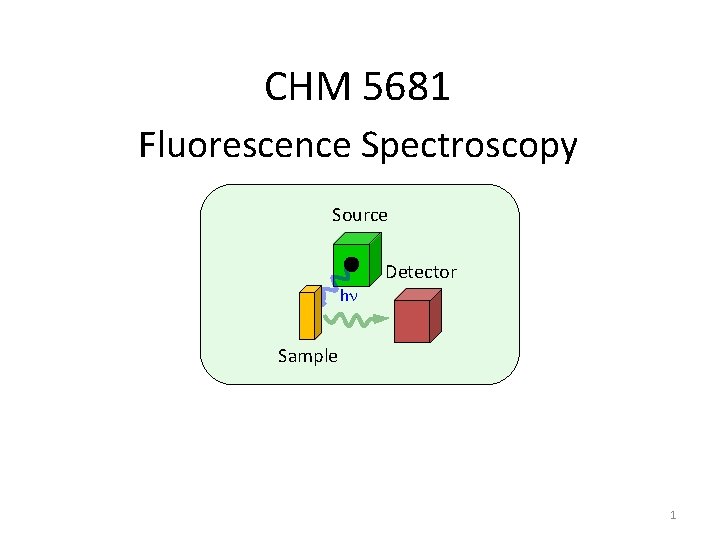
CHM 5681 Fluorescence Spectroscopy Source Detector hn Sample
![Emissive Molecules Phosphorescent Fluorescent Perylene OEP Pt. OEP Ir(ppy)3 BODIPY Fluorescein Rose Bengal [Ru(bpy)3]2+ Emissive Molecules Phosphorescent Fluorescent Perylene OEP Pt. OEP Ir(ppy)3 BODIPY Fluorescein Rose Bengal [Ru(bpy)3]2+](https://slidetodoc.com/presentation_image_h/22860962121d1875163ba66b0abe0a66/image-20.jpg)







- Slides: 74

CHM 5681 Fluorescence Spectroscopy Source Detector hn Sample 1

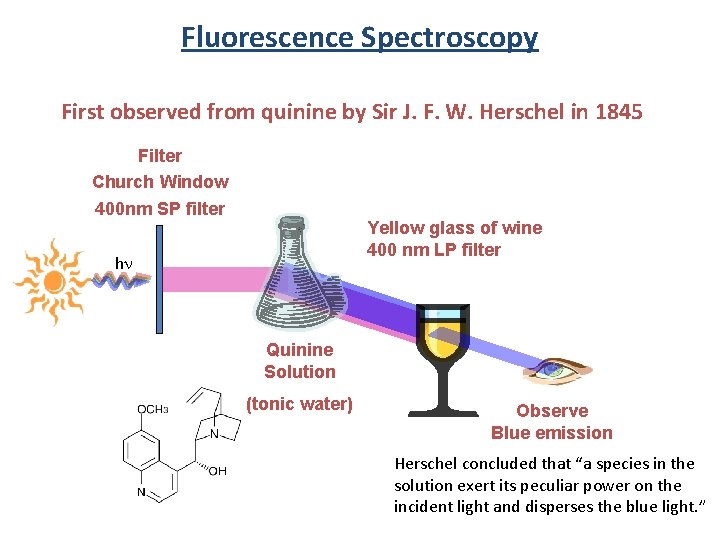
Fluorescence Spectroscopy First observed from quinine by Sir J. F. W. Herschel in 1845 Filter Church Window 400 nm SP filter Yellow glass of wine 400 nm LP filter hn Quinine Solution (tonic water) Observe Blue emission Herschel concluded that “a species in the solution exert its peculiar power on the incident light and disperses the blue light. ”

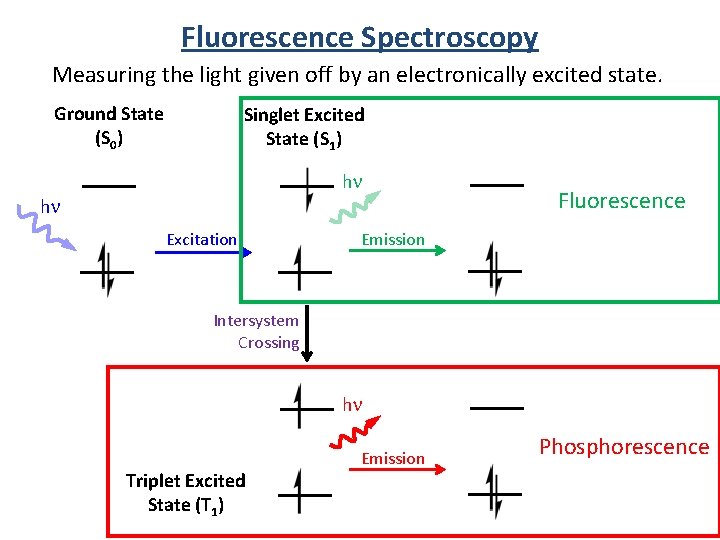
Fluorescence Spectroscopy Measuring the light given off by an electronically excited state. Ground State (S 0) Singlet Excited State (S 1) hn hn Excitation Fluorescence Emission Intersystem Crossing hn Triplet Excited State (T 1) Emission Phosphorescence

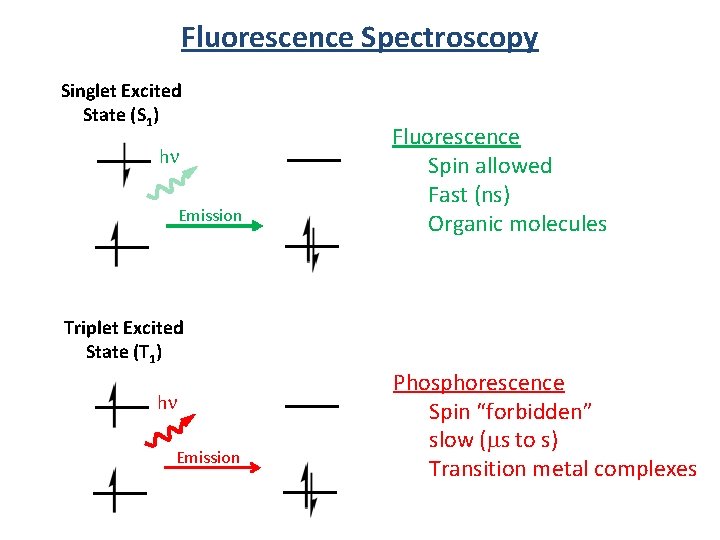
Fluorescence Spectroscopy Singlet Excited State (S 1) hn Emission Fluorescence Spin allowed Fast (ns) Organic molecules Triplet Excited State (T 1) hn Emission Phosphorescence Spin “forbidden” slow (ms to s) Transition metal complexes

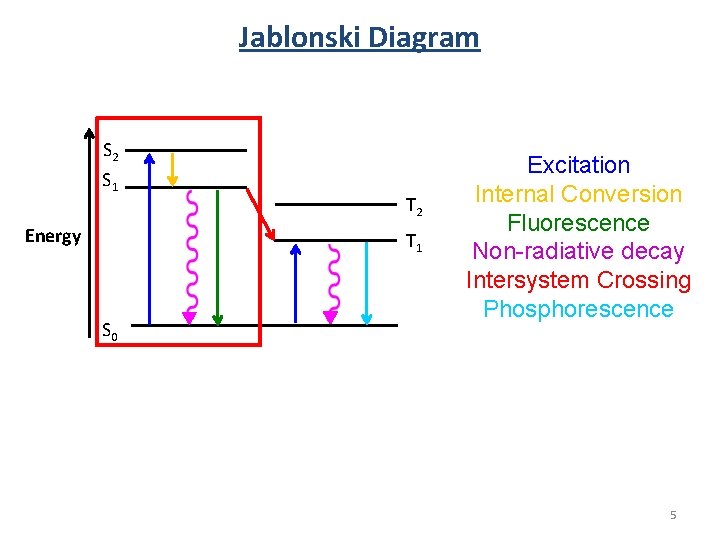
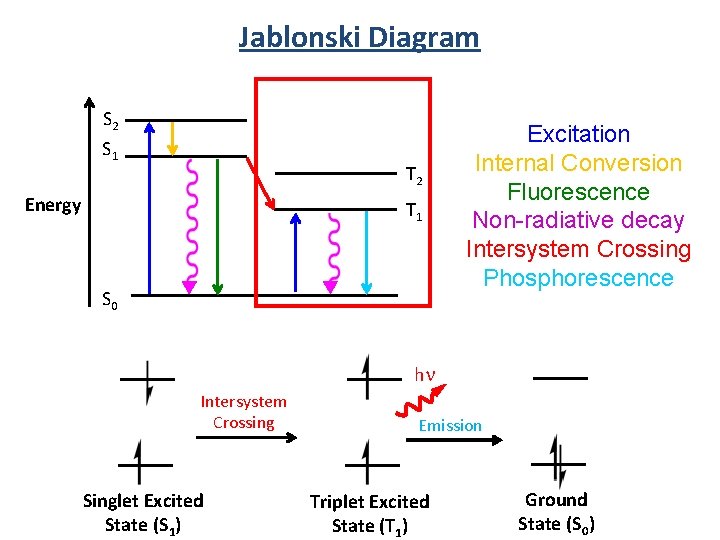
Jablonski Diagram S 2 S 1 Energy T 2 T 1 S 0 Excitation Internal Conversion Fluorescence Non-radiative decay Intersystem Crossing Phosphorescence 5

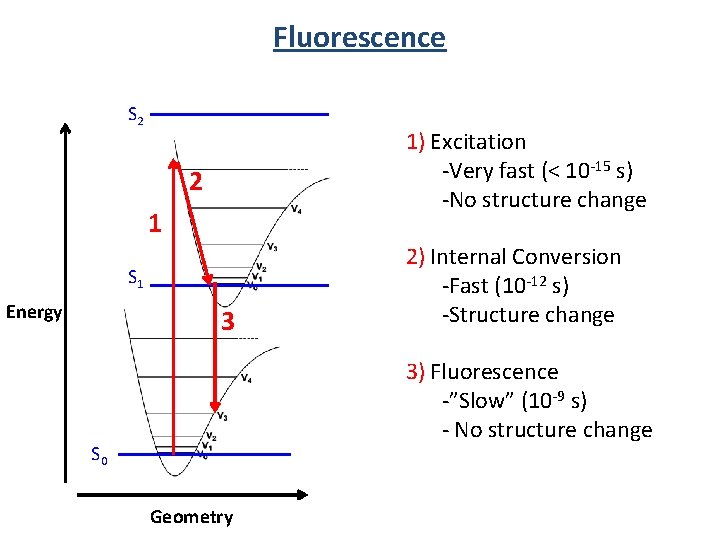
Fluorescence S 2 1) Excitation -Very fast (< 10 -15 s) -No structure change 2 1 S 1 Energy 3 2) Internal Conversion -Fast (10 -12 s) -Structure change 3) Fluorescence -”Slow” (10 -9 s) - No structure change S 0 Geometry

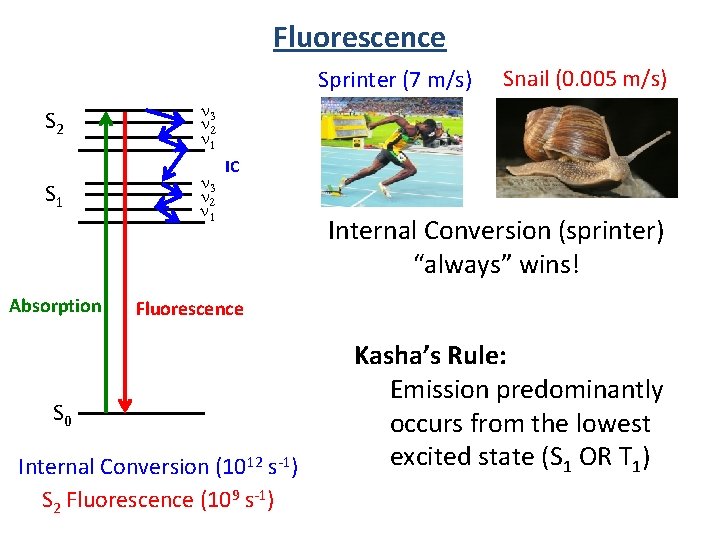
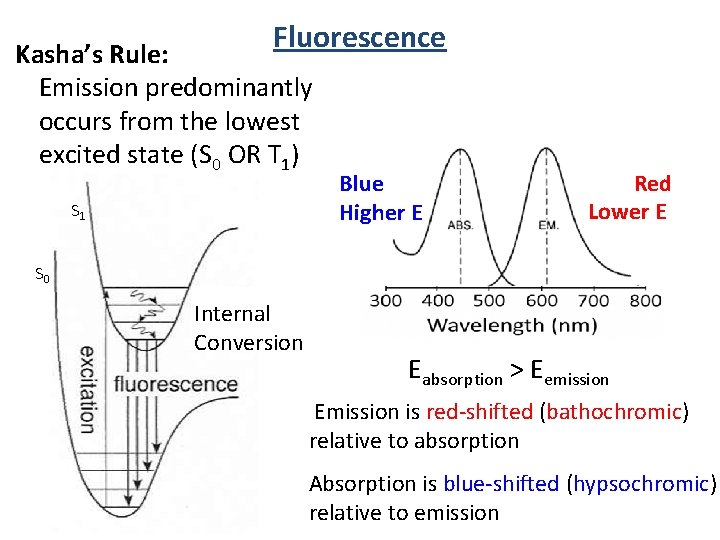
Fluorescence Sprinter (7 m/s) S 2 n 3 n 2 n 1 S 1 n 3 n 2 n 1 Absorption Snail (0. 005 m/s) IC Internal Conversion (sprinter) “always” wins! Fluorescence S 0 Internal Conversion (1012 s-1) S 2 Fluorescence (109 s-1) Kasha’s Rule: Emission predominantly occurs from the lowest excited state (S 1 OR T 1)

Fluorescence 1920 -2013 Kasha Laboratory Building AKA Institute of Molecular Biophysics Kasha’s Rule: Emission predominantly occurs from the lowest excited state (S 0 OR T 1)

Fluorescence Kasha’s Rule: Emission predominantly occurs from the lowest excited state (S 0 OR T 1) S 1 Blue Higher E Red Lower E S 0 Internal Conversion Eabsorption > Eemission Emission is red-shifted (bathochromic) relative to absorption Absorption is blue-shifted (hypsochromic) relative to emission

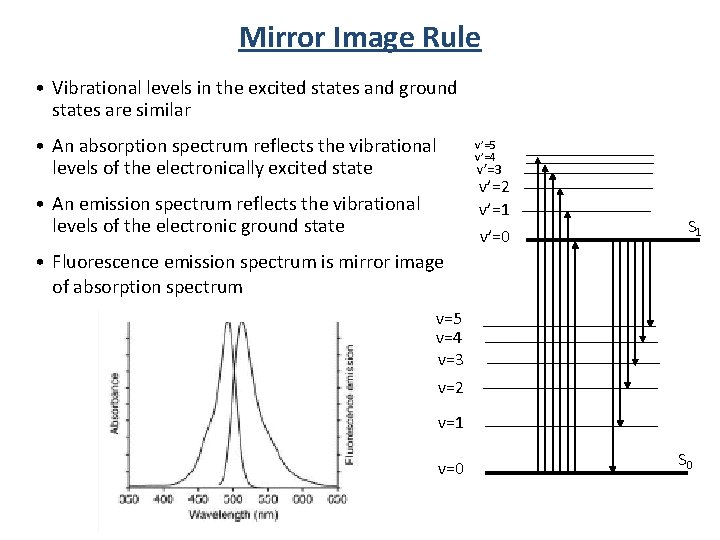
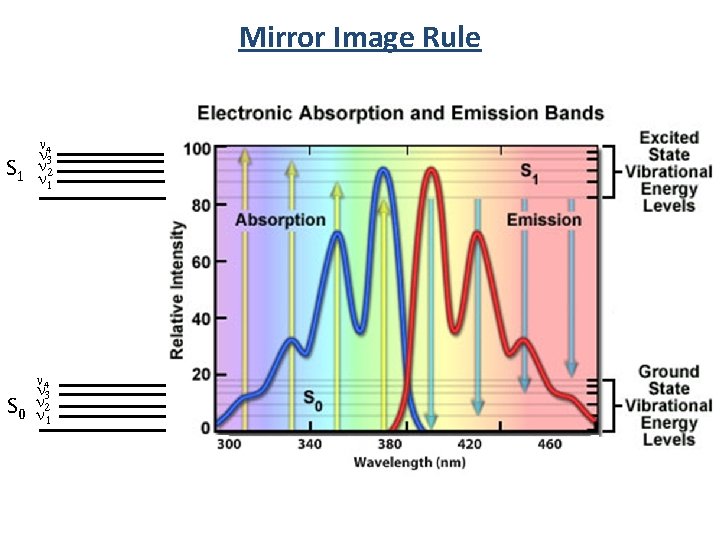
Mirror Image Rule • Vibrational levels in the excited states and ground states are similar • An absorption spectrum reflects the vibrational levels of the electronically excited state • An emission spectrum reflects the vibrational levels of the electronic ground state v’=5 v’=4 v’=3 v’=2 v’=1 v’=0 S 1 • Fluorescence emission spectrum is mirror image of absorption spectrum v=5 v=4 v=3 v=2 v=1 v=0 S 0

Mirror Image Rule n 4 n S 1 nn 32 1 n 4 n 3 n S 0 n 21

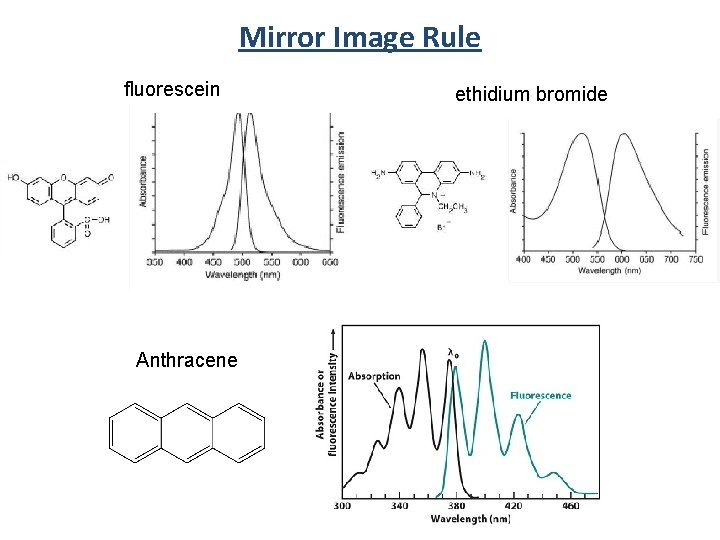
Mirror Image Rule fluorescein Anthracene ethidium bromide

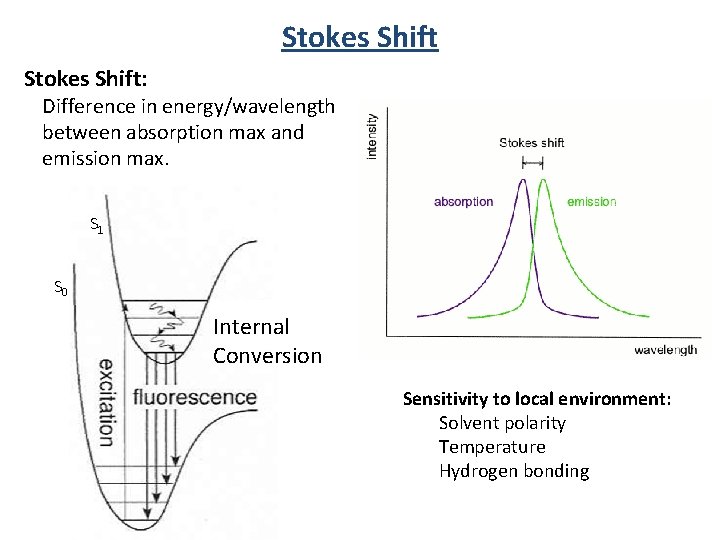
Stokes Shift: Difference in energy/wavelength between absorption max and emission max. S 1 S 0 Internal Conversion Sensitivity to local environment: Solvent polarity Temperature Hydrogen bonding

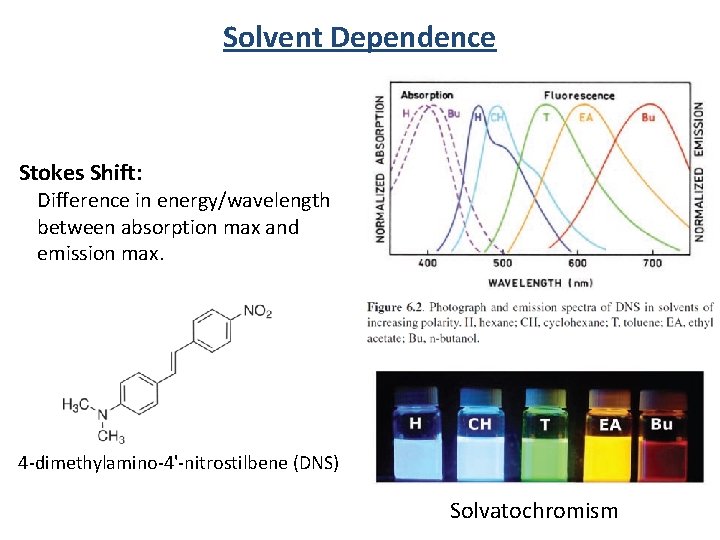
Solvent Dependence Stokes Shift: Difference in energy/wavelength between absorption max and emission max. 4 -dimethylamino-4'-nitrostilbene (DNS) Solvatochromism

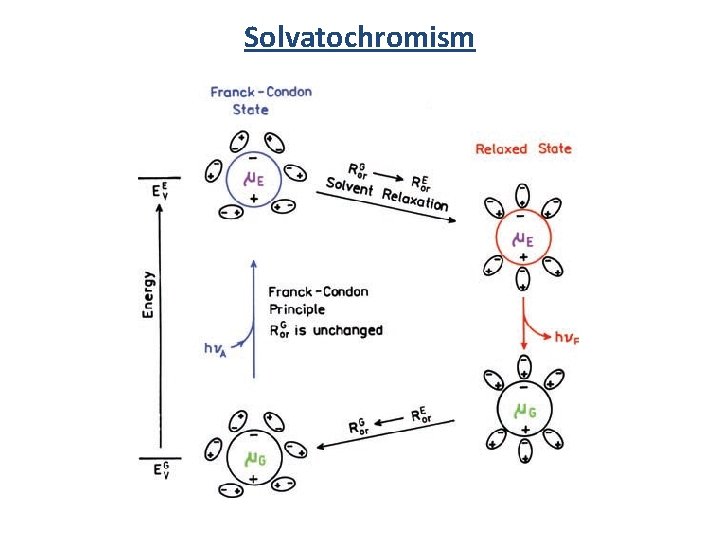
Solvatochromism

Jablonski Diagram S 2 S 1 T 2 Energy T 1 S 0 Excitation Internal Conversion Fluorescence Non-radiative decay Intersystem Crossing Phosphorescence hn Intersystem Crossing Singlet Excited State (S 1) Emission Triplet Excited State (T 1) Ground State (S 0)

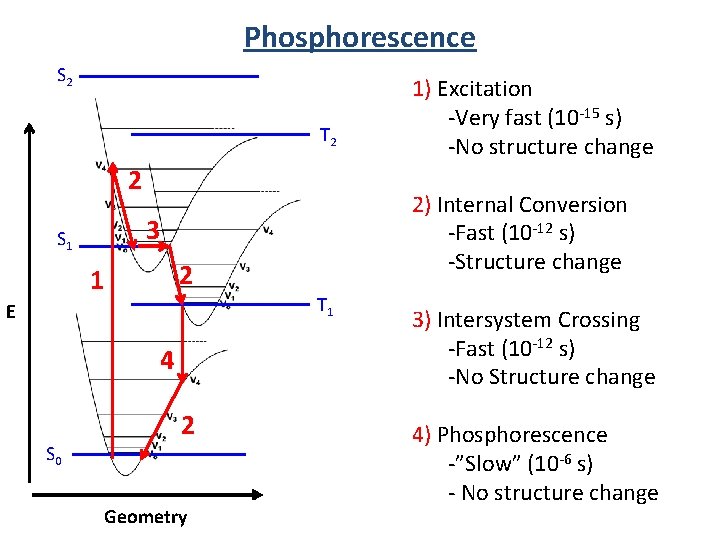
Phosphorescence S 2 T 2 2 2 1 T 1 E 4 S 0 2) Internal Conversion -Fast (10 -12 s) -Structure change 3 S 1 2 Geometry 1) Excitation -Very fast (10 -15 s) -No structure change 3) Intersystem Crossing -Fast (10 -12 s) -No Structure change 4) Phosphorescence -”Slow” (10 -6 s) - No structure change

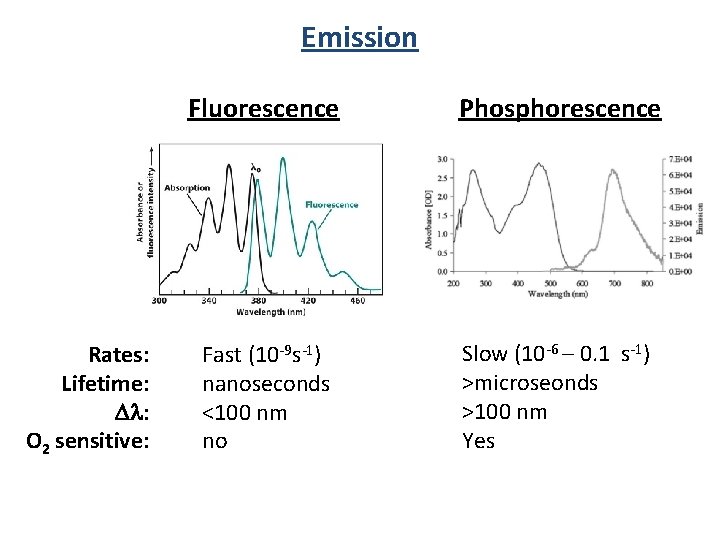
Emission Rates: Lifetime: Dl: O 2 sensitive: Fluorescence Phosphorescence Fast (10 -9 s-1) nanoseconds <100 nm no Slow (10 -6 – 0. 1 s-1) >microseonds >100 nm Yes

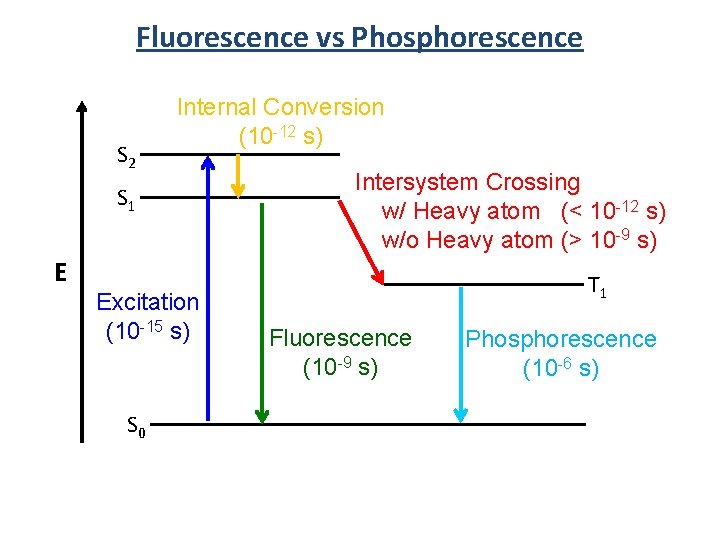
Fluorescence vs Phosphorescence S 2 Internal Conversion (10 -12 s) S 1 E Excitation (10 -15 s) S 0 Intersystem Crossing w/ Heavy atom (< 10 -12 s) w/o Heavy atom (> 10 -9 s) T 1 Fluorescence (10 -9 s) Phosphorescence (10 -6 s)
![Emissive Molecules Phosphorescent Fluorescent Perylene OEP Pt OEP Irppy3 BODIPY Fluorescein Rose Bengal Rubpy32 Emissive Molecules Phosphorescent Fluorescent Perylene OEP Pt. OEP Ir(ppy)3 BODIPY Fluorescein Rose Bengal [Ru(bpy)3]2+](https://slidetodoc.com/presentation_image_h/22860962121d1875163ba66b0abe0a66/image-20.jpg)
Emissive Molecules Phosphorescent Fluorescent Perylene OEP Pt. OEP Ir(ppy)3 BODIPY Fluorescein Rose Bengal [Ru(bpy)3]2+ Coumarin Anthracene + ICH 3 C 60

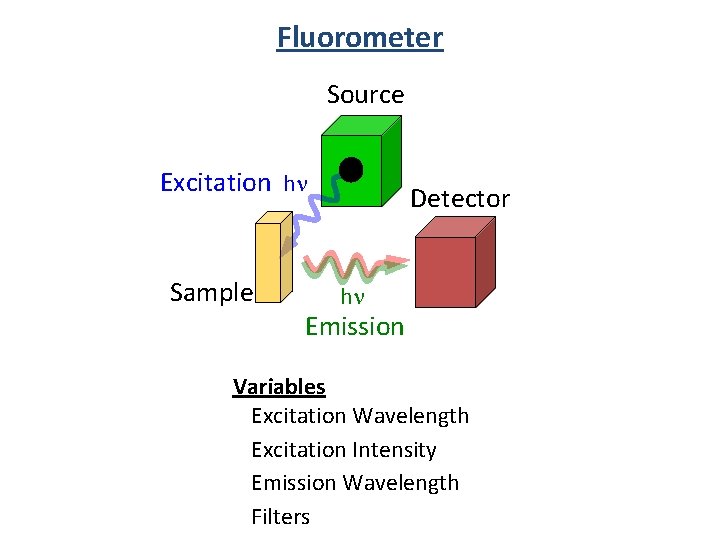
Fluorometer Source Excitation hn Sample Detector hn Emission Variables Excitation Wavelength Excitation Intensity Emission Wavelength Filters

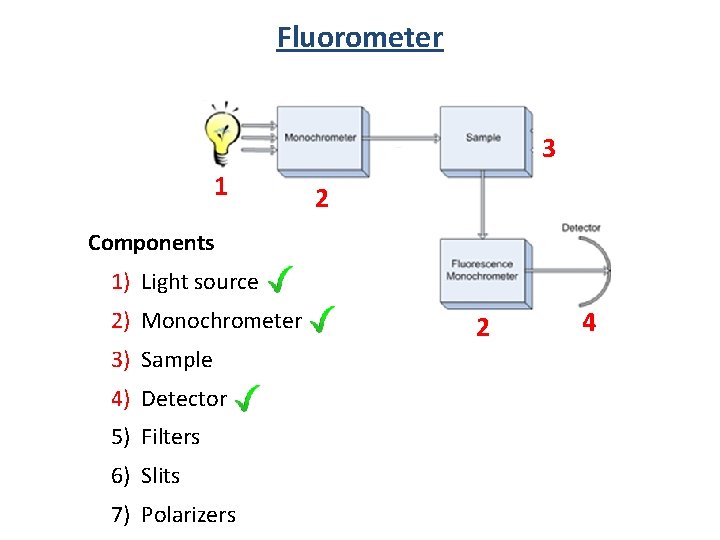
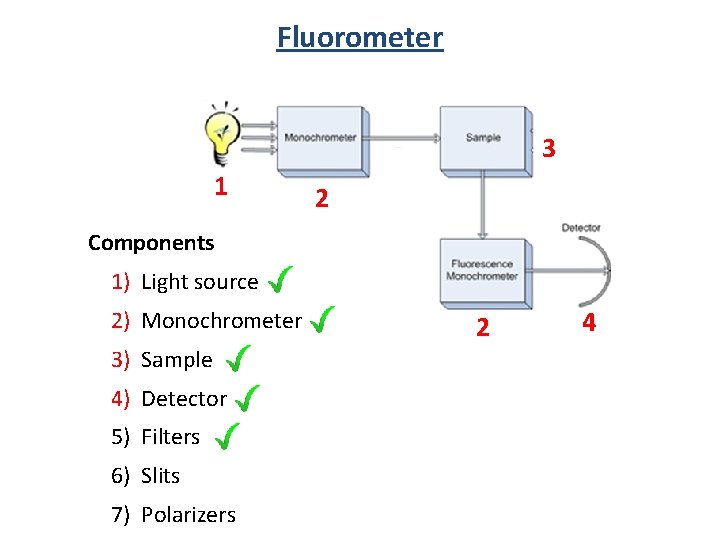
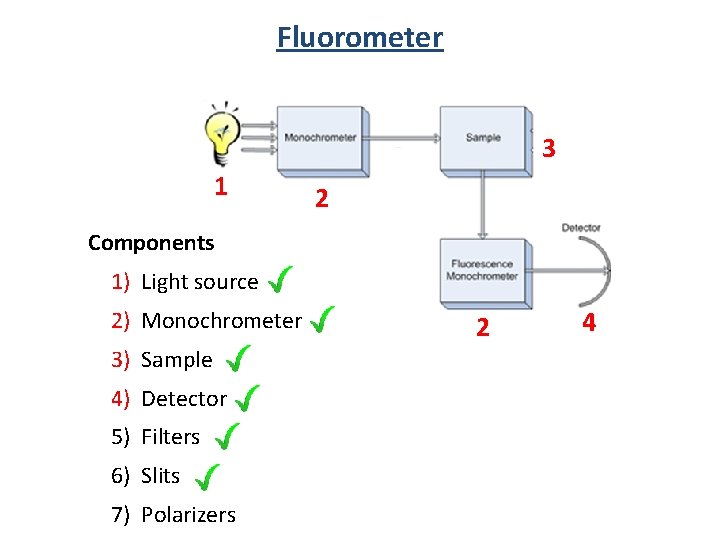
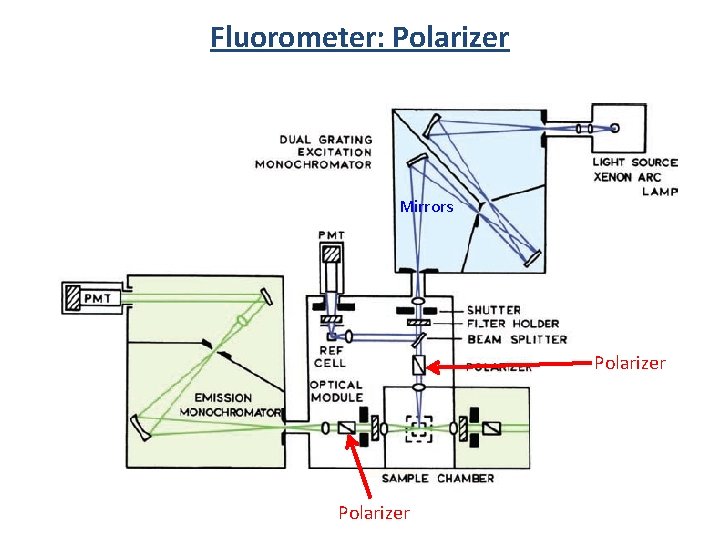
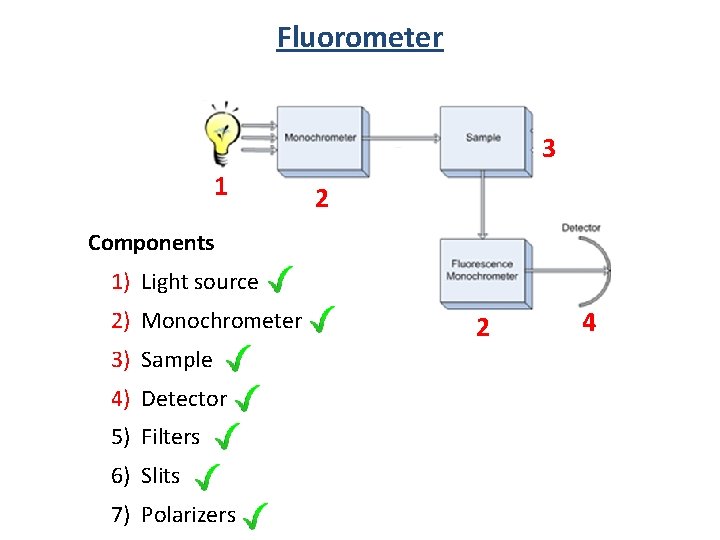
Fluorometer 3 1 2 Components 1) Light source 2) Monochrometer 3) Sample 4) Detector 5) Filters 6) Slits 7) Polarizers 2 4

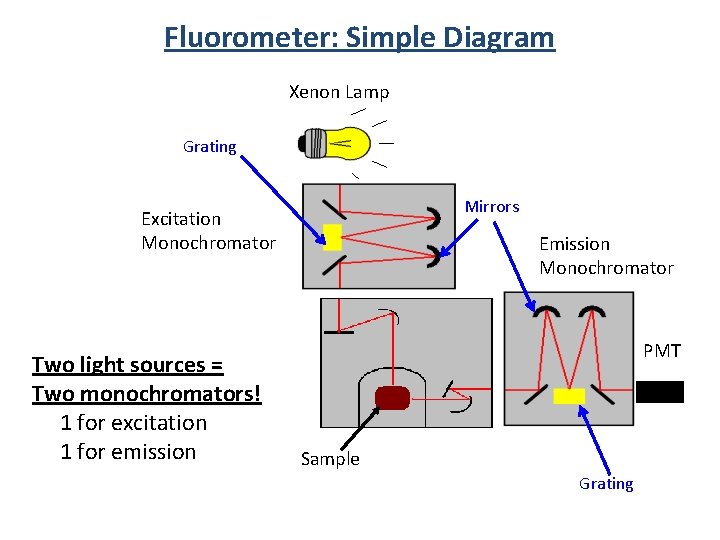
Fluorometer: Simple Diagram Xenon Lamp Grating Mirrors Excitation Monochromator Two light sources = Two monochromators! 1 for excitation 1 for emission Emission Monochromator PMT Sample Grating

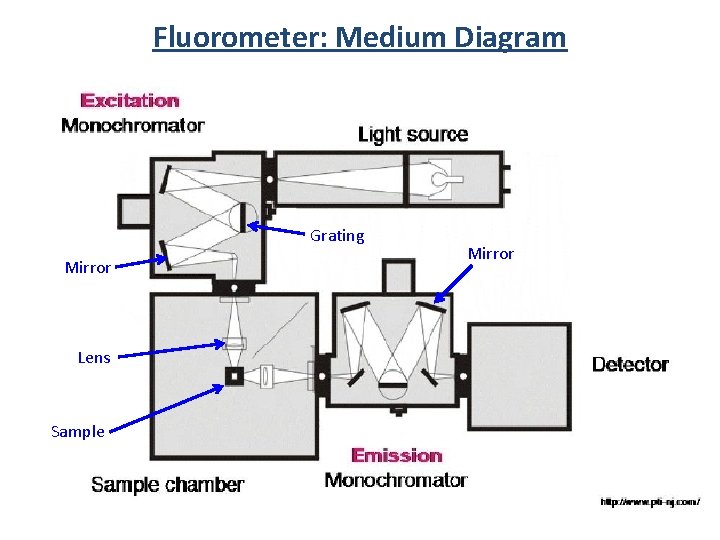
Fluorometer: Medium Diagram Grating Mirror Lens Sample Mirror

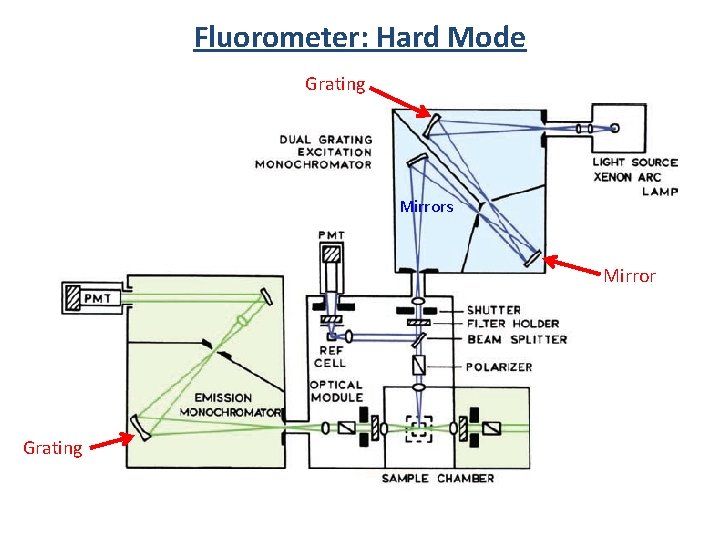
Fluorometer: Hard Mode Grating Mirrors Mirror Grating

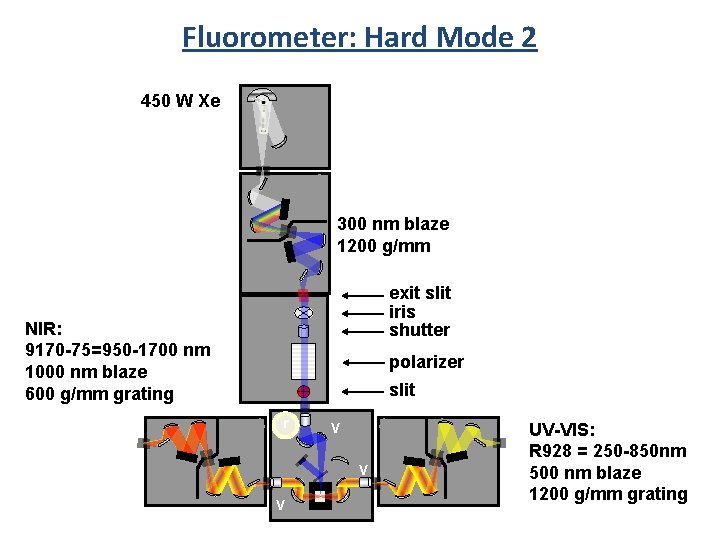
Fluorometer: Hard Mode 2 450 W Xe 300 nm blaze 1200 g/mm exit slit iris shutter NIR: 9170 -75=950 -1700 nm 1000 nm blaze 600 g/mm grating polarizer slit r V V V UV-VIS: R 928 = 250 -850 nm 500 nm blaze 1200 g/mm grating

Horiba JY Fluoromax-4 MAC Lab (Materials Characterization) CSL 116

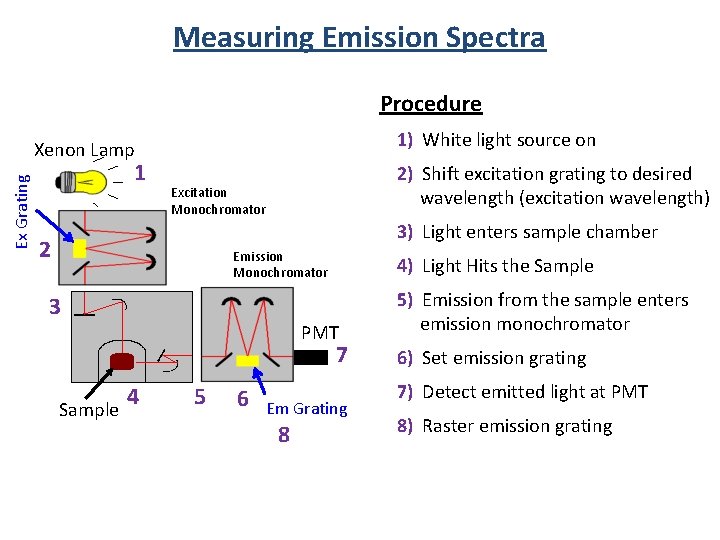
Measuring Emission Spectra Procedure 1) White light source on Ex Grating Xenon Lamp 1 2) Shift excitation grating to desired wavelength (excitation wavelength) Excitation Monochromator 3) Light enters sample chamber 2 Emission Monochromator 4) Light Hits the Sample 3 PMT 7 4 Sample 5 6 Em Grating 8 5) Emission from the sample enters emission monochromator 6) Set emission grating 7) Detect emitted light at PMT 8) Raster emission grating

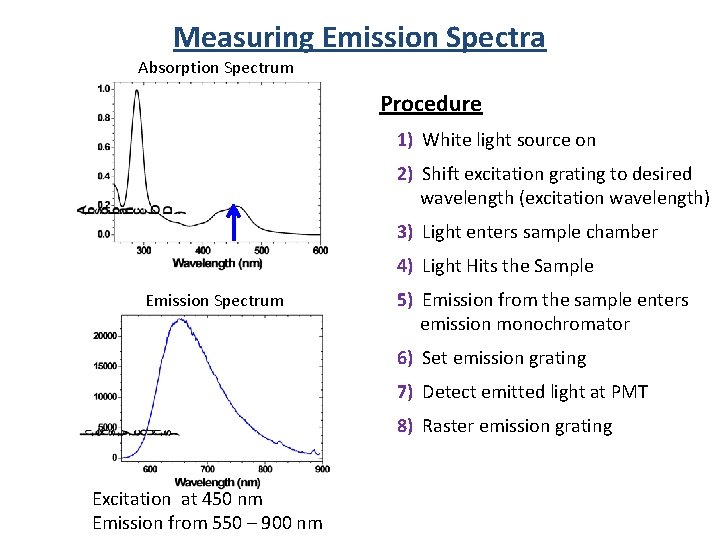
Measuring Emission Spectra Absorption Spectrum Procedure 1) White light source on 2) Shift excitation grating to desired wavelength (excitation wavelength) 3) Light enters sample chamber 4) Light Hits the Sample Emission Spectrum 5) Emission from the sample enters emission monochromator 6) Set emission grating 7) Detect emitted light at PMT 8) Raster emission grating Excitation at 450 nm Emission from 550 – 900 nm

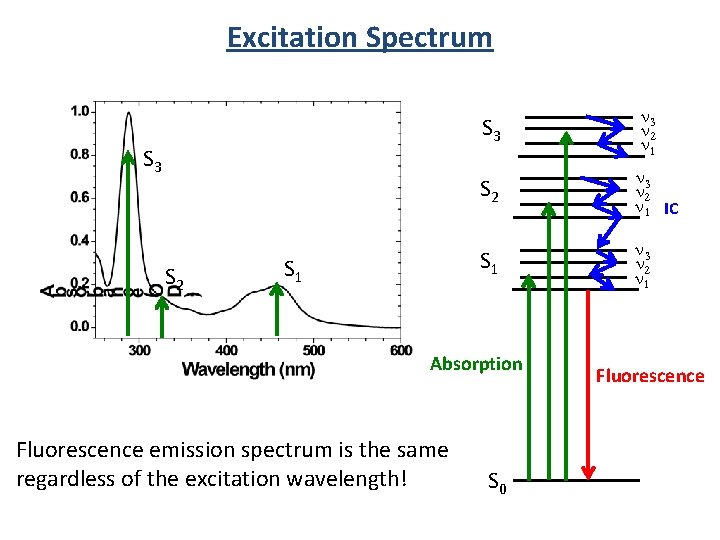
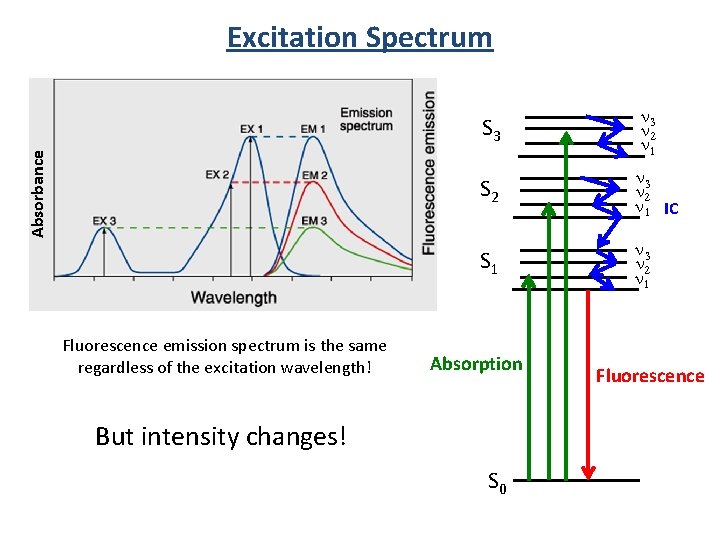
Excitation Spectrum S 3 S 2 S 1 S 3 n 2 n 1 S 2 n 3 n 2 n 1 IC S 1 n 3 n 2 n 1 Absorption Fluorescence emission spectrum is the same regardless of the excitation wavelength! S 0 Fluorescence

Absorbance Excitation Spectrum Fluorescence emission spectrum is the same regardless of the excitation wavelength! S 3 n 2 n 1 S 2 n 3 n 2 n 1 IC S 1 n 3 n 2 n 1 Absorption But intensity changes! S 0 Fluorescence

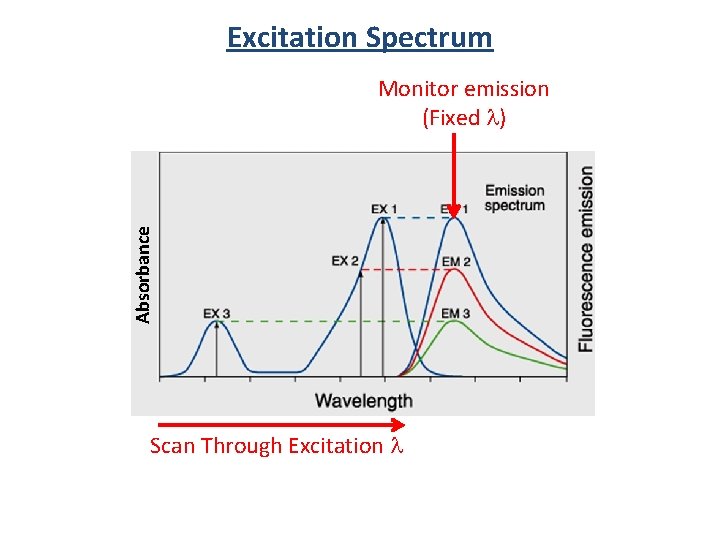
Excitation Spectrum Absorbance Monitor emission (Fixed l) Scan Through Excitation l

Measuring Excitation Spectra Procedure 1) Shift emission grating to desired wavelength (monitor emission max) Ex Grating Xenon Lamp 3 Excitation Monochromator 2 7 2) Shift excitation grating to stating wavelength 3) Light source on Emission Monochromator 4) Light Hits the Sample PMT 6 Sample 4 5 1 5) Emission from the sample enters emission monochromator 6) Detect emitted light at PMT Em Grating 7) Raster excitation grating

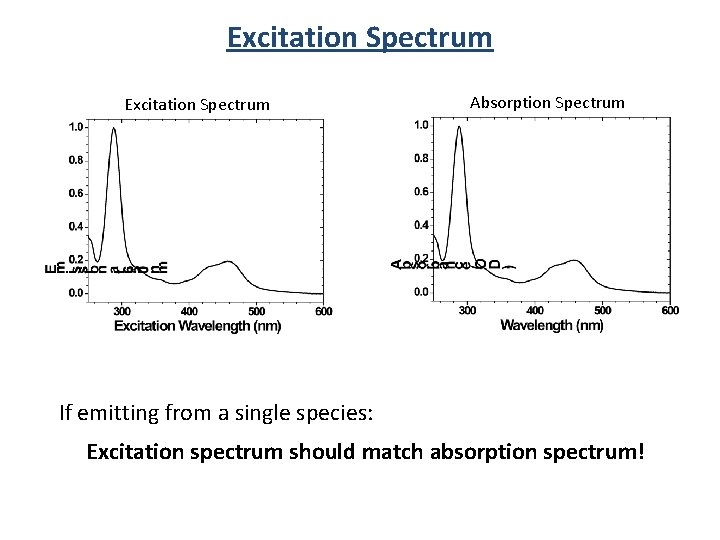
Excitation Spectrum Absorption Spectrum If emitting from a single species: Excitation spectrum should match absorption spectrum!

Fluorometer 3 1 2 Components 1) Light source 2) Monochrometer 3) Sample 4) Detector 5) Filters 6) Slits 7) Polarizers 2 4

Samples Solutions Powders Thin Films Crystals

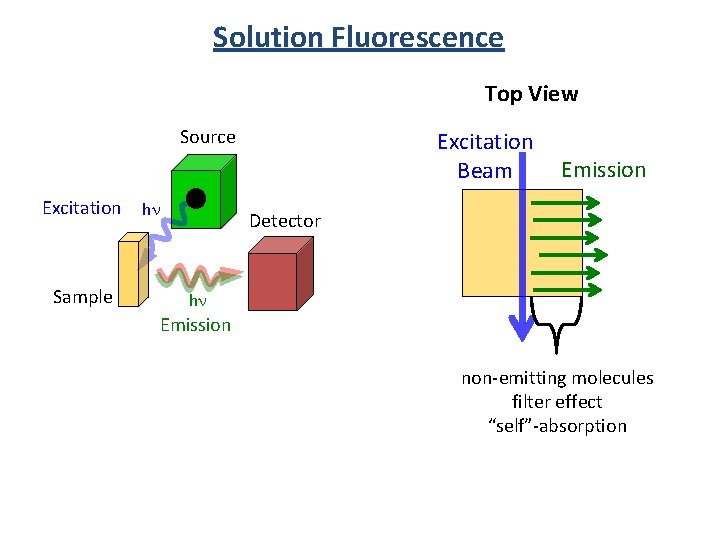
Solution Fluorescence Top View Source Excitation Sample hn Excitation Beam Emission Detector hn Emission non-emitting molecules filter effect “self”-absorption

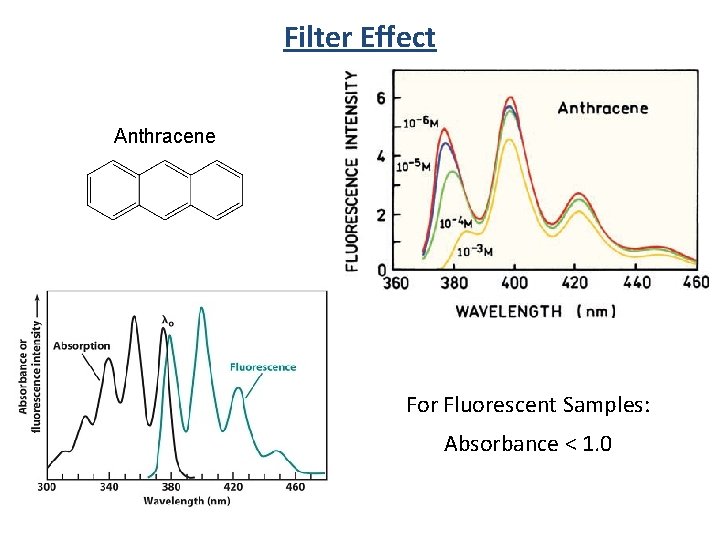
Filter Effect Anthracene For Fluorescent Samples: Absorbance < 1. 0

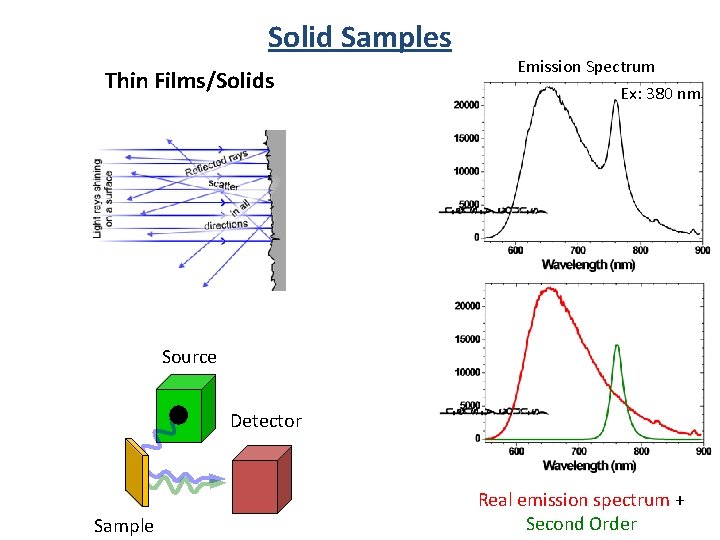
Solid Samples Thin Films/Solids Emission Spectrum Ex: 380 nm Source Detector Sample Real emission spectrum + Second Order

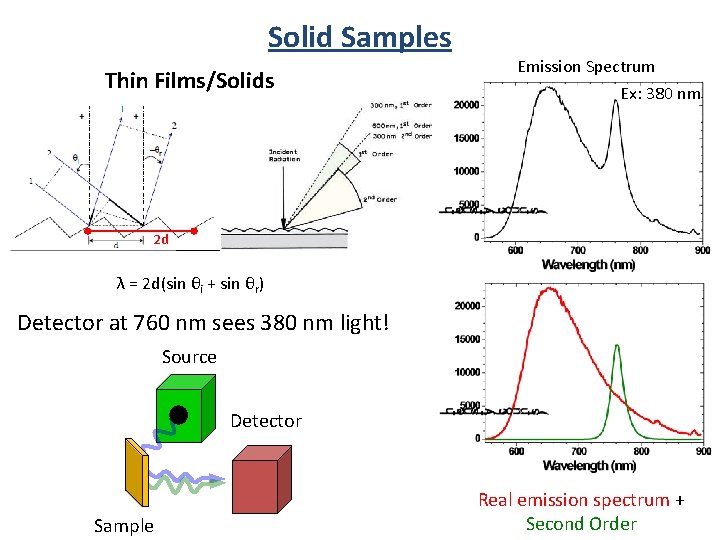
Solid Samples Thin Films/Solids Emission Spectrum Ex: 380 nm 2 d λ = 2 d(sin θi + sin θr) Detector at 760 nm sees 380 nm light! Source Detector Sample Real emission spectrum + Second Order

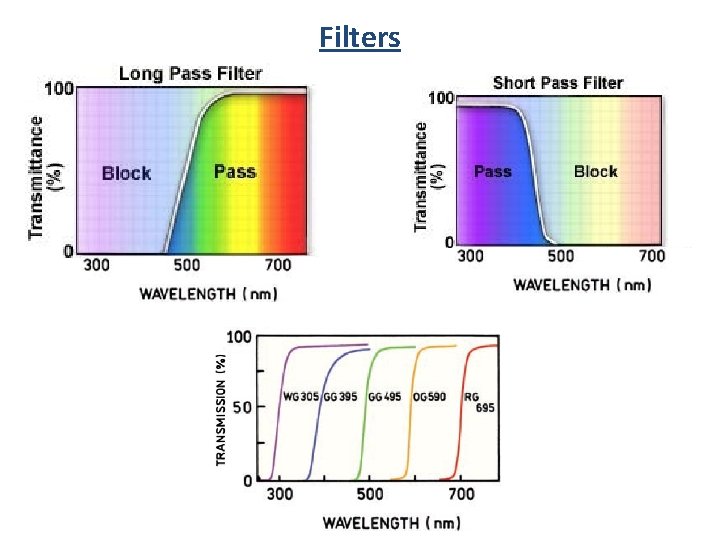
Filters

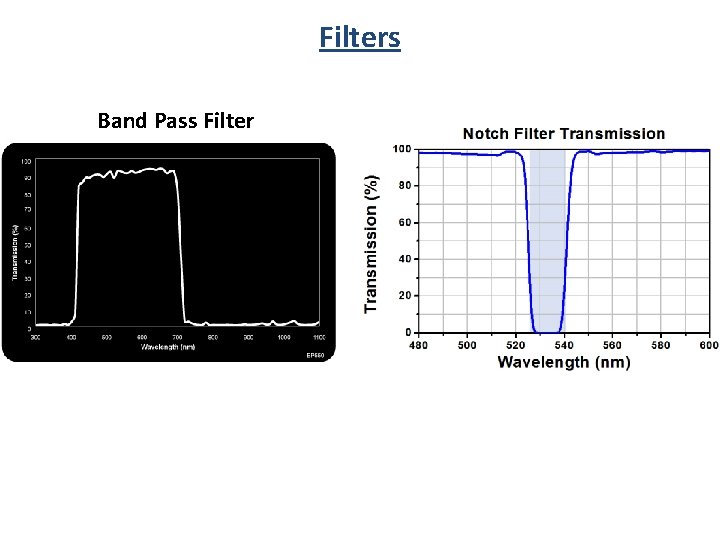
Filters Band Pass Filter

Fluorometer 3 1 2 Components 1) Light source 2) Monochrometer 3) Sample 4) Detector 5) Filters 6) Slits 7) Polarizers 2 4

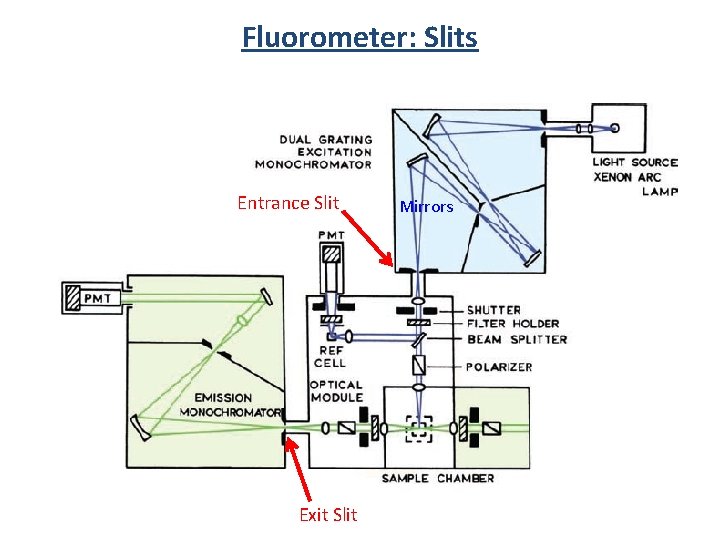
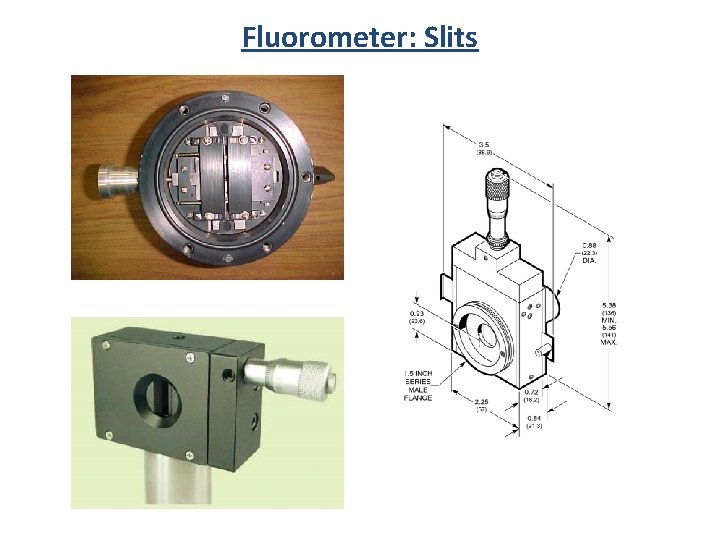
Fluorometer: Slits Entrance Slit Exit Slit Mirrors

Fluorometer: Slits

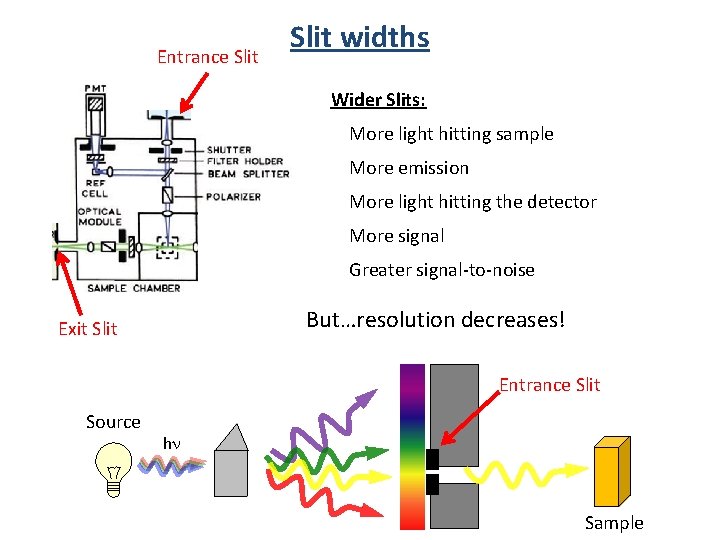
Entrance Slit widths Wider Slits: More light hitting sample More emission More light hitting the detector More signal Greater signal-to-noise But…resolution decreases! Exit Slit Entrance Slit Source hn Sample

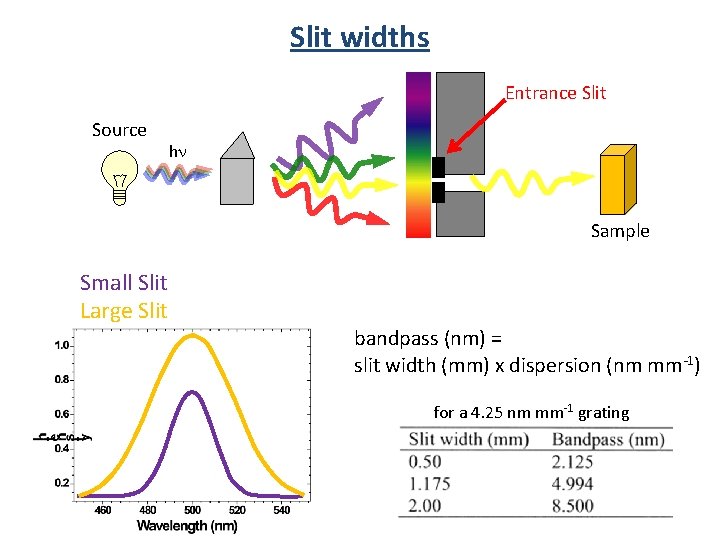
Slit widths Entrance Slit Source hn Sample Small Slit Large Slit bandpass (nm) = slit width (mm) x dispersion (nm mm-1) for a 4. 25 nm mm-1 grating

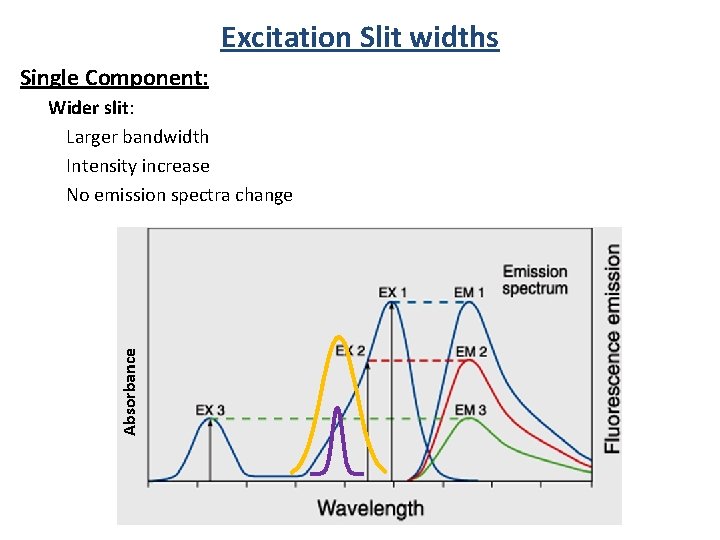
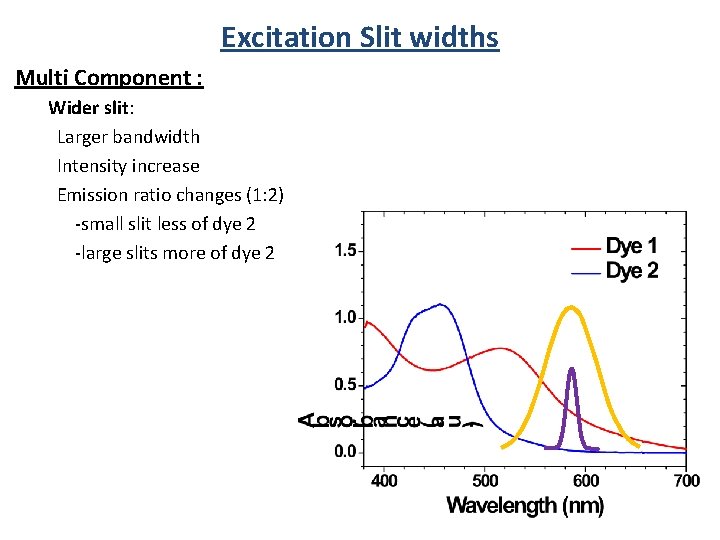
Excitation Slit widths Single Component: Absorbance Wider slit: Larger bandwidth Intensity increase No emission spectra change

Excitation Slit widths Multi Component : Wider slit: Larger bandwidth Intensity increase Emission ratio changes (1: 2) -small slit less of dye 2 -large slits more of dye 2

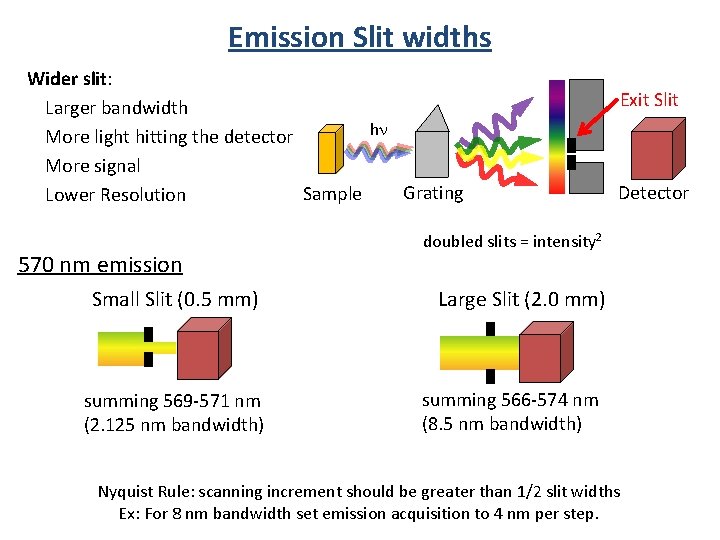
Emission Slit widths Wider slit: Larger bandwidth hn More light hitting the detector More signal Grating Sample Lower Resolution 570 nm emission Small Slit (0. 5 mm) summing 569 -571 nm (2. 125 nm bandwidth) Exit Slit Detector doubled slits = intensity 2 Large Slit (2. 0 mm) summing 566 -574 nm (8. 5 nm bandwidth) Nyquist Rule: scanning increment should be greater than 1/2 slit widths Ex: For 8 nm bandwidth set emission acquisition to 4 nm per step.

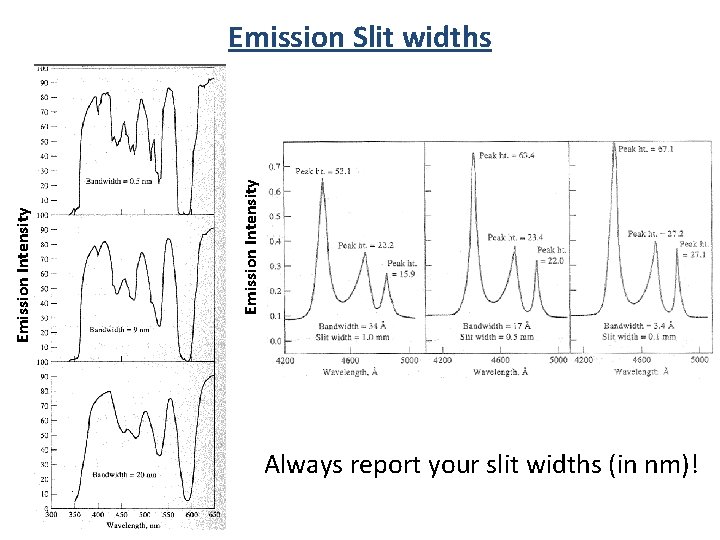
Emission Intensity Emission Slit widths Always report your slit widths (in nm)!

Fluorometer 3 1 2 Components 1) Light source 2) Monochrometer 3) Sample 4) Detector 5) Filters 6) Slits 7) Polarizers 2 4

Fluorometer: Polarizer Mirrors Polarizer

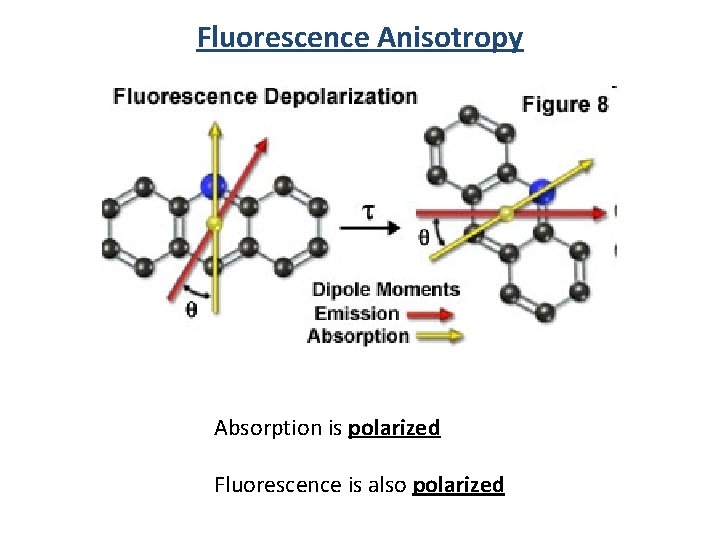
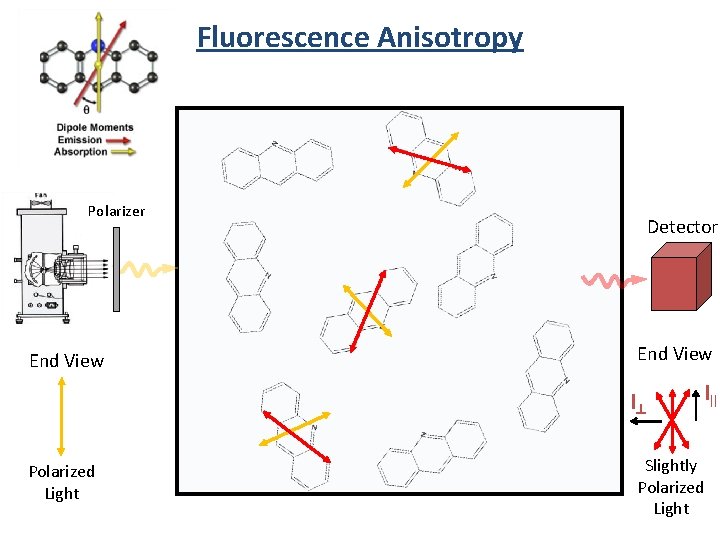
Fluorescence Anisotropy Absorption is polarized Fluorescence is also polarized


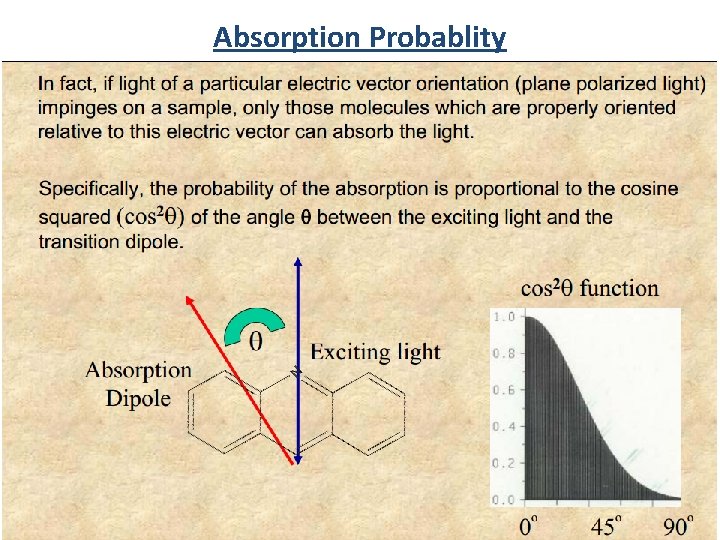
Absorption Probablity

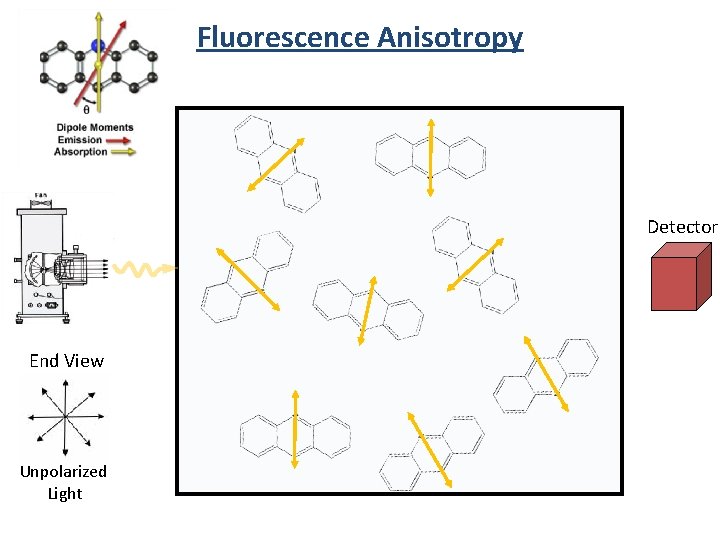
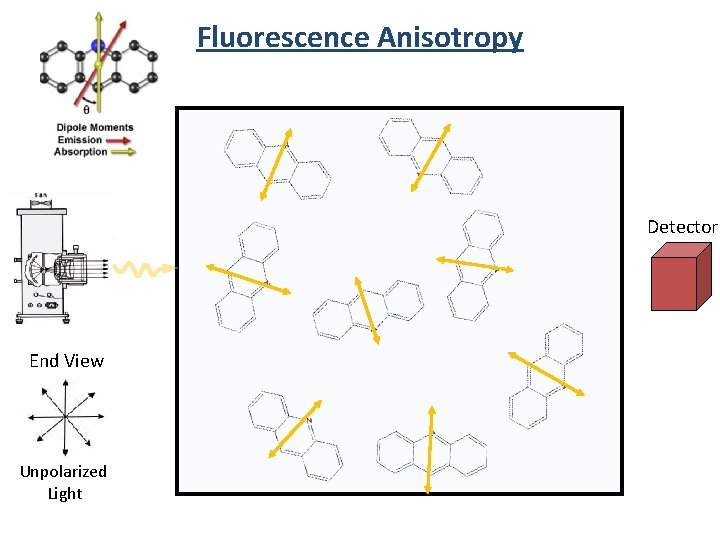
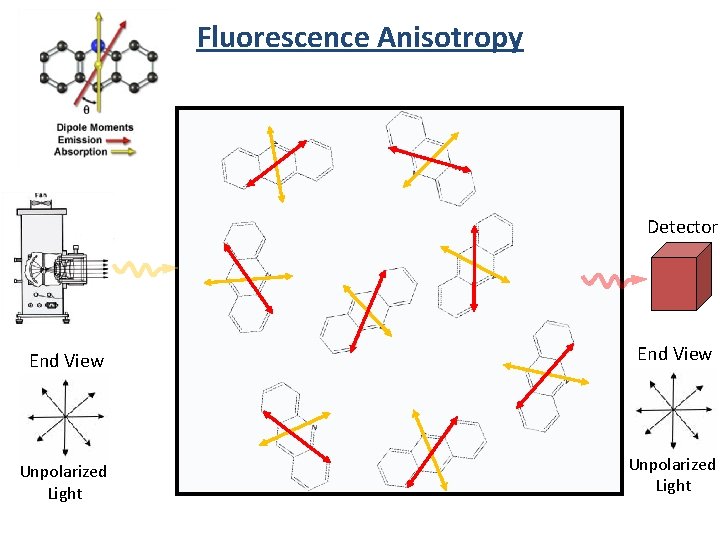
Fluorescence Anisotropy Detector End View Unpolarized Light

Fluorescence Anisotropy Detector End View Unpolarized Light

Fluorescence Anisotropy Detector End View Unpolarized Light

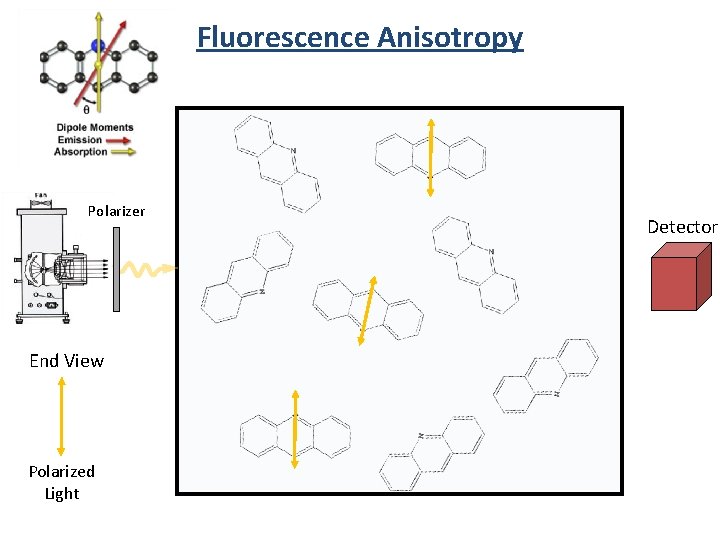
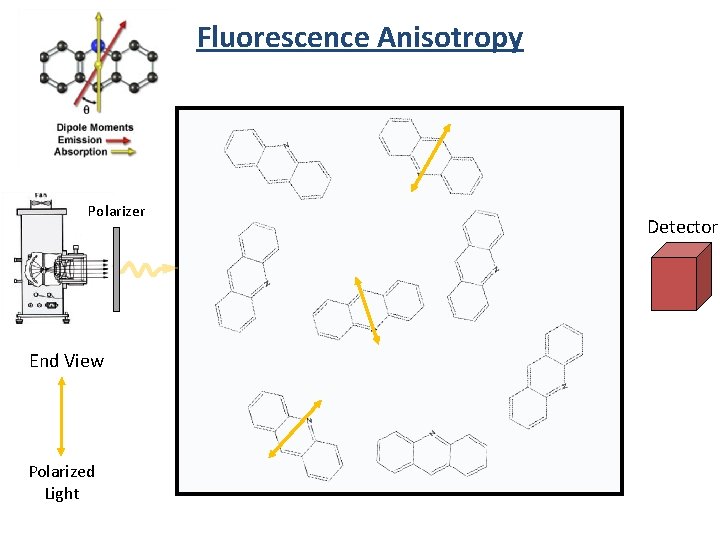
Fluorescence Anisotropy Polarizer End View Polarized Light Detector

Fluorescence Anisotropy Polarizer End View Polarized Light Detector

Fluorescence Anisotropy Polarizer End View Detector End View I^ Polarized Light I|| Slightly Polarized Light

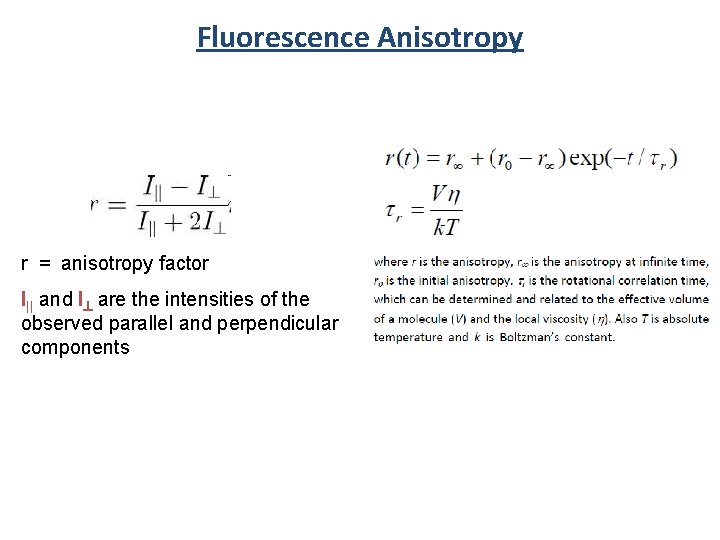
Fluorescence Anisotropy Sample I|| Detector I^ Polarized Excitation r = anisotropy factor I|| and I^ are the intensities of the observed parallel and perpendicular components

Fluorescence Anisotropy r = anisotropy factor I|| and I^ are the intensities of the observed parallel and perpendicular components

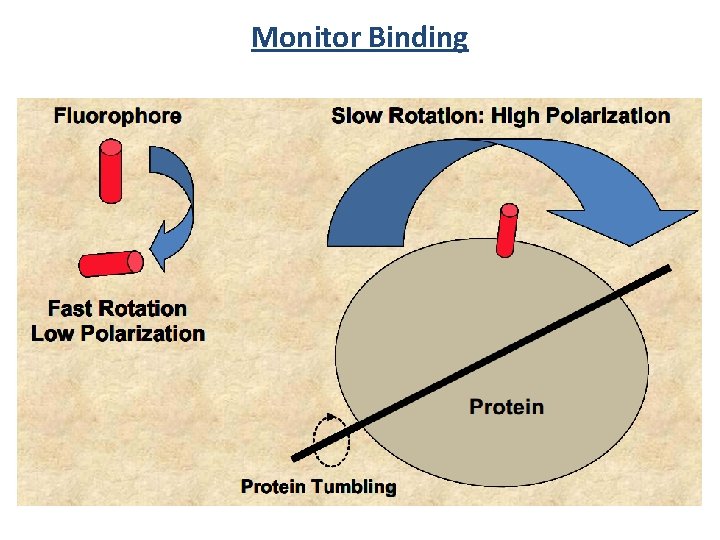
Monitor Binding

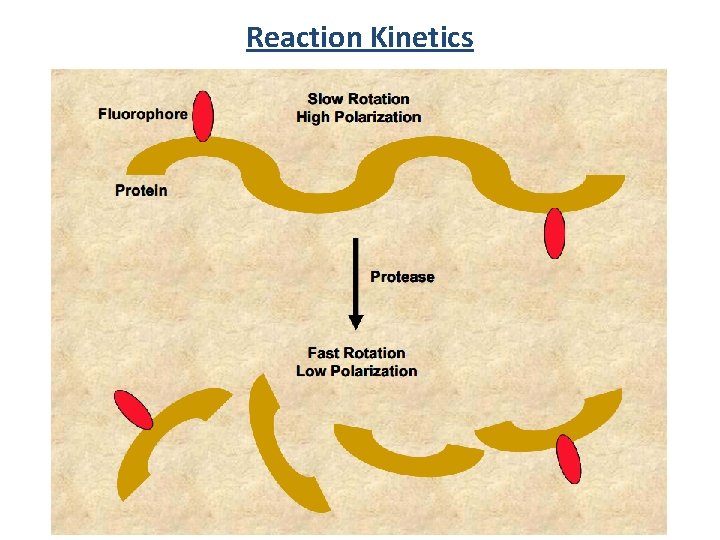
Reaction Kinetics

Fluorometer 3 1 2 Components 1) Light source 2) Monochrometer 3) Sample 4) Detector 5) Filters 6) Slits 7) Polarizers 2 4

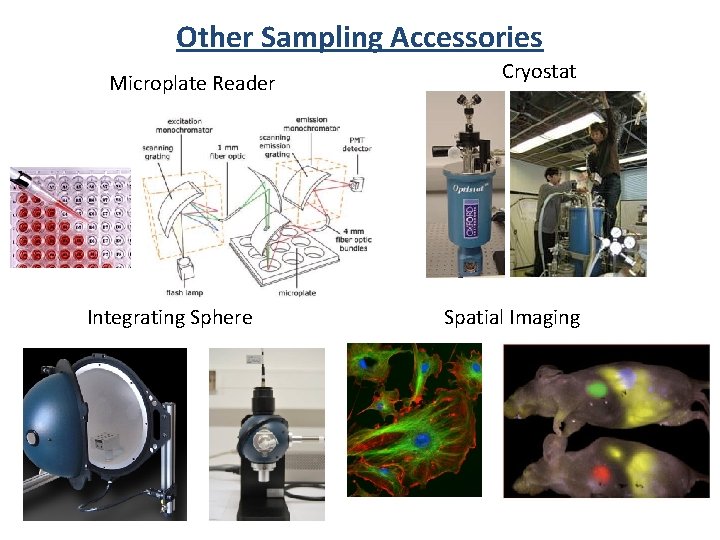
Other Sampling Accessories Microplate Reader Integrating Sphere Cryostat Spatial Imaging

Fluorescence Microscopy Detector “monochrometer” Filter Source “monochrometer” Filter Sample Translation stage

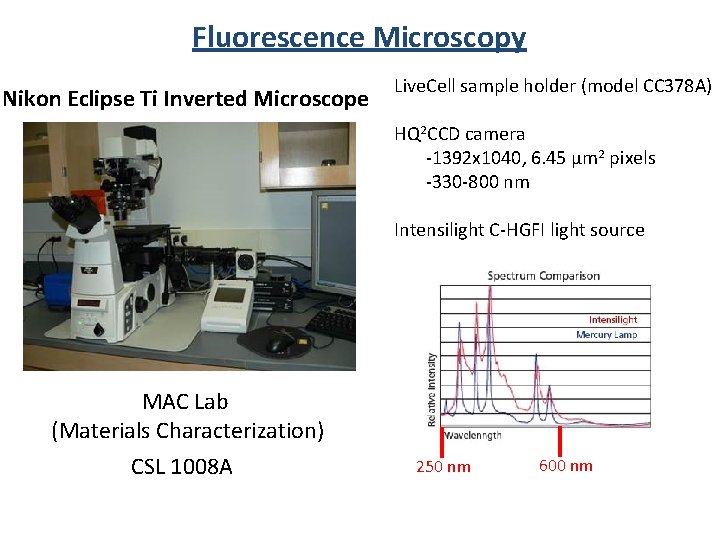
Fluorescence Microscopy Nikon Eclipse Ti Inverted Microscope Live. Cell sample holder (model CC 378 A) HQ 2 CCD camera -1392 x 1040, 6. 45 µm 2 pixels -330 -800 nm Intensilight C-HGFI light source MAC Lab (Materials Characterization) CSL 1008 A 250 nm 600 nm

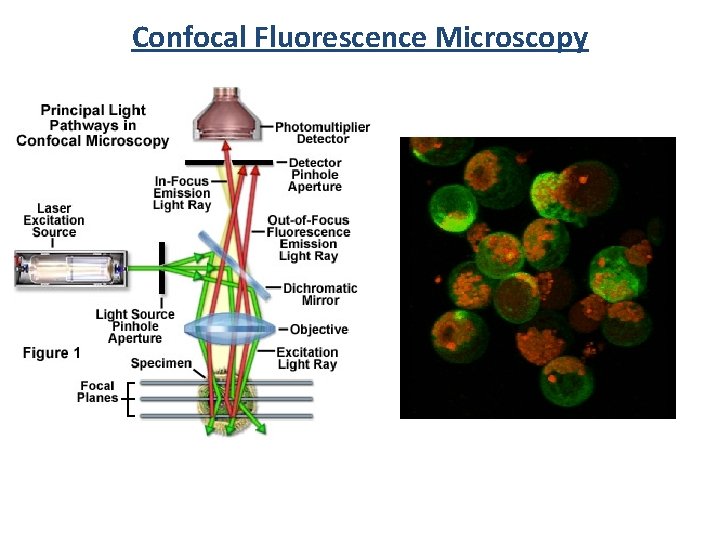
Confocal Fluorescence Microscopy

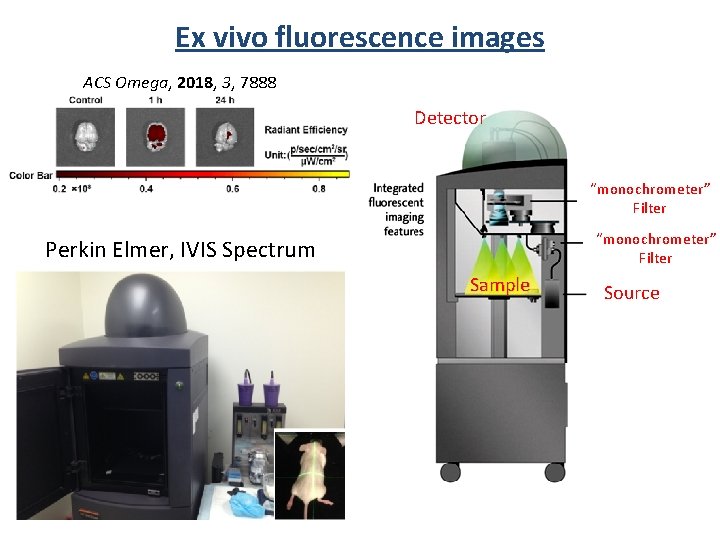
Ex vivo fluorescence images ACS Omega, 2018, 3, 7888 Detector “monochrometer” Filter Perkin Elmer, IVIS Spectrum Sample Source

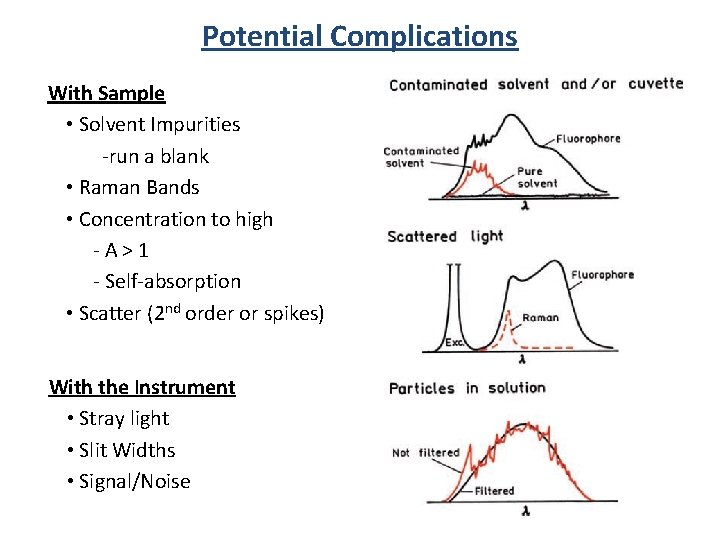
Potential Complications With Sample • Solvent Impurities -run a blank • Raman Bands • Concentration to high - A > 1 - Self-absorption • Scatter (2 nd order or spikes) With the Instrument • Stray light • Slit Widths • Signal/Noise

Fluorescence Spectroscopy End Any Questions?