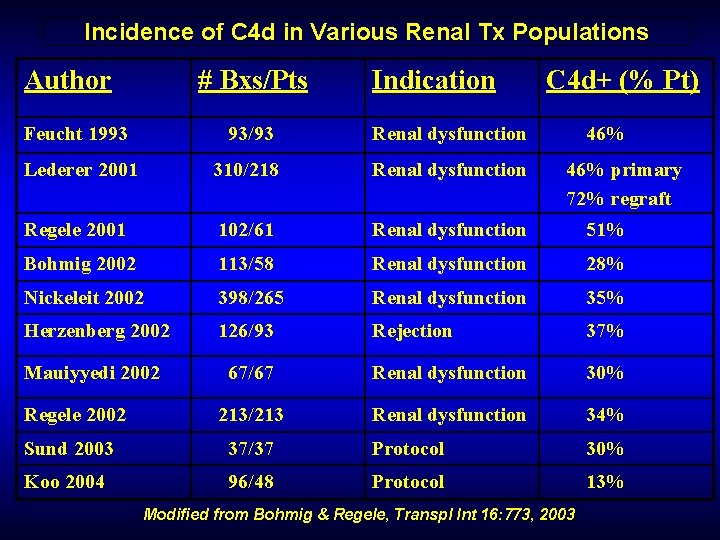
Incidence of C 4 d in Various Renal Tx Populations Author # Bxs/Pts Indication C 4 d+ (% Pt) Feucht 1993 93/93 Renal dysfunction Lederer 2001 310/218 Renal dysfunction Regele 2001 102/61 Renal dysfunction 51% Bohmig 2002 113/58 Renal dysfunction 28% Nickeleit 2002 398/265 Renal dysfunction 35% Herzenberg 2002 126/93 Rejection 37% Mauiyyedi 2002 67/67 Renal dysfunction 30% 213/213 Renal dysfunction 34% Regele 2002 46% primary 72% regraft Sund 2003 37/37 Protocol 30% Koo 2004 96/48 Protocol 13% Modified from Bohmig & Regele, Transpl Int 16: 773, 2003

Incidence of C 4 d in Renal Transplant Population @ EUH • Results: 23 of 252 pts (9%) were positive, using the criteria of Nickeleit and Mihatsch (Nephrol Dial Transpl 18: 2232 -2239, 2003). • Conclusion: The incidence of C 4 d deposition (and presumably antibody-mediated rejection) among the kidney transplant population at EUH appears less prevalent than that reported in the literature.


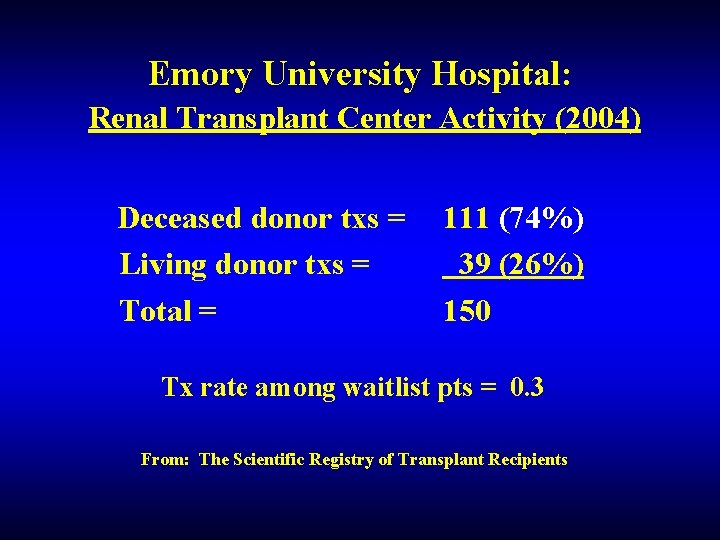
Emory University Hospital: Renal Transplant Center Activity (2004) Deceased donor txs = Living donor txs = Total = 111 (74%) 39 (26%) 150 Tx rate among waitlist pts = 0. 3 From: The Scientific Registry of Transplant Recipients

Emory University Hospital: Transplant Recipient Characteristics (2004) Ethnicity/race of waitlist pts (end of 2004): EUH(%) African-American White Hispanic/Latino Asian Other 63 32 2 3 <1 USA average(%) 36 39 16 8 1 From: The Scientific Registry of Transplant Recipients

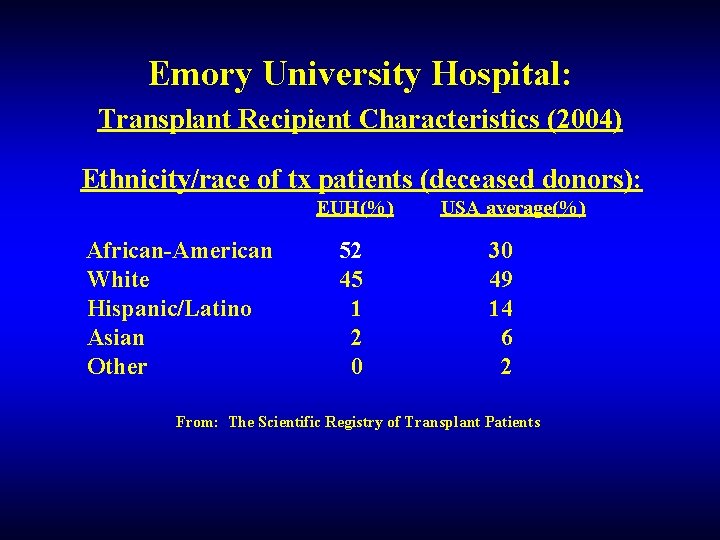
Emory University Hospital: Transplant Recipient Characteristics (2004) Ethnicity/race of tx patients (deceased donors): EUH(%) African-American White Hispanic/Latino Asian Other 52 45 1 2 0 USA average(%) 30 49 14 6 2 From: The Scientific Registry of Transplant Patients

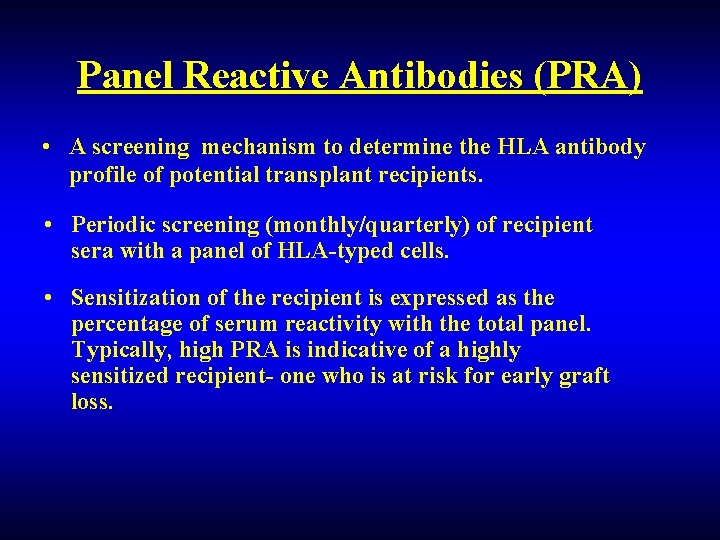
Panel Reactive Antibodies (PRA) • A screening mechanism to determine the HLA antibody profile of potential transplant recipients. • Periodic screening (monthly/quarterly) of recipient sera with a panel of HLA-typed cells. • Sensitization of the recipient is expressed as the percentage of serum reactivity with the total panel. Typically, high PRA is indicative of a highly sensitized recipient- one who is at risk for early graft loss.

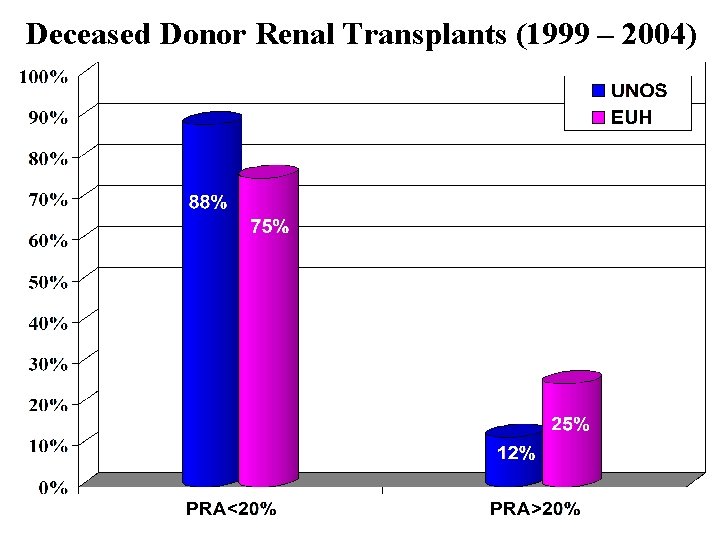
Deceased Donor Renal Transplants (1999 – 2004)

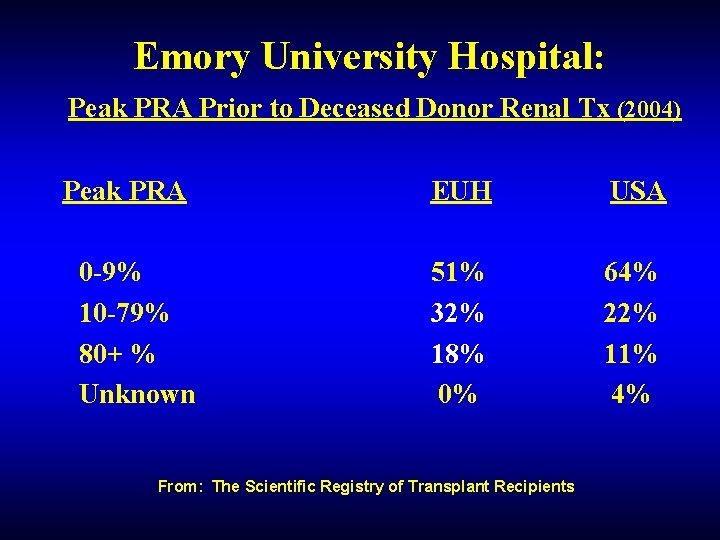
Emory University Hospital: Peak PRA Prior to Deceased Donor Renal Tx (2004) Peak PRA 0 -9% 10 -79% 80+ % Unknown EUH USA 51% 32% 18% 0% 64% 22% 11% 4% From: The Scientific Registry of Transplant Recipients

% Graft Survival Cadaveric Renal Allograft Survival (1998 – 2003) 100 99 90 94 UNOS 97 Emory N = >500 90 93 N = 20791 80 81 70 60 50 0 3 mos UNOS/SRTR 2003 1 Years 2 3



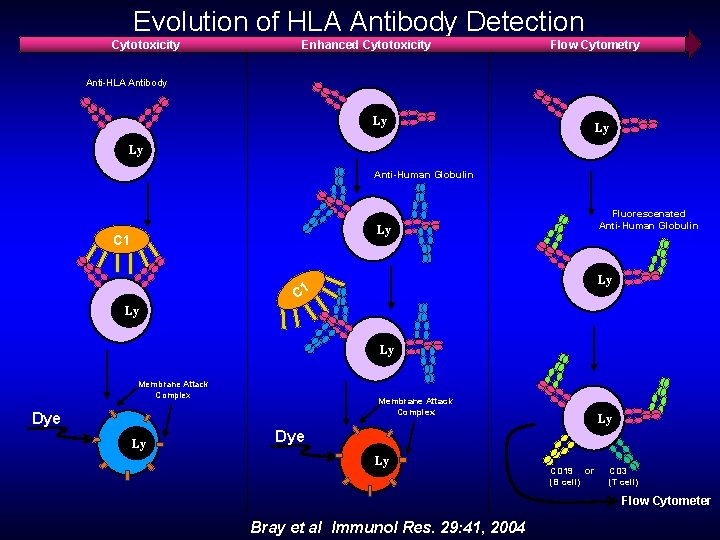
Evolution of HLA Antibody Detection Cytotoxicity Enhanced Cytotoxicity Flow Cytometry Anti-HLA Antibody Ly Ly Ly Anti-Human Globulin Fluorescenated Anti-Human Globulin Ly C 1 Ly Ly Membrane Attack Complex Dye Ly Ly Dye Ly CD 19 or (B cell) CD 3 (T cell) Flow Cytometer Bray et al Immunol Res. 29: 41, 2004

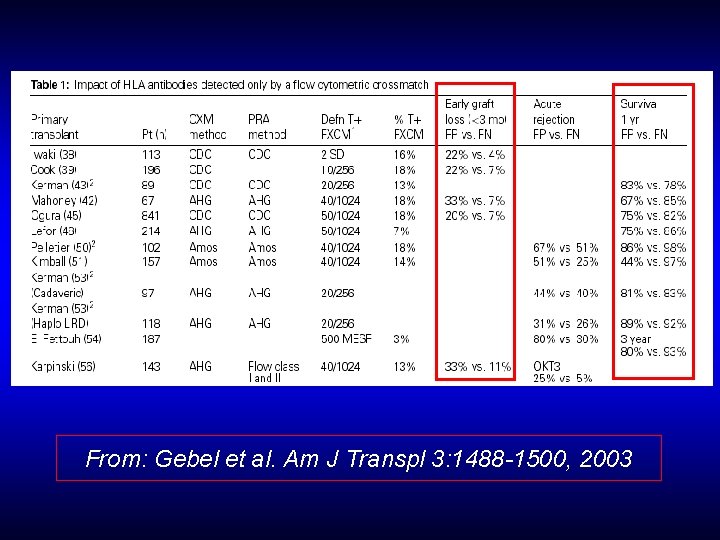
From: Gebel et al. Am J Transpl 3: 1488 -1500, 2003

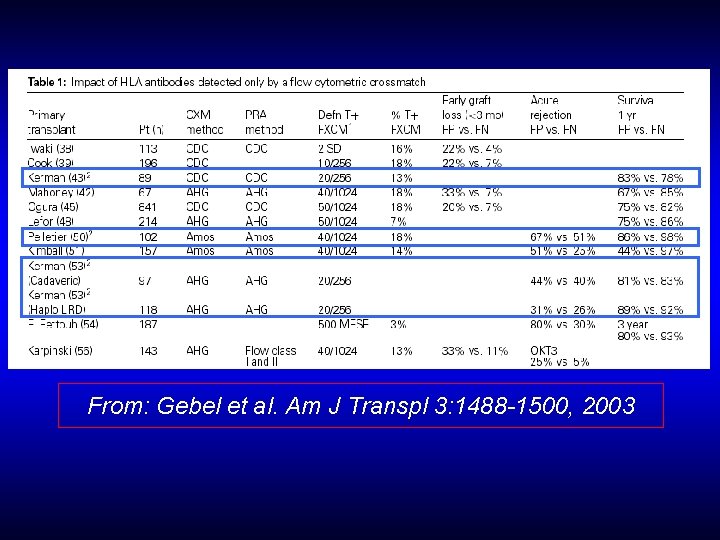
From: Gebel et al. Am J Transpl 3: 1488 -1500, 2003

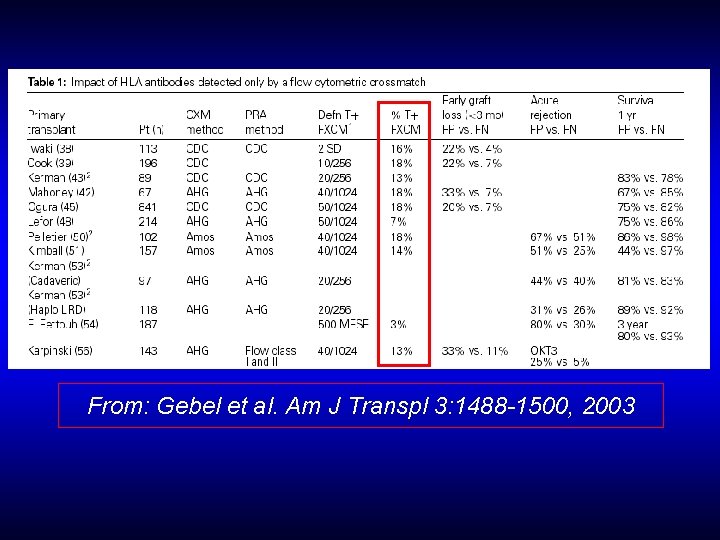
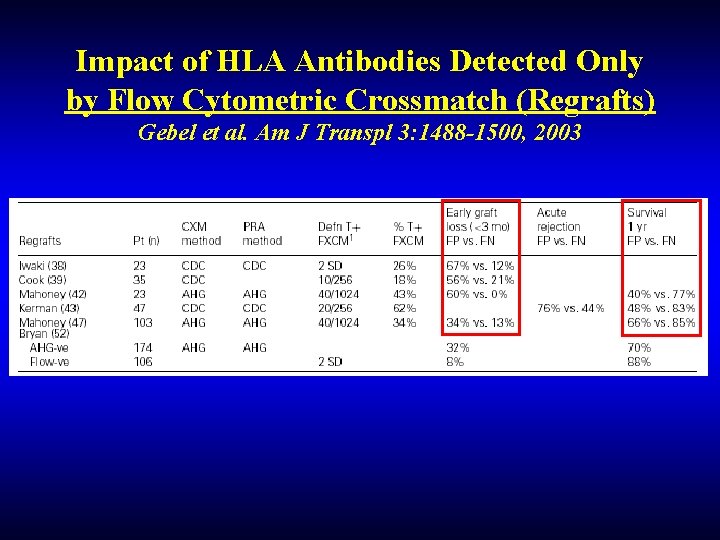
Impact of HLA Antibodies Detected Only by Flow Cytometric Crossmatch (Regrafts) Gebel et al. Am J Transpl 3: 1488 -1500, 2003

In 2002, of the >150 labs participating in the ASHI-CAP class I crossmatch surveys (MX 1 -A, B, C), only 68– 70% reported AHG augmented CDC and 47– 52% flow-based crossmatches.

From: Gebel et al. Am J Transpl 3: 1488 -1500, 2003

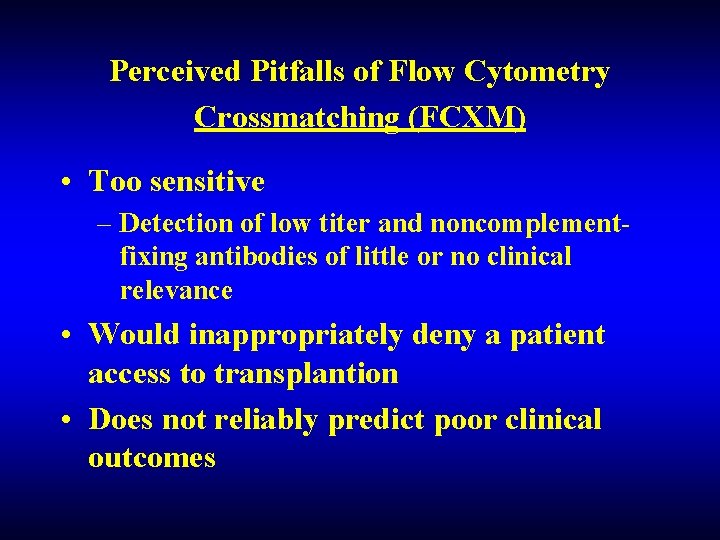
Perceived Pitfalls of Flow Cytometry Crossmatching (FCXM) • Too sensitive – Detection of low titer and noncomplementfixing antibodies of little or no clinical relevance • Would inappropriately deny a patient access to transplantion • Does not reliably predict poor clinical outcomes

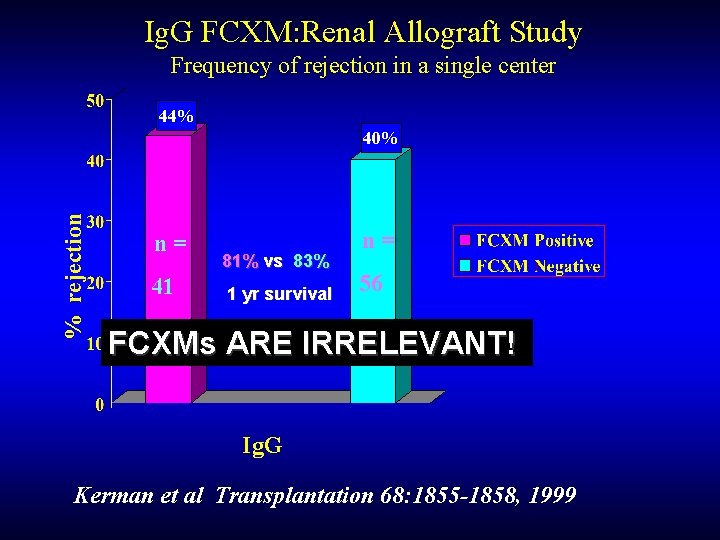
Ig. G FCXM: Renal Allograft Study Frequency of rejection in a single center 44% % rejection 40% n= 41 81% vs 83% 1 yr survival n= 56 FCXMs ARE IRRELEVANT! Ig. G Kerman et al Transplantation 68: 1855 -1858, 1999

In 2002, of the >150 labs participating in the ASHI-CAP class I crossmatch surveys (MX 1 -A, B, C), only 68– 70% reported AHG augmented CDC and 47– 52% flow-based crossmatches.

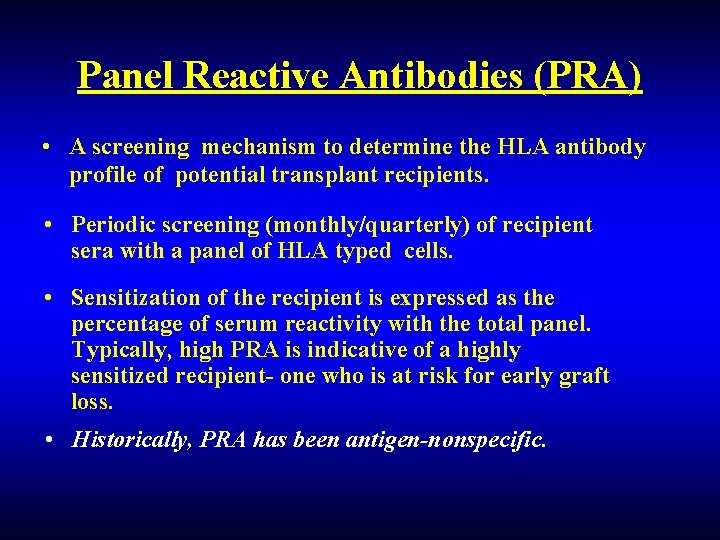
Panel Reactive Antibodies (PRA) • A screening mechanism to determine the HLA antibody profile of potential transplant recipients. • Periodic screening (monthly/quarterly) of recipient sera with a panel of HLA typed cells. • Sensitization of the recipient is expressed as the percentage of serum reactivity with the total panel. Typically, high PRA is indicative of a highly sensitized recipient- one who is at risk for early graft loss. • Historically, PRA has been antigen-nonspecific.

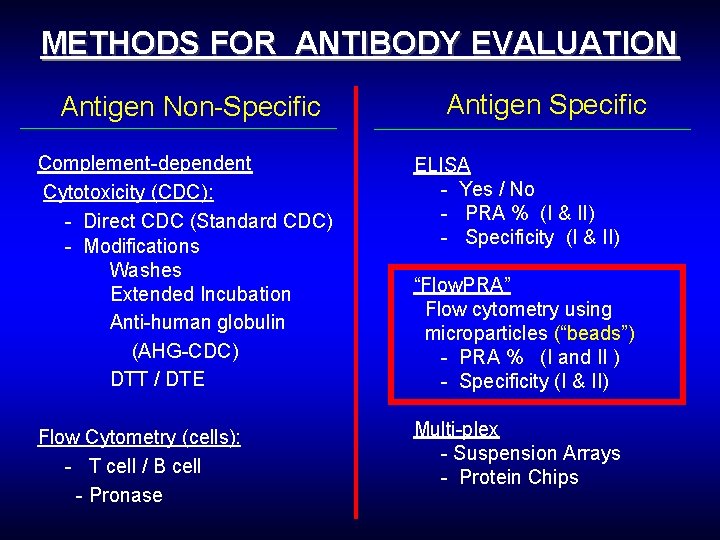
METHODS FOR ANTIBODY EVALUATION Antigen Non-Specific Antigen Specific Complement-dependent Cytotoxicity (CDC): - Direct CDC (Standard CDC) - Modifications Washes Extended Incubation Anti-human globulin (AHG-CDC) DTT / DTE ELISA - Yes / No - PRA % (I & II) - Specificity (I & II) Flow Cytometry (cells): - T cell / B cell - Pronase Multi-plex - Suspension Arrays - Protein Chips “Flow. PRA” Flow cytometry using microparticles (“beads”) - PRA % (I and II ) - Specificity (I & II)

Flow Microparticles One Lambda www. onelambda. com

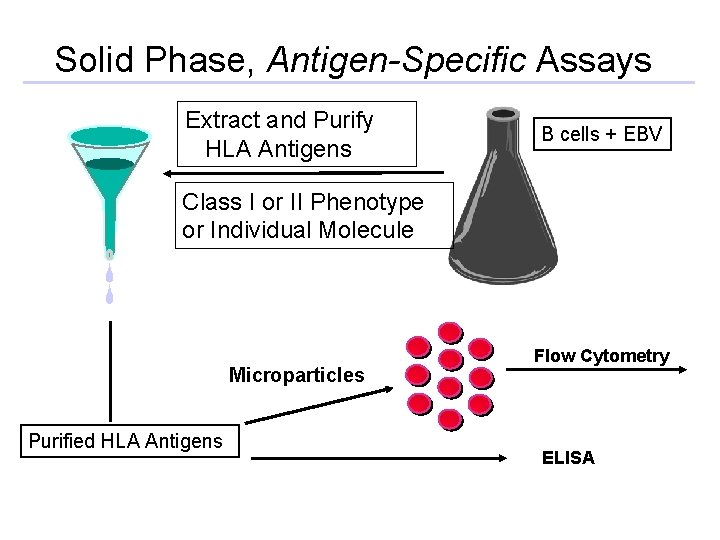
Solid Phase, Antigen-Specific Assays Extract and Purify HLA Antigens B cells + EBV Class I or II Phenotype or Individual Molecule Microparticles Purified HLA Antigens Flow Cytometry ELISA

Microparticles ELISA Coated with 30 HLA I or 30 HLA II antigens 90%

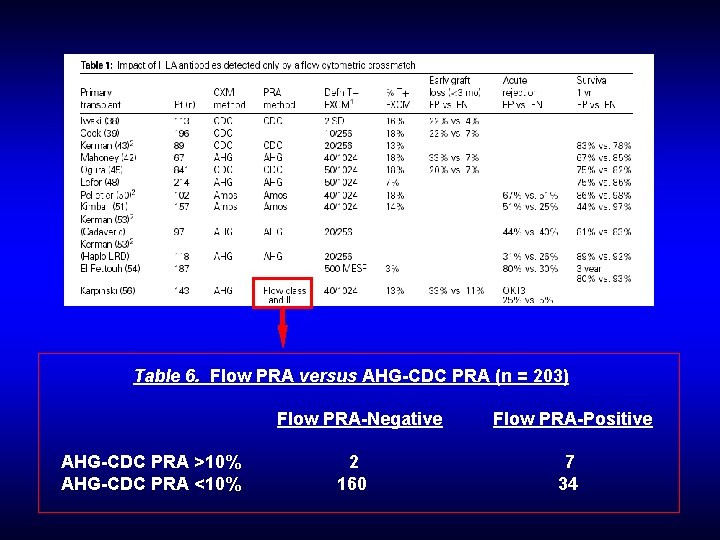
Table 6. Flow PRA versus AHG-CDC PRA (n = 203) Flow PRA-Negative AHG-CDC PRA >10% AHG-CDC PRA <10% 2 160 Flow PRA-Positive 7 34

PRA ANALYSIS BY DIFFERING METHODLOGIES POSITIVE NEGATIVE CDC 102 162 AHG-CDC 116 (+13%) 148 ELISA 127 (+10%) 137 Flow. PRA 139 (+10%) 125 Gebel and Bray, Transplantation 69: 1370 -1374, 2000.

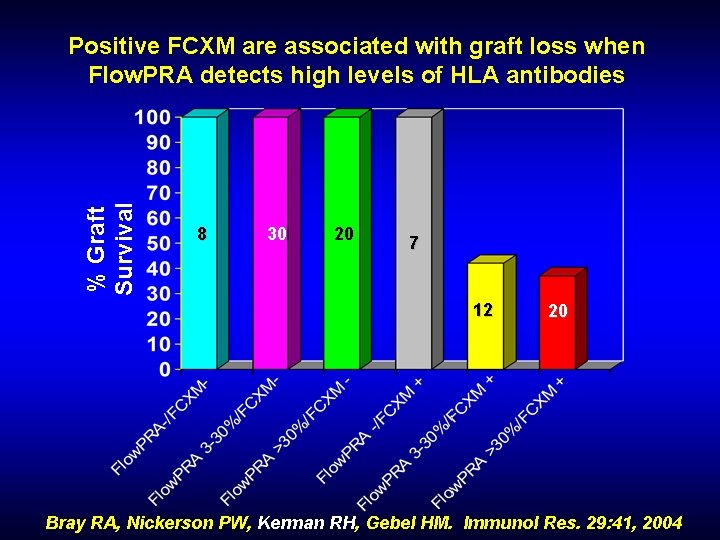
% Graft Survival Positive FCXM are associated with graft loss when Flow. PRA detects high levels of HLA antibodies 8 30 20 7 12 20 Bray RA, Nickerson PW, Kerman RH, Gebel HM. Immunol Res. 29: 41, 2004

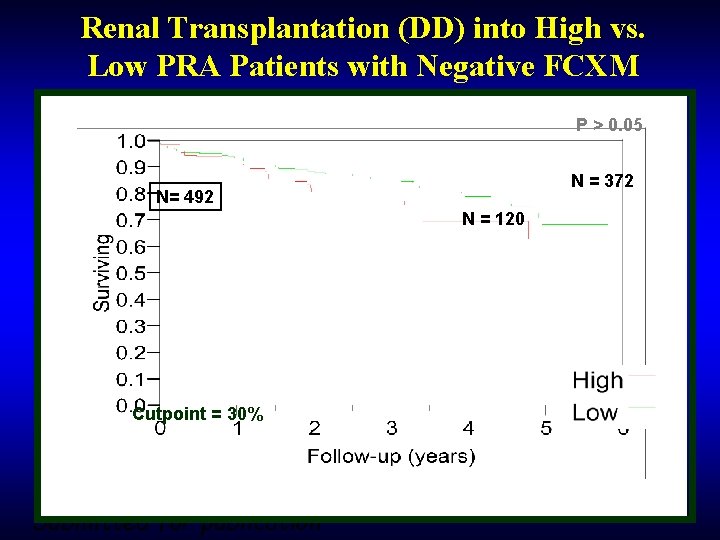
Renal Transplantation (DD) into High vs. Low PRA Patients with Negative FCXM P > 0. 05 N = 372 N= 492 N = 120 Cutpoint = 30% Submitted for publication

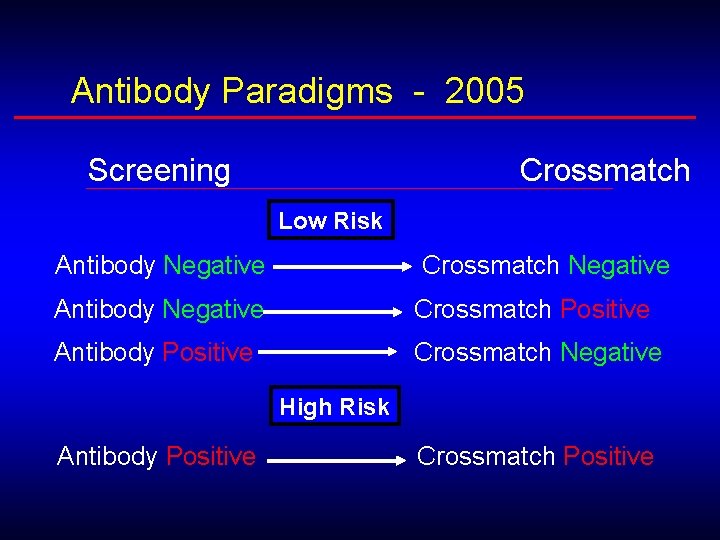
Antibody Paradigms - 2005 Screening Crossmatch Low Risk Antibody Negative Crossmatch Negative Antibody Negative Crossmatch Positive Antibody Positive Crossmatch Negative High Risk Antibody Positive Crossmatch Positive

PRA • PRA can be a qualitative and/or quantitative assessment of alloimmunization in transplant patients. • Optimally, PRA testing should identify the specificity of an antibody and provide the “transplantability” index of a patient. • More succinctly, PRA testing should correlate with the final crossmatch.

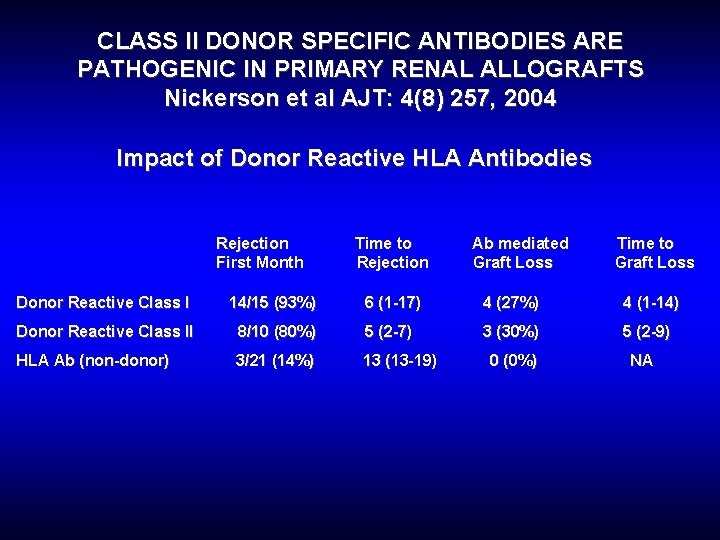
CLASS II DONOR SPECIFIC ANTIBODIES ARE PATHOGENIC IN PRIMARY RENAL ALLOGRAFTS Nickerson et al AJT: 4(8) 257, 2004 Impact of Donor Reactive HLA Antibodies Rejection First Month Time to Rejection Ab mediated Graft Loss Time to Graft Loss Donor Reactive Class I 14/15 (93%) 6 (1 -17) 4 (27%) 4 (1 -14) Donor Reactive Class II 8/10 (80%) 5 (2 -7) 3 (30%) 5 (2 -9) HLA Ab (non-donor) 3/21 (14%) 13 (13 -19) 0 (0%) NA

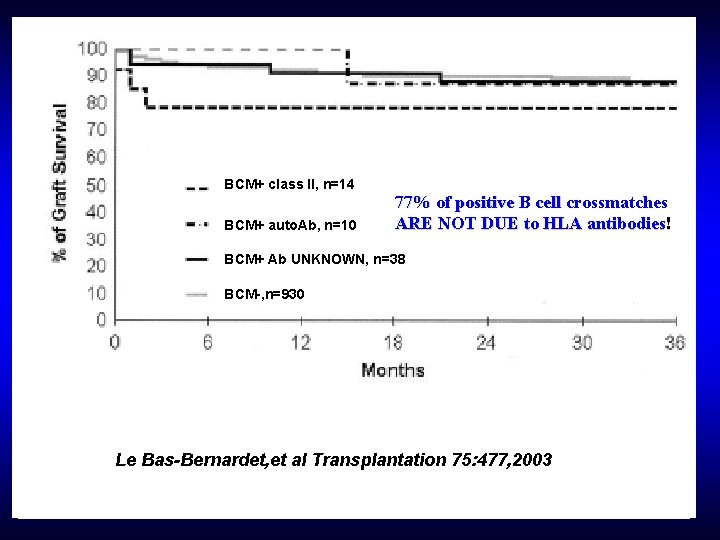
BCM+ class II, n=14 BCM+ auto. Ab, n=10 77% of positive B cell crossmatches ARE NOT DUE to HLA antibodies! BCM+ Ab UNKNOWN, n=38 BCM-, n=930 Le Bas-Bernardet, et al Transplantation 75: 477, 2003

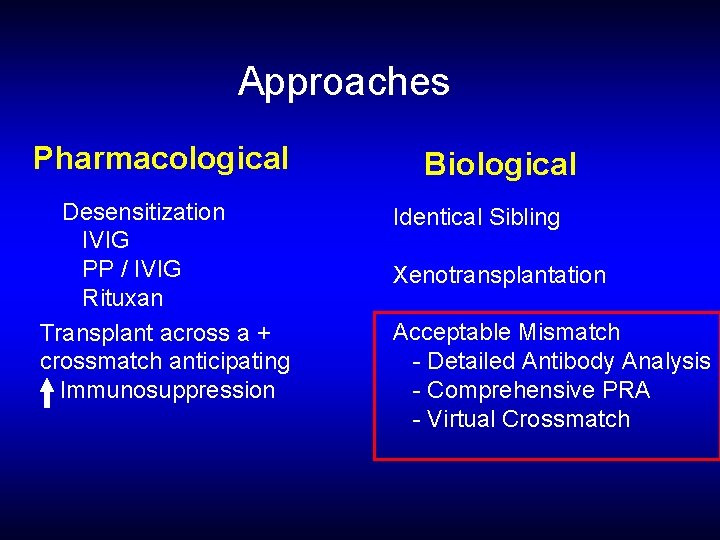
Approaches Pharmacological Desensitization IVIG PP / IVIG Rituxan Transplant across a + crossmatch anticipating Immunosuppression Biological Identical Sibling Xenotransplantation Acceptable Mismatch - Detailed Antibody Analysis - Comprehensive PRA - Virtual Crossmatch

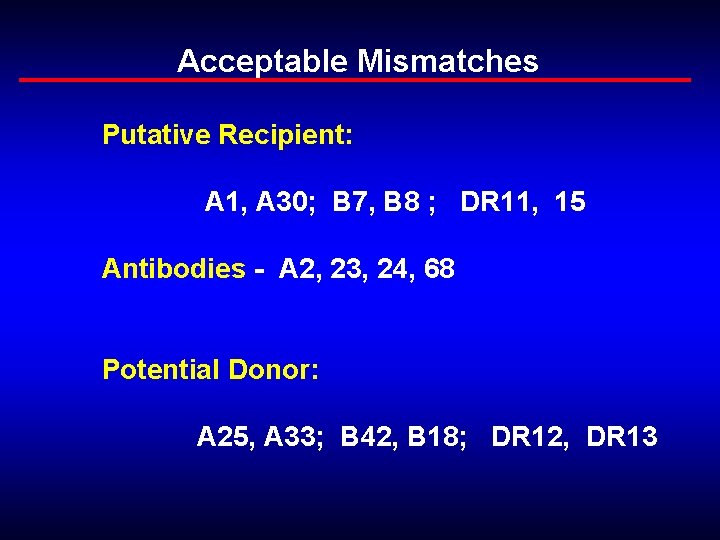
Acceptable Mismatches Putative Recipient: A 1, A 30; B 7, B 8 ; DR 11, 15 Antibodies - A 2, 23, 24, 68 Potential Donor: A 25, A 33; B 42, B 18; DR 12, DR 13

Strategic Approaches - Based on recognition that matching is not for everyone- 85% of DD Txs are mismatched. - Focus on appropriate mismatching rather than looking for an HLA “match”. - Requires detailed evaluation of the patient’s HLA antibodies. - Shifts emphasis to antibody evaluation and away from crossmatching to identify acceptable mismatches.

Desensitization Protocols Aren’t For Everyone - High Titer HLA Antibodies >512 - Refractory Specificities DR 52, DR 53 - Fragile Patients - Restricted to Living Donors - $$$$$$s

Recommendations to define the ‘non-sensitized’ patient: • Validate patient history for the lack of sensitizing events. • Confirm that a patient is nonsensitized using a solid phase assay documented to be more sensitive than CDC assays.

Recommendations to evaluate the ‘sensitized’ patient: • To optimize detection of low titer HLA antibodies, monitoring should be performed using sensitive solid-phase assays. • Monitoring should include evaluation for both antibodies to class I and class II HLA antigens. • A crossmatch test must be performed before transplantation using, as a minimum, an enhanced CDC technique. • The final crossmatch technique should be of equal sensitivity to the solid-phase assay used to screen for the presence of HLA antibody. • A B-cell crossmatch should be included in the final crossmatch. • Peak sera should be included in the final crossmatch. • Auto-crossmatches should be utilized to aid in the interpretation of allo-crossmatches.

END OF LECTURE
 Res extra commercium
Res extra commercium Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Renal corpuscle
Renal corpuscle Traer familiar command
Traer familiar command Ppp
Ppp Holder of sensitized material
Holder of sensitized material Bone marrow transplantation sri lanka
Bone marrow transplantation sri lanka Law of transplantation
Law of transplantation Patrick evrard transplantation
Patrick evrard transplantation Cultural transplantation examples
Cultural transplantation examples Types of transplant
Types of transplant Stem cell or bone marrow transplantation thailand
Stem cell or bone marrow transplantation thailand Market risk credit risk operational risk
Market risk credit risk operational risk Investasi adalah
Investasi adalah Risk projection in software engineering
Risk projection in software engineering Risk mitigation avoidance
Risk mitigation avoidance How to calculate relative risk
How to calculate relative risk Residual risk and secondary risk pmp
Residual risk and secondary risk pmp Inherent risks examples
Inherent risks examples Absolute risk vs relative risk
Absolute risk vs relative risk Activity sheet 1 how diversified are these portfolios
Activity sheet 1 how diversified are these portfolios Medium term risk
Medium term risk Risk financing transfer adalah
Risk financing transfer adalah The biggest risk is not taking any risk
The biggest risk is not taking any risk Key risk indicators financial risk management
Key risk indicators financial risk management Ir x cr x dr
Ir x cr x dr Business vs financial risk
Business vs financial risk Relative risk and attributable risk
Relative risk and attributable risk Risk map
Risk map Relative risk and attributable risk
Relative risk and attributable risk A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c The 8 habits by stephen covey
The 8 habits by stephen covey 7 habits habit 2
7 habits habit 2 Ten habits of highly effective people
Ten habits of highly effective people Highly qualified teacher michigan
Highly qualified teacher michigan Maturity continuum model victories by stephen
Maturity continuum model victories by stephen Closed question and open question
Closed question and open question Profilmate
Profilmate Who do you think are people media
Who do you think are people media Statternly
Statternly The competent manager
The competent manager Fine clothes to the jew poem
Fine clothes to the jew poem The prodigal bishop analysis
The prodigal bishop analysis 7 defective habits
7 defective habits 7 habits of highly effective teens lessons
7 habits of highly effective teens lessons