Fluorescence Polarization Immunoassay FPIA Fluorescence polarization is ideal



- Slides: 14

Fluorescence Polarization Immunoassay FPIA

• Fluorescence polarization is ideal for the study of small molecule fluorescent ligands binding to receptors. • In fluorescent polarization method of assay, polarized light excites a fluorescent label. As this label is bound, the degree of polarization of the fluorescent emission from the label increases.

disadvantages • Disadvantages of this method are nonlinear assay when the concentration of the test compound is related to polarization of the fluorescent emission. • Also, this type of assay has a limiting assayed can be accurately measured. • FPIA is utilized to provide accurate and sensitive measurement of small toxicology analytes such as therapeutic drugs, and drugs of abuse, toxicology and some hormones

FPIA reagent components • S: Antibody Reagent: Antiserum to analyte; • T: Tracer: Fluorescein-labeled analyte; • P: Pretreatment detergent: facilitate release of drug from serum proteins.

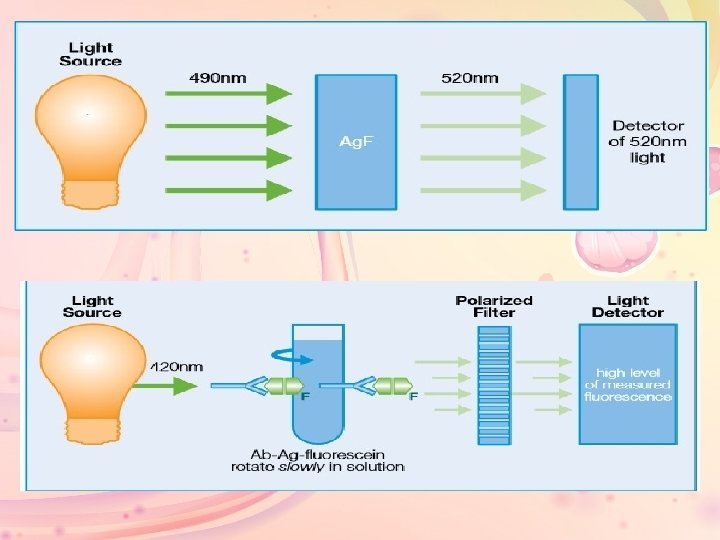
• FPIA utilizes three key concepts to measure specific analytes in a homogeneous format: fluorescence, rotation of molecules in solution, and polarized light. • Fluorescence: Fluorescein is a fluorescent label. It absorbs light energy at 490 nm and releases this energy at a higher wavelength (520 nm) as fluorescent light


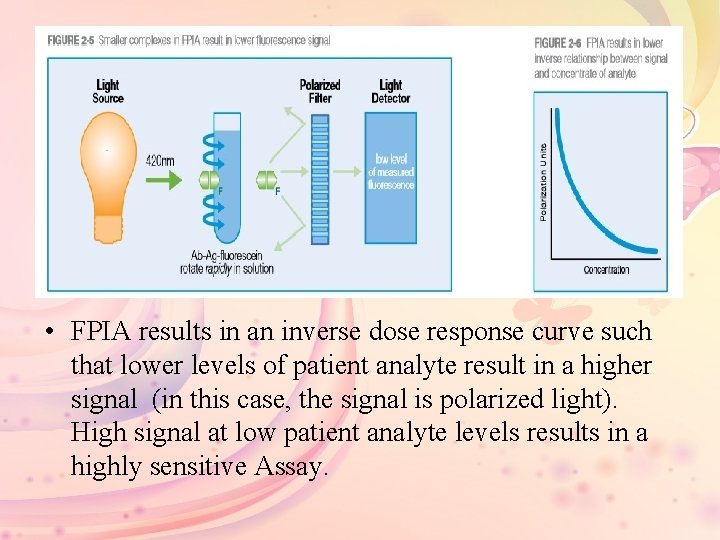
• FPIA results in an inverse dose response curve such that lower levels of patient analyte result in a higher signal (in this case, the signal is polarized light). High signal at low patient analyte levels results in a highly sensitive Assay.

• fluorescence polarization immunoassays combine two technologist to determine analyte concentration: – competitive protein binding – fluorescence polarization • competitive binding requires two antigen or analyte systems: Ø Analyte from the sample Ø Tracer-labeled analyte provided by the assay reagents • The analyte from the sample competes with the analyte tracer for binding sites on the antibody. Each analyte system attaches to binding sites based on its relative concentration.

• If high concentrations of the sample analyte are present more specimen analyte binds to the antibody, leaving the analyte tracer unbound. • If low concentrations of the sample analytes are present less specimen analyte binds to the antibody leaving analyte tracer bound.

• Polarized light can be used to produce a polarized flurorescent emission from the analyte-tracer. • The average polarization of the emitted fluorescence is related to the speed of rotation of the molecule. • The rate of molecule rotation in liquid is related to the size of the molecule. Small, unbound analytes rotate more rapidly than the larger analyteantibody complex. • Axsym system undergo to this priniciple.

FPIA reaction sequence: • A typical FPIA reaction would occur as follows: – Sample, pretreatment reagent and line diluent are read as a blank. – Sample, pretreatment reagent, and line diluent are combined and incubated at reaction temperature. An initial read is taken by the FPIA optics system as a blank for the assay.

• Competitive binding occurs: • Antibody, tracer, line diluent and more sample are added to the reaction mixture and incubated. • The analyte from the sample competes with the analyte-tracer for binding sites on the antibodies. • If the sample contains a high concentration of the analyte, the sample analyte binds to the antibodies, leaving the analyte-tracer unbound. • If the sample contains a low concentration (or no concentration) of the analyte, then few or no sample analyte molecules bind to the antibodies, leaving the antibodies open for the analyte tracer.

• Change in polarization of emitted fluorescence indicates analyte concentration. • FPIA optics detect and measure the change in polarization of emitted fluorescence, which is inversely rotational to the concentration of the analyte in the specimen.

• If the sample contains a high concentration of the analyte, then many analyte-tracer molecules are left unbound. When excited by polarized vertical light, these small fluorescent molecules rotate rapidly, emitting light in many different planes. The result is a decrease in polarization. • If the sample contains a low concentration (or no concentration ) of the analyte, then the analyte-tracer binds to the antibodies, resulting in few unbound analyte-tracer molecules. These large molecules (complexes of analyte-tracer bound to antibody) rotate slower. Rotation is slowed enough so that light is emitted in a single plane. The result is an increase in polarization.
 Fpia
Fpia Fpia
Fpia Fpia
Fpia Meia immunoassay
Meia immunoassay Labeled immunoassay
Labeled immunoassay Principle of elisa ppt
Principle of elisa ppt Protein fragment complementation assay
Protein fragment complementation assay Principle of fluorescence spectroscopy
Principle of fluorescence spectroscopy Jablonski diagramm
Jablonski diagramm Cold vapor atomic fluorescence spectrometry
Cold vapor atomic fluorescence spectrometry Fluorescence microscope uses
Fluorescence microscope uses Fluorescence-activated cell sorting (facs)
Fluorescence-activated cell sorting (facs) Jablonksi diagram
Jablonksi diagram Fluorescence bandpass filter
Fluorescence bandpass filter Confocal fluorescence microscopy
Confocal fluorescence microscopy