Chromatography and Instrumentation in Organic Chemistry 1 Chromatography














- Slides: 28

Chromatography and Instrumentation in Organic Chemistry

1. Chromatography • The word chromatography comes from two Greek words which mean “colour writing” • It was invented by a Russian botanist Mikhail Twsett in 1903 (separating pigments in plants) • Chromatography now widely used to separate substances in a mixture • Also used to measure the amounts of substances in a mixture

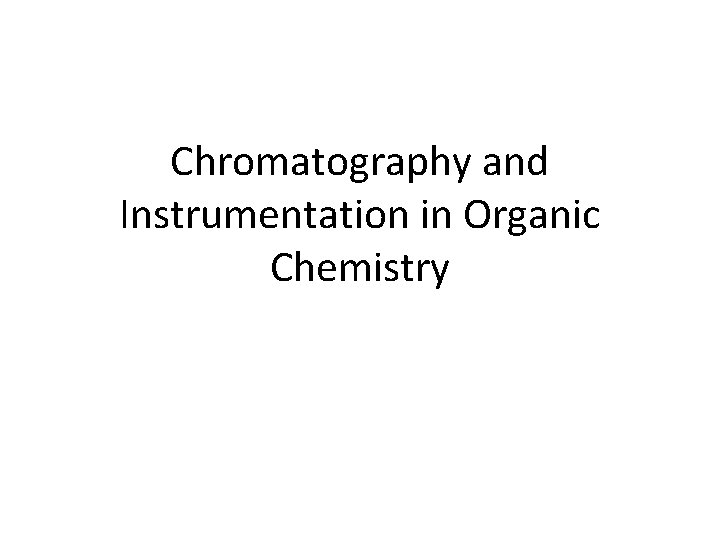
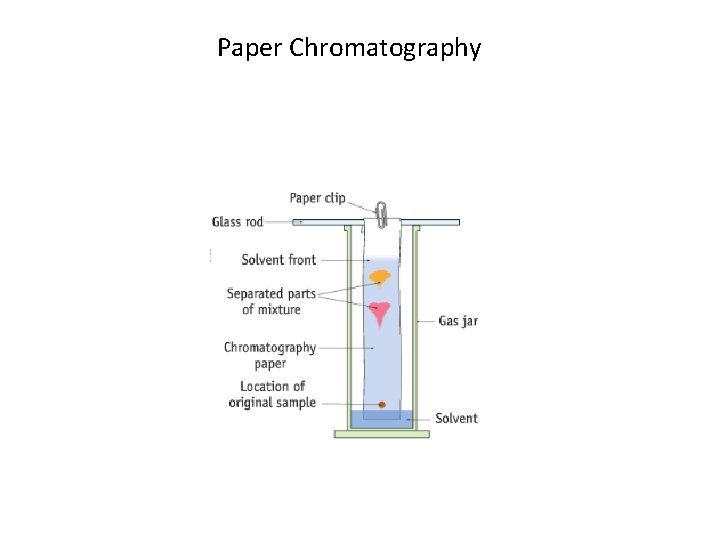
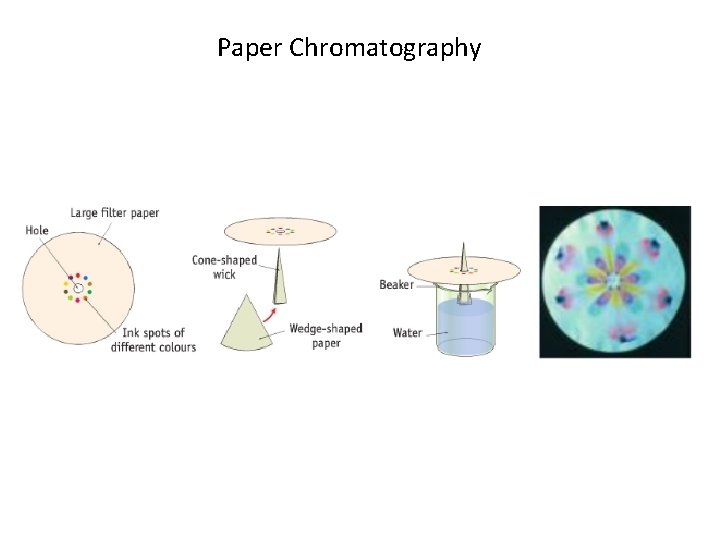
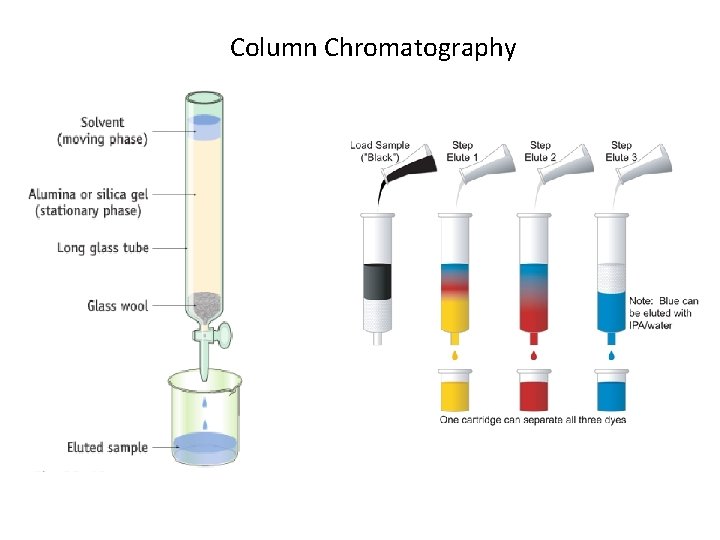
1. Chromatography • In chromatography, a mobile phase carries substances in a mixture at different rates through a stationary phase • Examples of mobile phase: water, ethanol • Examples of stationary phase: paper, aluminium oxide Chromatography = a separation technique in which a mobile phase carrying a mixture moves in contact with a selectively adsorbent stationary phase

Paper Chromatography

Paper Chromatography

Thin-layer Chromatography (TLC)

Column Chromatography

Column Chromatography

Column Chromatography

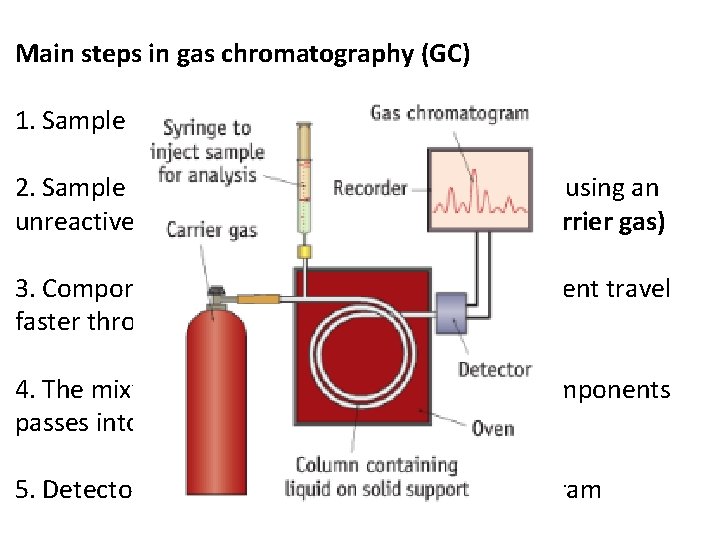
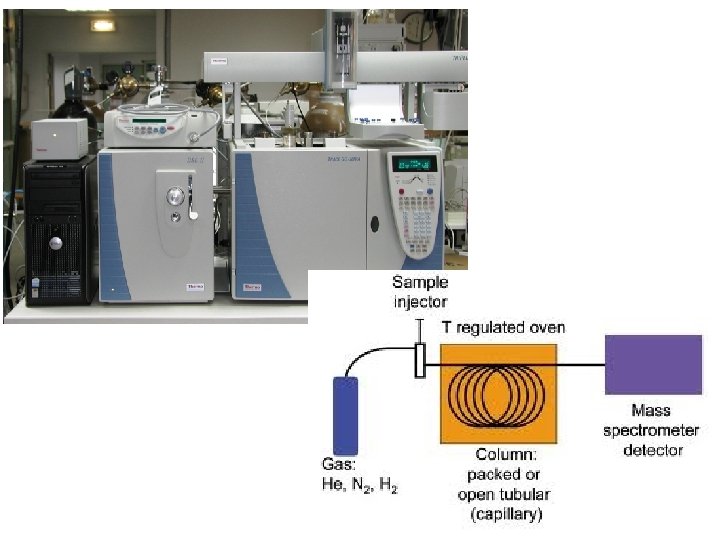
Chromatography in industrial laboratories 1. Gas chromatography (GC) • Stationary phase = high boiling point liquid (eg. Longchain alkane) spread on solid particles (eg. silica gel) • Mobile phase = a gas • Both are packed into a long, coiled tube called a column • The column is several metres in length • Column is inside an oven


Main steps in gas chromatography (GC) 1. Sample is injected using syringe 2. Sample is vaporised and carried through tube using an unreactive gas eg. Nitrogen, helium or argon (carrier gas) 3. Components that are more soluble in the solvent travel faster through the tube 4. The mixture is separated and the separate components passes into a detector 5. Detector plots a chart called a gas chromatogram

• GC is used to measure alcohol in blood and urine samples • Also used to analyse drug-test samples from athletes

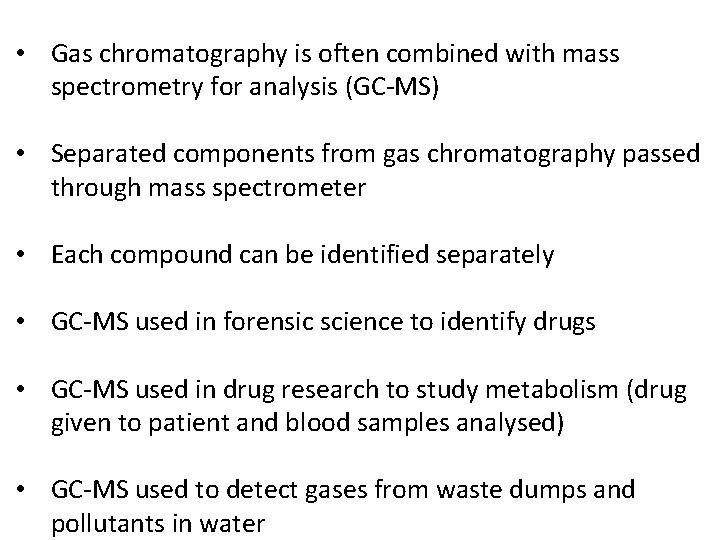
• Gas chromatography is often combined with mass spectrometry for analysis (GC-MS) • Separated components from gas chromatography passed through mass spectrometer • Each compound can be identified separately • GC-MS used in forensic science to identify drugs • GC-MS used in drug research to study metabolism (drug given to patient and blood samples analysed) • GC-MS used to detect gases from waste dumps and pollutants in water

• GC-MS even used on Mars!


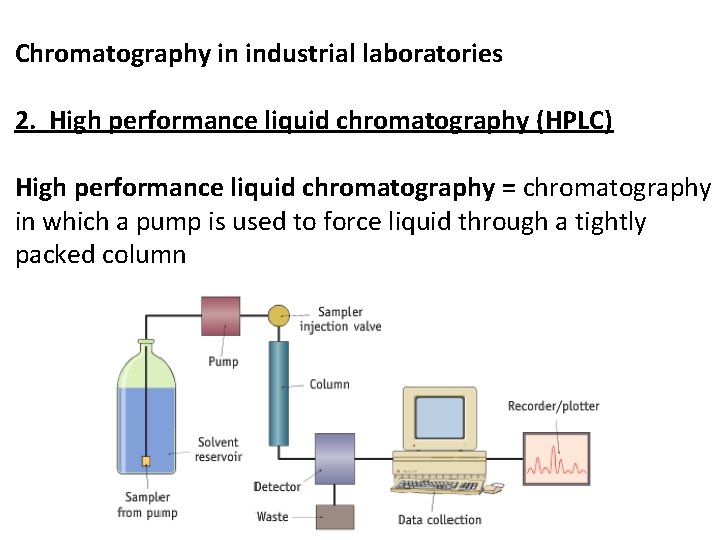
Chromatography in industrial laboratories 2. High performance liquid chromatography (HPLC) • Mobile phase = liquid • Stationary phase = small solid particles • Better separation occurs when particles in stationary phase are very small • Pump needed to force liquid through column

Chromatography in industrial laboratories 2. High performance liquid chromatography (HPLC) High performance liquid chromatography = chromatography in which a pump is used to force liquid through a tightly packed column

Chromatography in industrial laboratories 2. High performance liquid chromatography (HPLC) • HPLC column is shorter than GC column because separation is more efficient • HPLC column only 10 – 30 cm long • HPLC column made of stainless steel to withstand pressure • HPLC uses lower temperature than GC so can analyse substances that would be damaged by higher temperatures • HPLC used to analyse foods

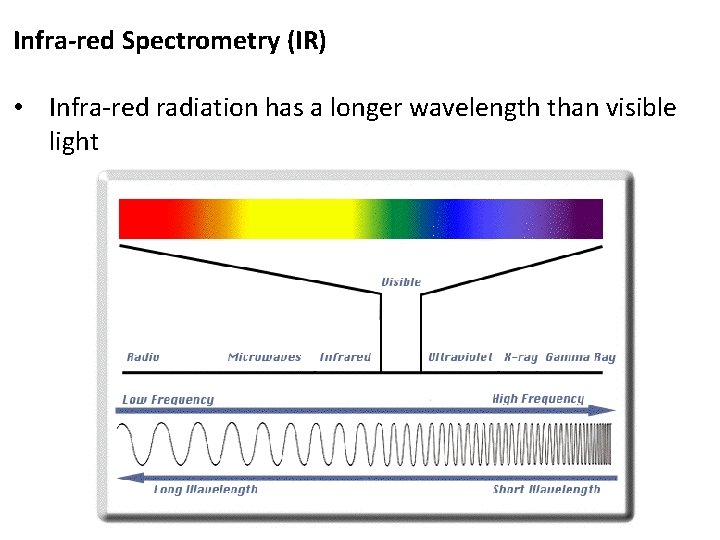
Infra-red Spectrometry (IR) • Infra-red radiation has a longer wavelength than visible light

Infra-red Spectrometry (IR) • Infra-red radiation is invisible but given off by hot objects

Infra-red Spectrometry (IR) • Organic compounds absorb infra-red radiation • Infra-red radiation absorbed by the vibrations of the bonds of molecules Eg. A C=O bond will absorb a different frequency of infra-red radiation to an O-H bond • Every organic compound has its own IR spectrum



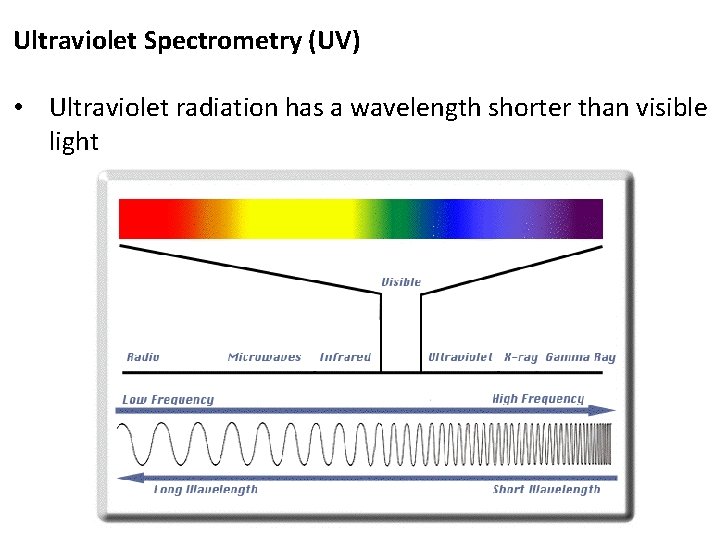
Ultraviolet Spectrometry (UV) • Ultraviolet radiation has a wavelength shorter than visible light

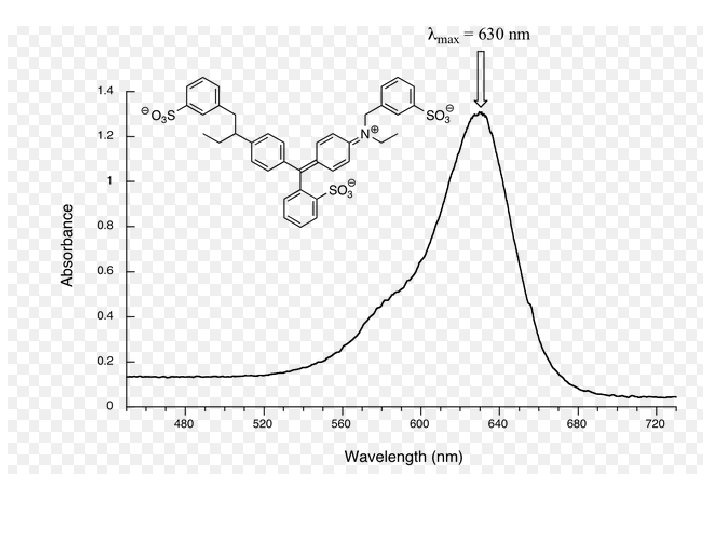
Ultraviolet Spectrometry (UV) • UV spectrometry analyses how different compounds absorb UV light • UV spectrometry used to detect the presence of particular functional groups in molecules • Also used to measure the concentration of substances in solution eg. Drugs


 Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Affinity chromatography instrumentation
Affinity chromatography instrumentation Gel chromatography instrumentation
Gel chromatography instrumentation Difference between fats and oil
Difference between fats and oil Structure and bonding in organic chemistry
Structure and bonding in organic chemistry Organic synthesis via enolates
Organic synthesis via enolates Father of organic chemistry
Father of organic chemistry Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry C10h22oh
C10h22oh Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Pericyclic
Pericyclic David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Ario practice problems
Ario practice problems Functional group vs substituent
Functional group vs substituent Organic chemistry lab report format
Organic chemistry lab report format Alkane organic chemistry
Alkane organic chemistry Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Introduction to organic chemistry
Introduction to organic chemistry Organic chemistry wade
Organic chemistry wade Ethyl methyl chart
Ethyl methyl chart How is cracking done
How is cracking done Organic biochemistry
Organic biochemistry Organic chemistry myanmar
Organic chemistry myanmar


















































