Chapter 9 Lecture Conceptual Integrated Science Second Edition





































- Slides: 94

Chapter 9 Lecture Conceptual Integrated Science Second Edition Atoms and the Periodic Table © 2013 Pearson Education, Inc.

This lecture will help you understand: • • • Atoms Are Ancient and Empty The Elements Protons and Neutrons Isotopes and Atomic Mass The Periodic Table Physical and Conceptual Models Identifying Atoms Using the Spectroscope The Quantum Hypothesis Electron Waves The Shell Model © 2013 Pearson Education, Inc.

Atoms Are Ancient and Empty • Atoms are – ancient. • The origin of most atoms goes back to the birth of the universe. – mostly empty space. • Elements heavier than hydrogen and much of the helium were produced in the interiors of stars. © 2013 Pearson Education, Inc.

Atoms Are Ancient and Empty CHECK YOUR NEIGHBOR Which of these statements about the atom are incorrect? A. Atoms have been around since the beginning of the universe. B. Atoms are mostly empty space. C. Atoms are perpetually moving. D. Atoms are manufactured in plants and in humans during pregnancy. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

The Elements • An element is a material made of only one kind of atom. For example, pure gold is an element because it is made of only gold atoms. • An atom is the fundamental unit of an element. The term "element" is used when referring to macroscopic quantities. The term "atom" is used when discussing the submicroscopic. © 2013 Pearson Education, Inc.

The Elements • Atoms: – Atoms make up all matter around us. – To date, 115 distinct kinds of atoms are known — 90 are found in nature, and the rest are synthesized. • An element is any material that consists of only one type of atom. © 2013 Pearson Education, Inc.

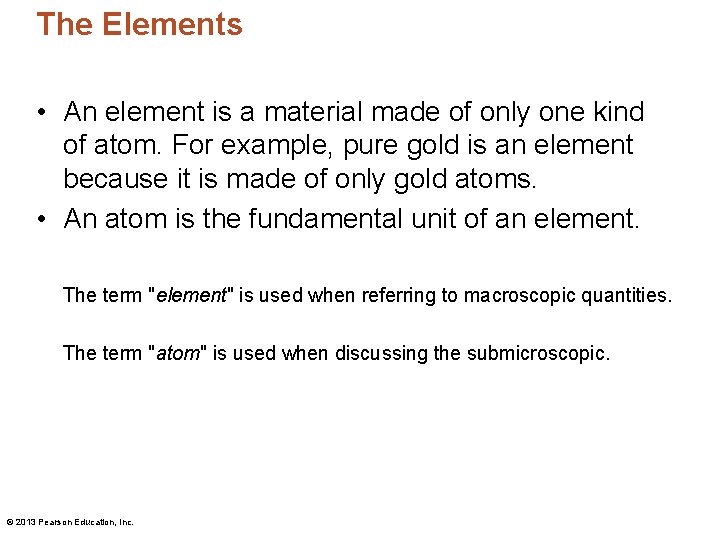
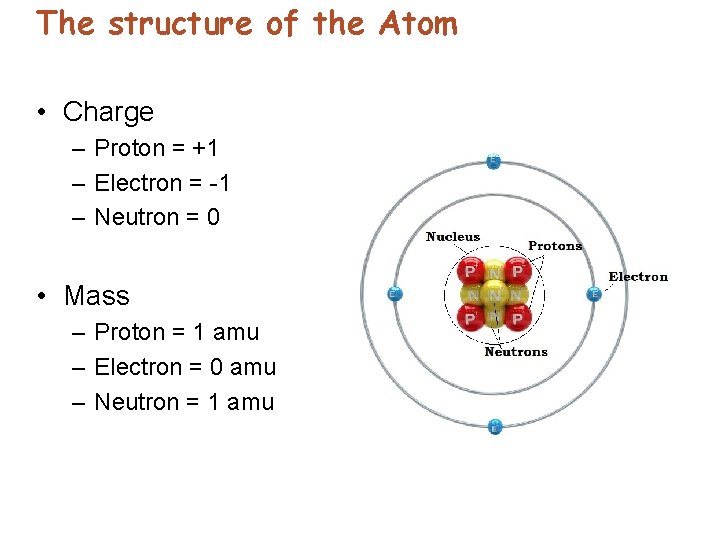
Protons and Neutrons • Protons – carry a positive charge—the same quantity of charge as electrons. – are about 1800 times as massive as electrons. – have the same number of protons in the nucleus as electrons that surround the nucleus of an electrically neutral atom. © 2013 Pearson Education, Inc.

Protons and Neutrons • Positively charged central core – nucleus – Protons – carry positive charge. Each proton has a charge equal to the charge of an electron. – Neutrons – no electrical charge. Mass slightly more than protons.

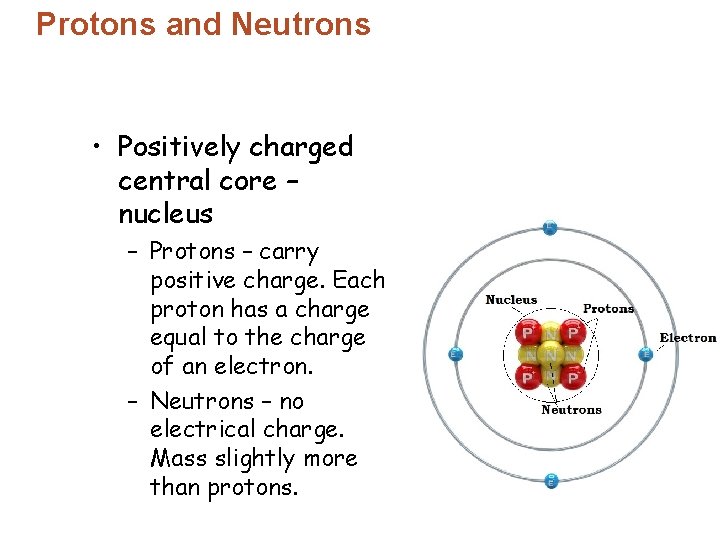
Protons and Neutrons • Electrons – are identical. – repel the electrons of neighboring atoms. – have electrical repulsion that prevents atomic closeness. © 2013 Pearson Education, Inc.

Protons and Neutrons • Electrons – move in the space around the nucleus. – Negative charge attracts to the nucleus’ positive charge. • In a neutral atom – number of protons = number of electrons. • Protons have a much greater mass than electrons.

Protons and Neutrons • The atomic number is the number of protons in each element listed in the periodic table. • Neutrons – accompany protons in the nucleus. – have about the same mass as protons but no charge, so they are electrically neutral. • Both protons and neutrons are nucleons. © 2013 Pearson Education, Inc.

The structure of the Atom • Charge – Proton = +1 – Electron = -1 – Neutron = 0 • Mass – Proton = 1 amu – Electron = 0 amu – Neutron = 1 amu

Atomic Numbers • Henry Moseley (1887 – 1915) – Found that atoms of each element contain a unique positive charge in their nucleus. – Atom’s identity comes from the number of protons in its nucleus. • Atomic number – number of protons – Unique to every element. – Example : Nitrogen = 7 protons. Hydrogen = 1 proton. – In a neutral atom the number of protons always equals number of electrons.

Sample Problem #1 • How many protons and electrons are present in a Sodium atom?

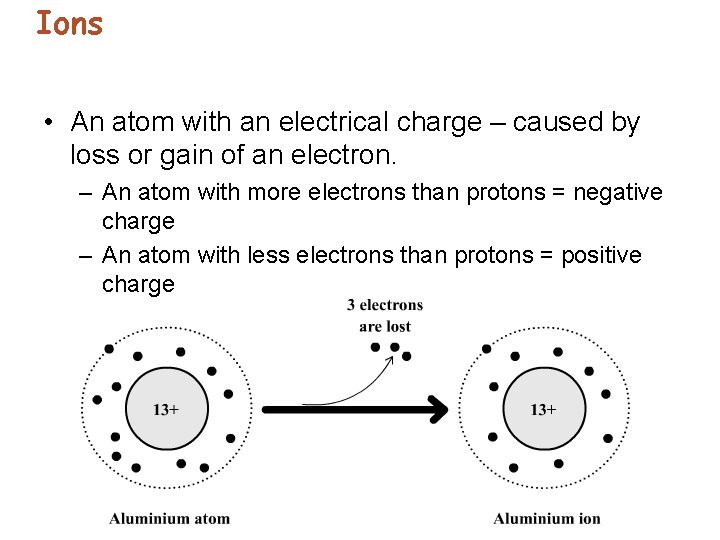
Ions • An atom with an electrical charge – caused by loss or gain of an electron. – An atom with more electrons than protons = negative charge – An atom with less electrons than protons = positive charge

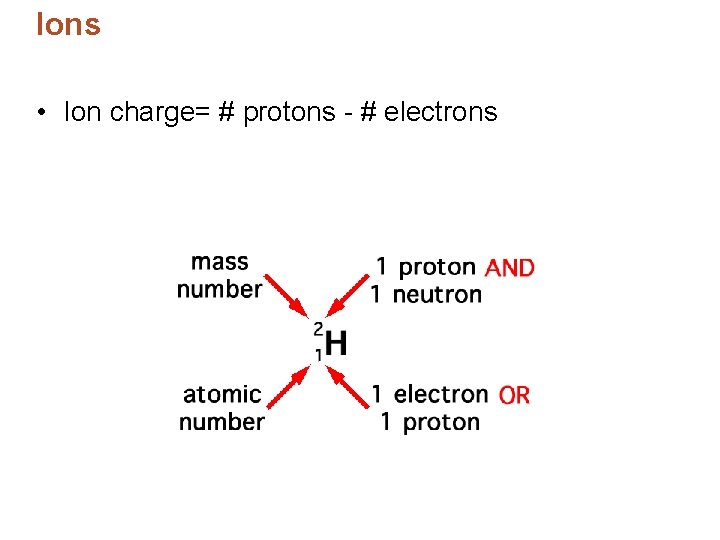
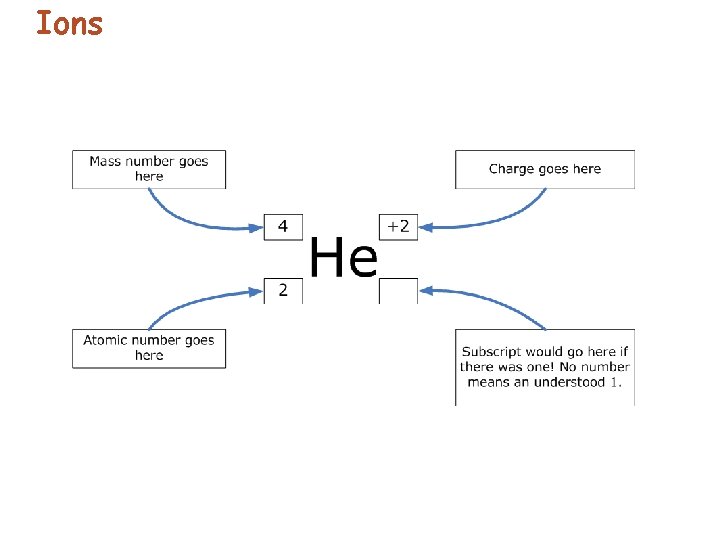
Ions • Ion charge= # protons - # electrons

Ions

Sample Problem #2 • What is the chemical symbol for the ion with 18 protons and 15 electrons?

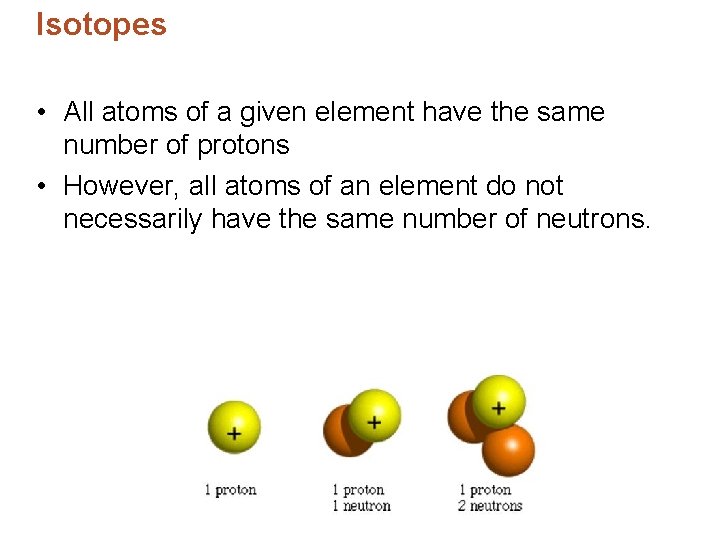
Isotopes • All atoms of a given element have the same number of protons • However, all atoms of an element do not necessarily have the same number of neutrons.

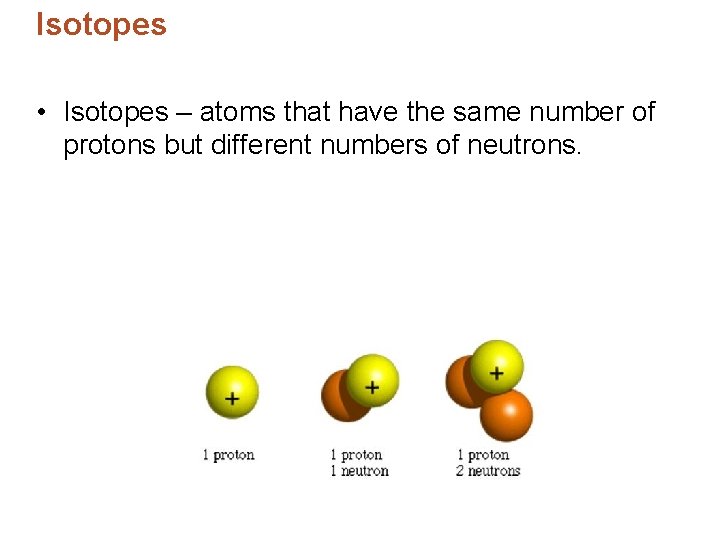
Isotopes • Isotopes – atoms that have the same number of protons but different numbers of neutrons.

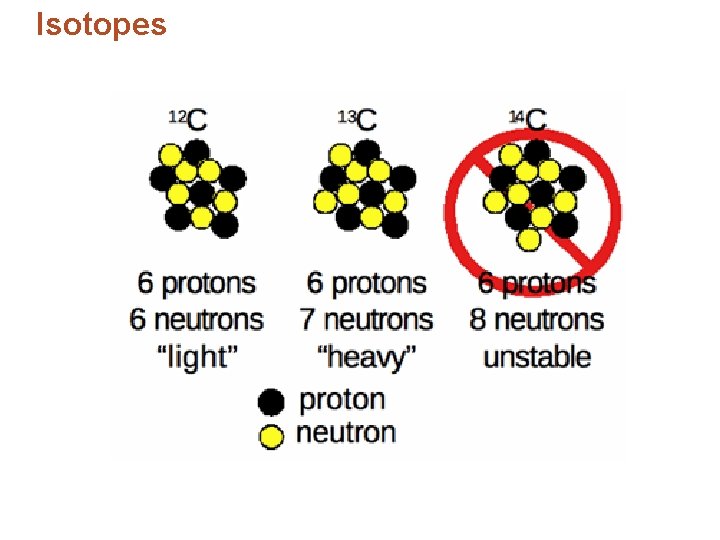
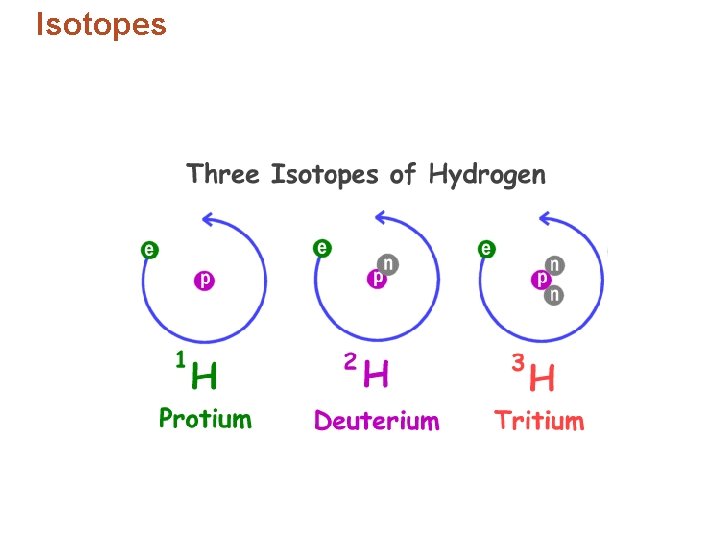
Isotopes • In nature elements are found as mixtures of isotopes, but always in the same percentages. – Example: hydrogen found as 99. 9844% atoms with no neutrons and 0. 0156% atoms with one neutron. • Isotopes are almost indistinguishable. • Chemical properties depend primarily on electrons and protons. • Difference in mass – “heavy, ” “light”

Isotopes

Isotopes

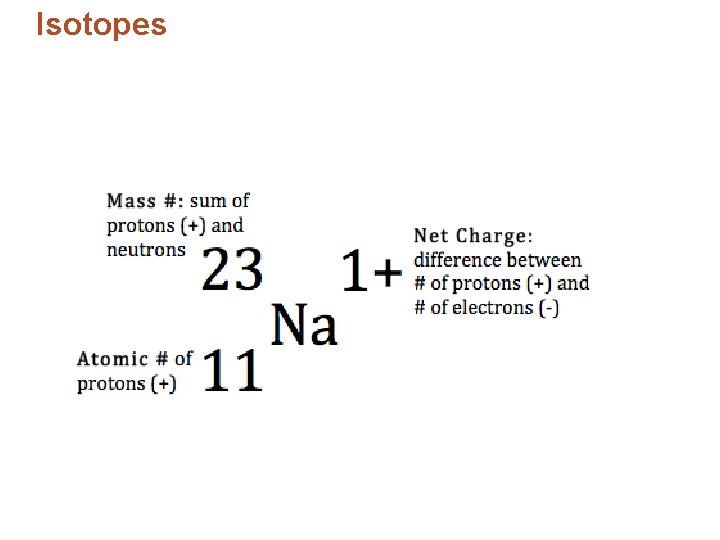
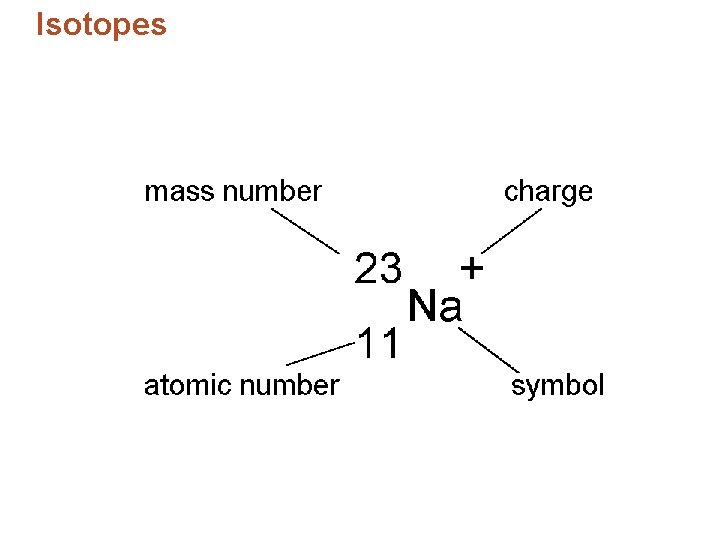
Isotopes • Identifying isotopes: – Number added after the element’s name. – Isotope’s mass number – the sum of the isotope’s number of protons and neutrons. – Example: chlorine-37, 17 protons and 20 neutrons.

Isotopes

Isotopes

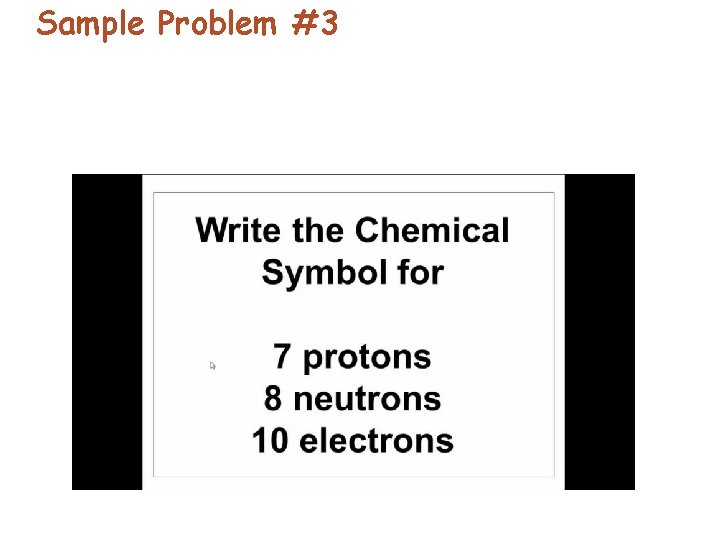
Sample Problem #3

Isotopes and Atomic Mass • Isotopes – are atoms of the same element that contain the same number of protons but different numbers of neutrons in the nucleus. – are identified by mass number, which is the total number of protons and neutrons in the nucleus. – differ only in mass, not electric charge; therefore, isotopes share many characteristics. • Total number of neutrons in isotope: Mass number atomic number © 2013 Pearson Education, Inc.

Isotopes and Atomic Mass • Atomic mass is – the total mass of the atom(s) [protons, neutrons, and electrons]. – listed in the periodic table in atomic mass units. • One atomic mass unit is equal to 1. 661 10– 24 gram or 1. 661 10– 27 kg. © 2013 Pearson Education, Inc.

Isotopes and Atomic Mass CHECK YOUR NEIGHBOR The atomic number of an element matches the number of A. B. C. D. protons in the nucleus of an atom. electrons in a neutral atom. both of the above none of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

Isotopes and Atomic Mass CHECK YOUR NEIGHBOR A nucleus with an atomic number of 44 and a mass number of 100 must have A. B. C. D. 44 neutrons. 56 neutrons. 100 neutrons. none of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

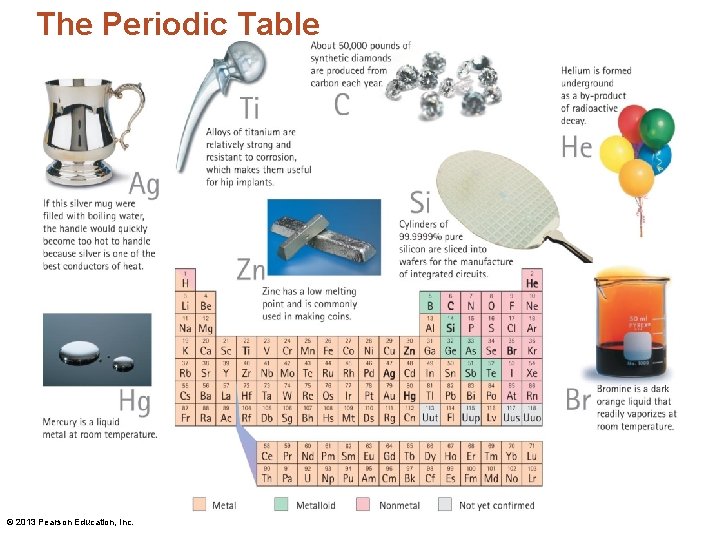
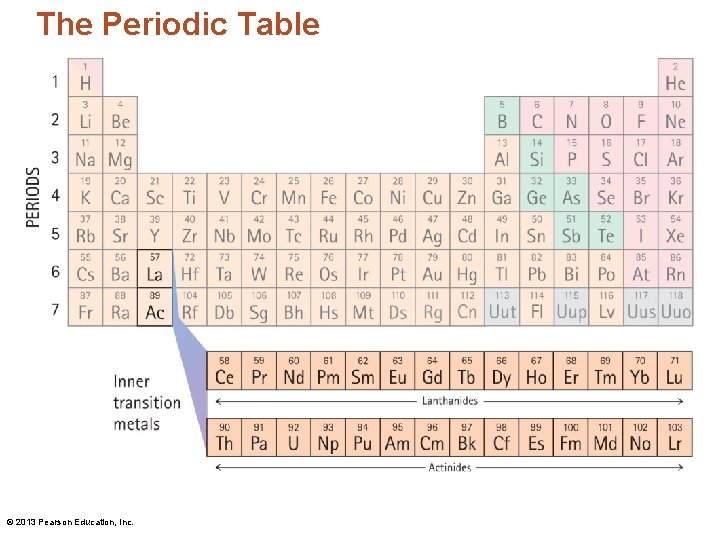
The Periodic Table • The periodic table is a listing of all the known elements. • It is NOT something to be memorized. • Instead, we learn how to READ the periodic table. • A chemist uses the periodic table much as a writer uses a dictionary. NEITHER needs be memorized! © 2013 Pearson Education, Inc.

The Periodic Table © 2013 Pearson Education, Inc.

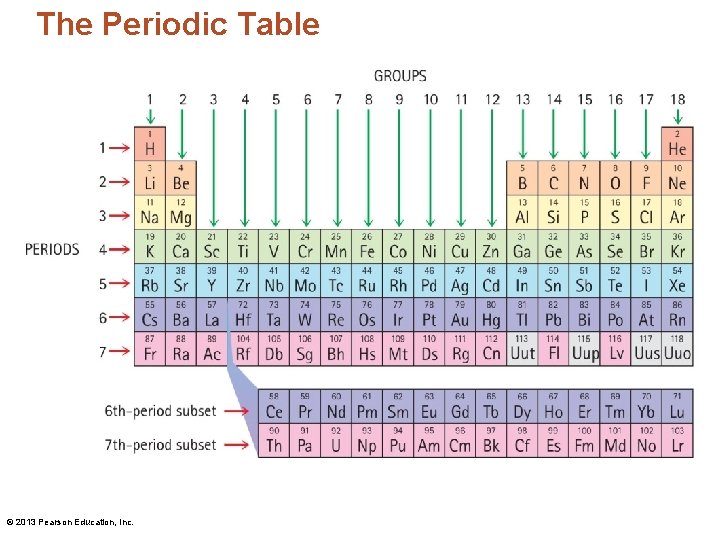
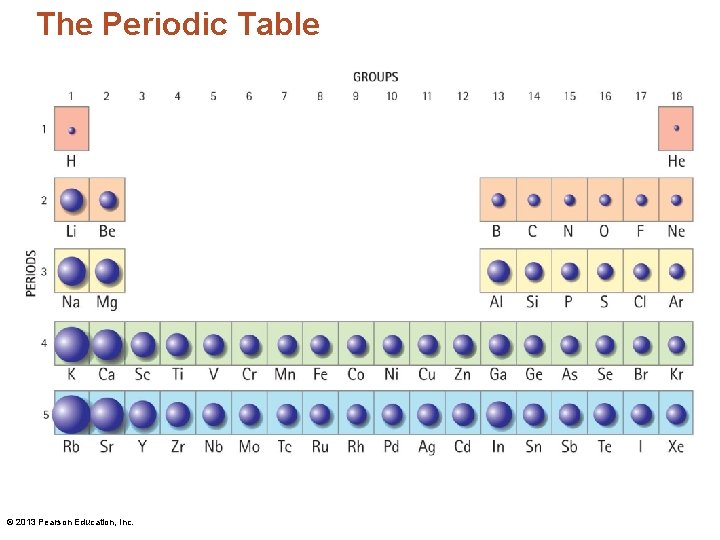
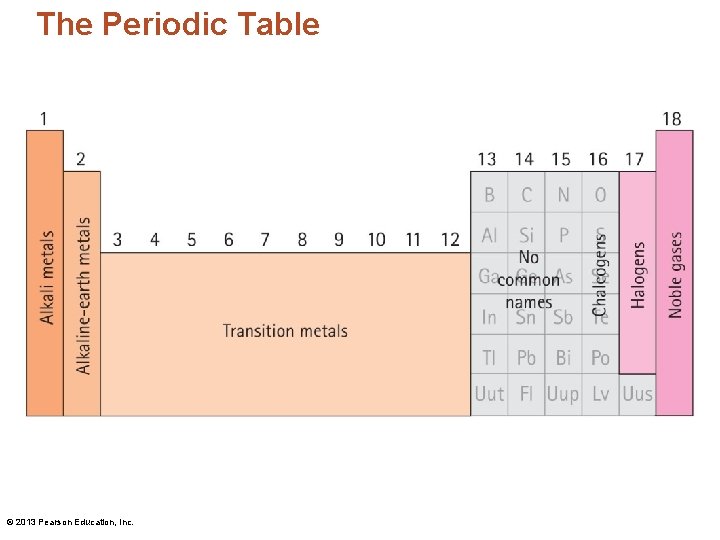
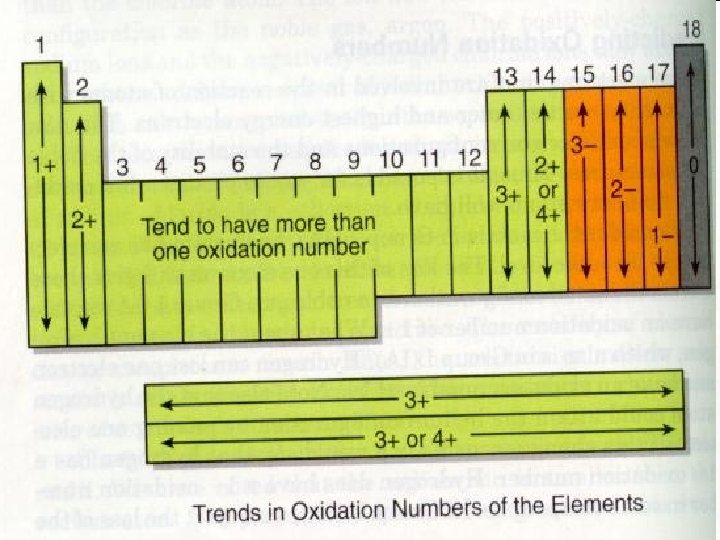
The Periodic Table • The elements are highly organized within the periodic table. • Each vertical column is called a group/family. • Each horizontal row is called a period. © 2013 Pearson Education, Inc.

The Periodic Table © 2013 Pearson Education, Inc.

The Periodic Table © 2013 Pearson Education, Inc.

The Periodic Table © 2013 Pearson Education, Inc.

The Periodic Table © 2013 Pearson Education, Inc.

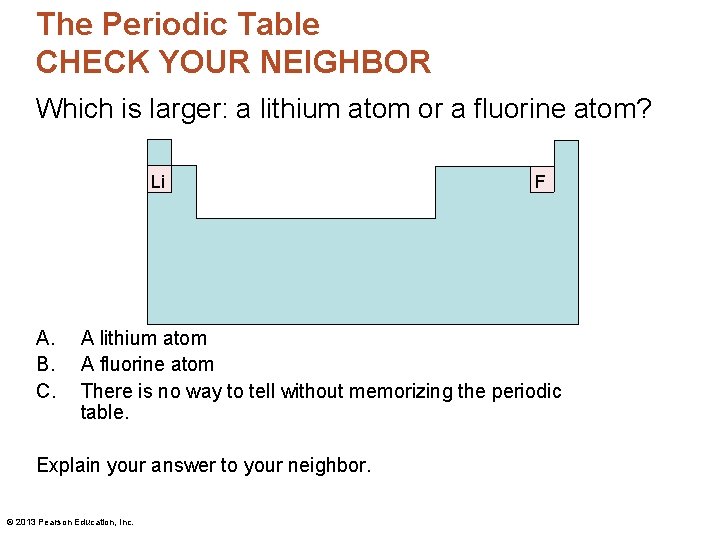
The Periodic Table CHECK YOUR NEIGHBOR Which is larger: a lithium atom or a fluorine atom? Li A. B. C. F A lithium atom A fluorine atom There is no way to tell without memorizing the periodic table. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

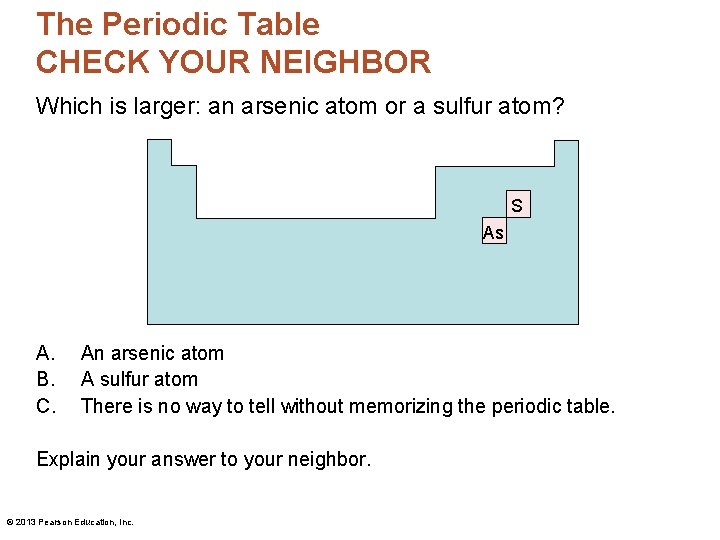
The Periodic Table CHECK YOUR NEIGHBOR Which is larger: an arsenic atom or a sulfur atom? S As A. B. C. An arsenic atom A sulfur atom There is no way to tell without memorizing the periodic table. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

Physical and Conceptual Models • A physical model replicates an object at a convenient scale. – Model trains, cars, planes, buildings • A conceptual model describes a system. – An atom is best described by a conceptual model. © 2013 Pearson Education, Inc.

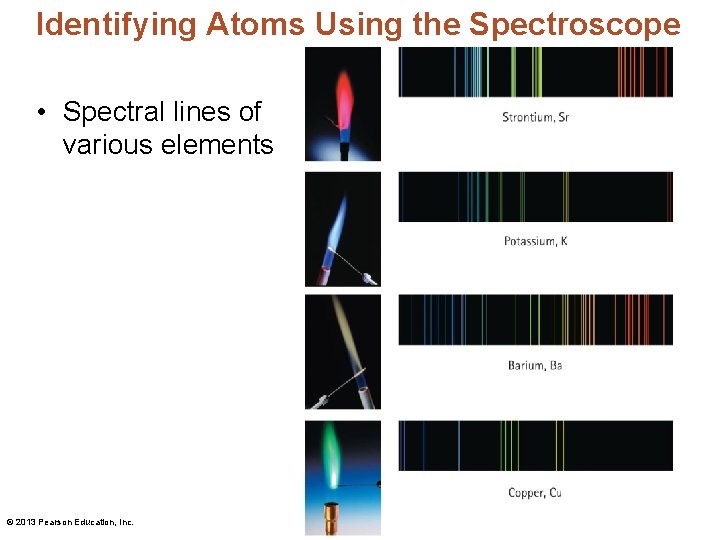
Identifying Atoms Using the Spectroscope • A spectroscope – is an instrument that separates and spreads light into its component frequencies. – allows the analysis of light emitted by elements when they are made to glow. It identifies each element by its characteristic pattern. – Each element emits a distinctive glow when energized and displays a distinctive spectrum. © 2013 Pearson Education, Inc.

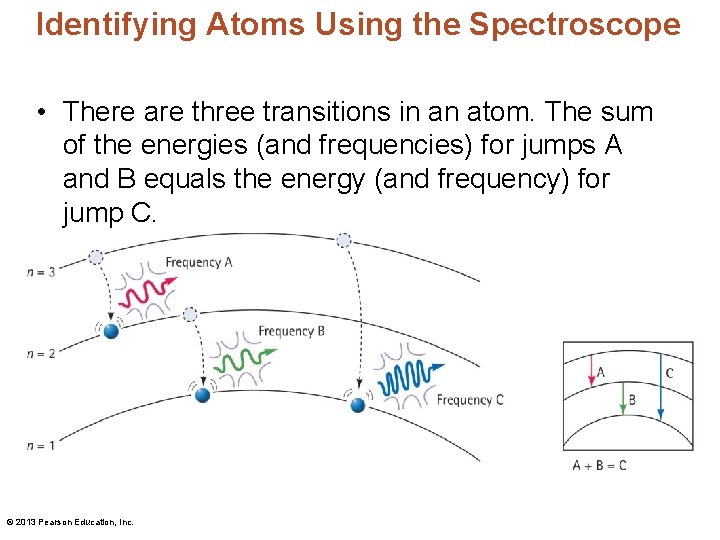
Identifying Atoms Using the Spectroscope • An atomic spectrum is an element's fingerprint— a pattern of discrete (distinct) frequencies of light. • Discoveries of the atomic spectrum of hydrogen: – A researcher in the 1800 s noted that hydrogen has a more orderly atomic spectrum than others. – Johann Balmer expressed line positions by a mathematical formula. – Johannes Rydberg noted that the sum of the frequencies of two lines often equals the frequency of a third line. © 2013 Pearson Education, Inc.

Identifying Atoms Using the Spectroscope • Spectral lines of various elements © 2013 Pearson Education, Inc.

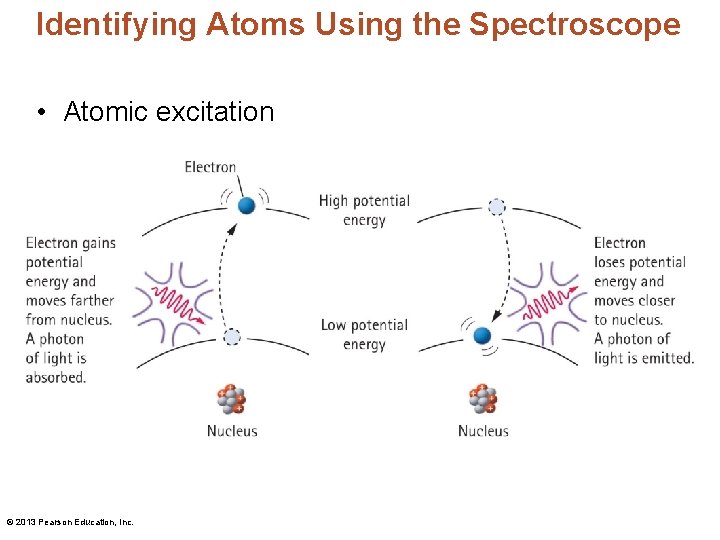
Identifying Atoms Using the Spectroscope • Atomic excitation © 2013 Pearson Education, Inc.

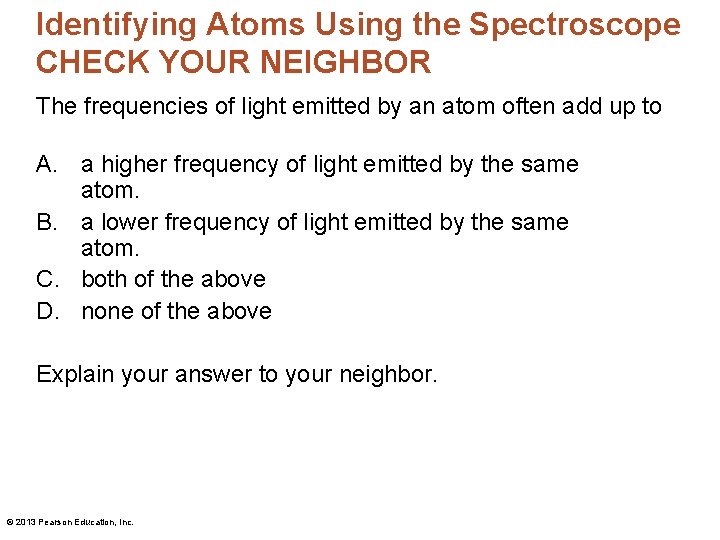
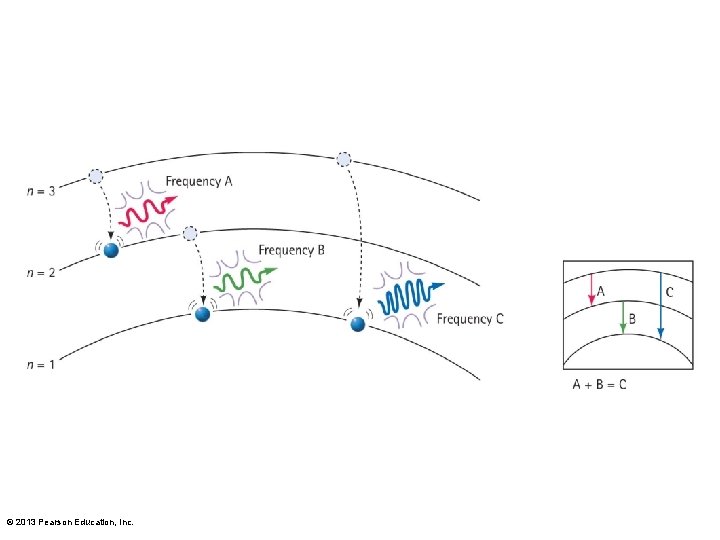
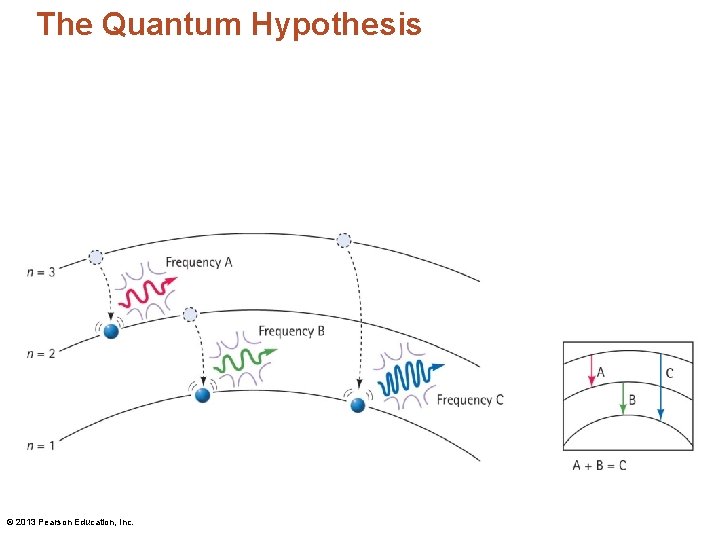
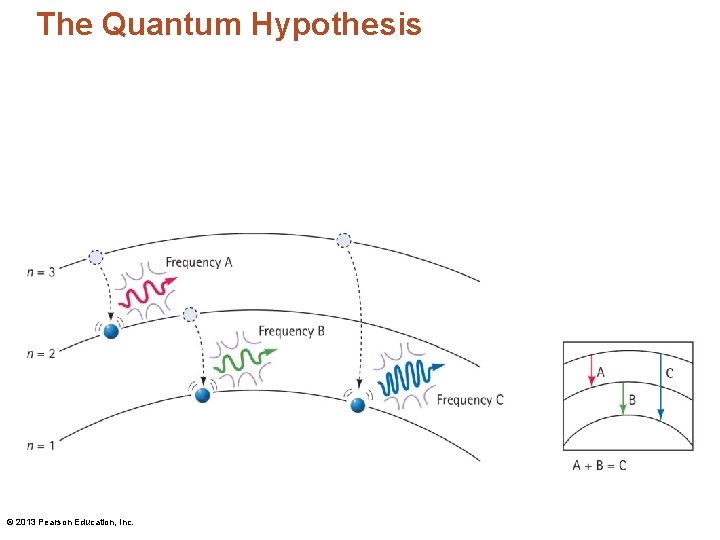
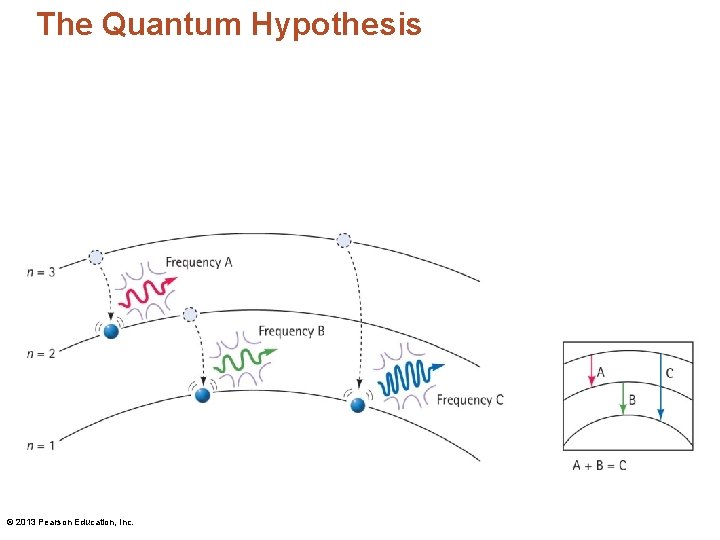
Identifying Atoms Using the Spectroscope • There are three transitions in an atom. The sum of the energies (and frequencies) for jumps A and B equals the energy (and frequency) for jump C. © 2013 Pearson Education, Inc.

Identifying Atoms Using the Spectroscope CHECK YOUR NEIGHBOR Each spectral line in an atomic spectrum represents A. a specific frequency of light emitted by an element. B. one of the many colors of an element. C. a pattern characteristic of the element. D. all of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

Identifying Atoms Using the Spectroscope CHECK YOUR NEIGHBOR The hydrogen spectrum consists of many spectral lines. How can this simple element have so many lines? A. One electron can be boosted to many different energy levels. B. The electron can move at a variety of speeds. C. The electron can vibrate at a variety of frequencies. D. Many standing electron waves can fit in the shell of the hydrogen atom. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

Identifying Atoms Using the Spectroscope CHECK YOUR NEIGHBOR When an atom is excited, its A. electrons are boosted to higher energy levels. B. atoms are charged with light energy. C. atoms are made to shake, rattle, and roll. D. none of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

Identifying Atoms Using the Spectroscope CHECK YOUR NEIGHBOR The frequencies of light emitted by an atom often add up to A. a higher frequency of light emitted by the same atom. B. a lower frequency of light emitted by the same atom. C. both of the above D. none of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

The Quantum Hypothesis • Max Planck, a German physicist, hypothesized that warm bodies emit radiant energy in discrete bundles called quanta. The energy in each energy bundle is proportional to the frequency of the radiation. • Einstein stated that light itself is quantized. A beam of light is not a continuous stream of energy but consists of countless small discrete quanta of energy, with each quantum called a photon. © 2013 Pearson Education, Inc.

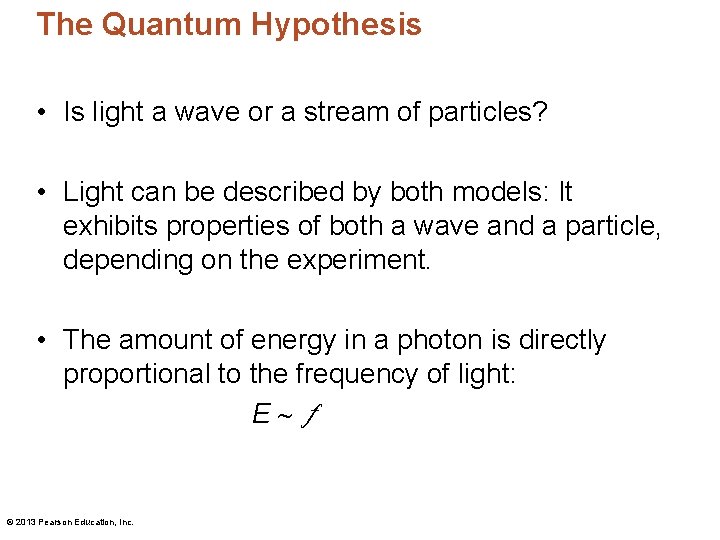
The Quantum Hypothesis • Is light a wave or a stream of particles? • Light can be described by both models: It exhibits properties of both a wave and a particle, depending on the experiment. • The amount of energy in a photon is directly proportional to the frequency of light: E © 2013 Pearson Education, Inc.

The Quantum Hypothesis CHECK YOUR NEIGHBOR In the relationship E , the symbol stands for the frequency of emitted light, and E stands for the A. B. C. D. potential energy of the electron emitting the light. energy of the photon. kinetic energy of the photon. all of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

The Quantum Hypothesis CHECK YOUR NEIGHBOR Which of these has the most energy per photon? A. B. C. D. Red light Green light Blue light All have the same. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

The Quantum Hypothesis CHECK YOUR NEIGHBOR Which of these photons has the least energy? A. B. C. D. Infrared Visible Ultraviolet All have the same. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

The Quantum Hypothesis • Using the quantum hypothesis – Danish physicist Niels Bohr explained the formation of atomic spectra as follows: • The potential energy of an electron depends on its distance from the nucleus. • When an atom absorbs a photon of light, it absorbs energy. Then a low-potential-energy electron is boosted to become a high-potential-energy electron. © 2013 Pearson Education, Inc.

The Quantum Hypothesis © 2013 Pearson Education, Inc.

The Quantum Hypothesis • Using the quantum hypothesis (continued): – When an electron in any energy level drops closer to the nucleus, it emits a photon of light. – Bohr reasoned that there must be a number of distinct energy levels within the atom. Each energy level has a principal quantum number n, where n is always an integer. The lowest level is n = 1 and is closest to the nucleus. – Electrons release energy in discrete amounts that form discrete lines in the atom's spectrum. © 2013 Pearson Education, Inc.

The Quantum Hypothesis © 2013 Pearson Education, Inc.

The Quantum Hypothesis CHECK YOUR ANSWER Which of the following is a quantum number? A. B. C. D. 0. 02 0. 2 2 2. 5 Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

The Quantum Hypothesis • Bohr's model explains why atoms don't collapse – Electrons can lose only specific amounts of energy equivalent to transitions between levels. – An atom reaches the lowest energy level called the ground state, where the electron can't lose more energy and can't move closer to the nucleus. © 2013 Pearson Education, Inc.

The Quantum Hypothesis © 2013 Pearson Education, Inc.

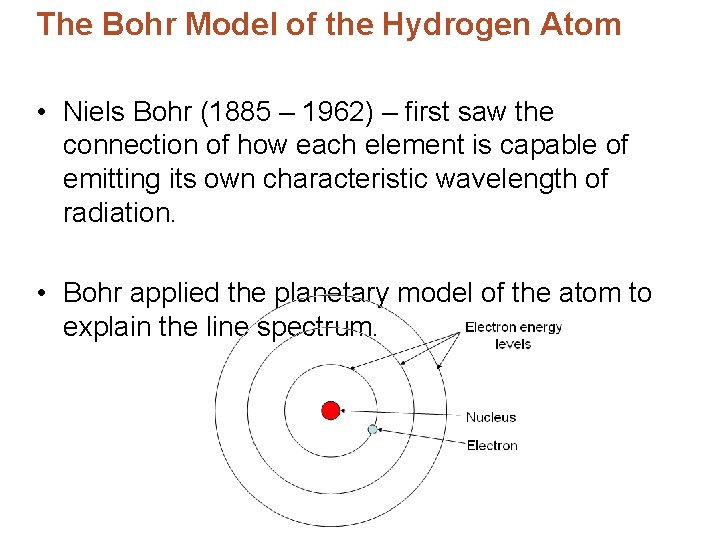
The Bohr Model of the Hydrogen Atom • Niels Bohr (1885 – 1962) – first saw the connection of how each element is capable of emitting its own characteristic wavelength of radiation. • Bohr applied the planetary model of the atom to explain the line spectrum.

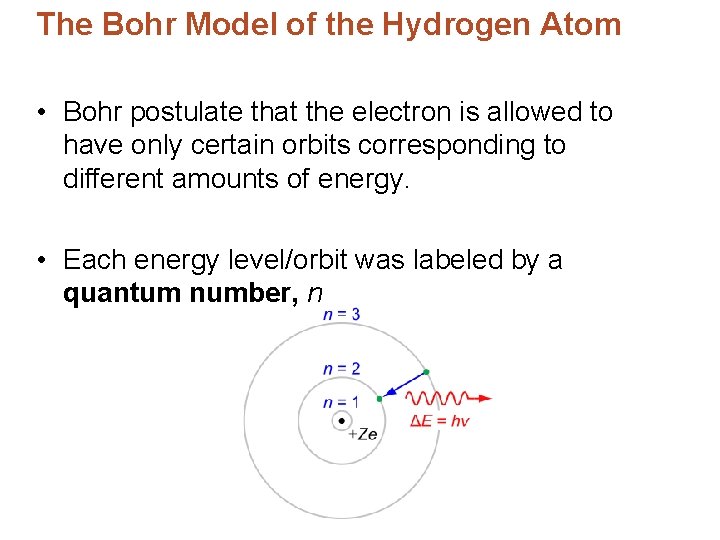
The Bohr Model of the Hydrogen Atom • Bohr postulate that the electron is allowed to have only certain orbits corresponding to different amounts of energy. • Each energy level/orbit was labeled by a quantum number, n

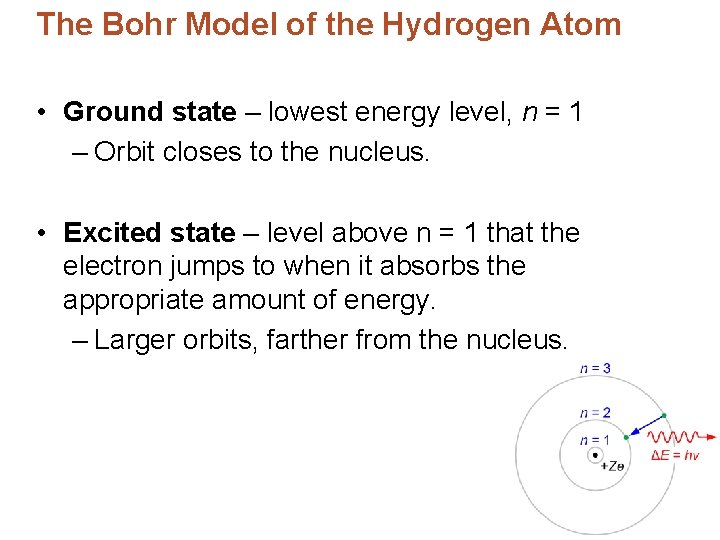
The Bohr Model of the Hydrogen Atom • Ground state – lowest energy level, n = 1 – Orbit closes to the nucleus. • Excited state – level above n = 1 that the electron jumps to when it absorbs the appropriate amount of energy. – Larger orbits, farther from the nucleus.

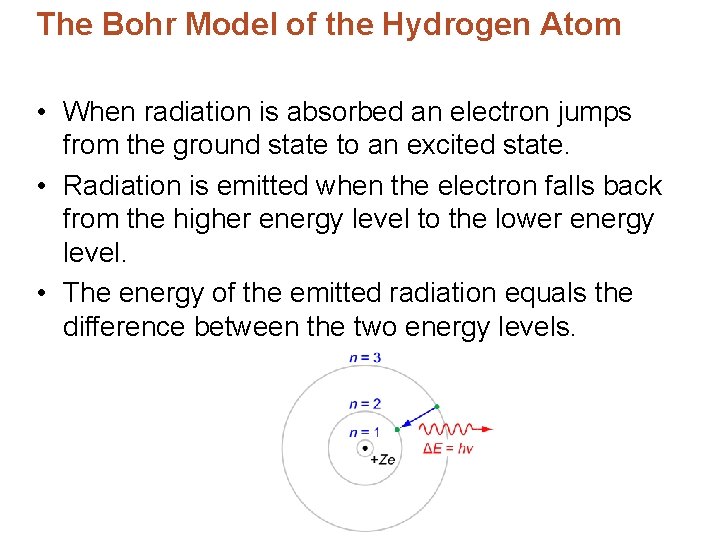
The Bohr Model of the Hydrogen Atom • When radiation is absorbed an electron jumps from the ground state to an excited state. • Radiation is emitted when the electron falls back from the higher energy level to the lower energy level. • The energy of the emitted radiation equals the difference between the two energy levels.

The Quantum Hypothesis • Planetary model of the atom: – Photons are emitted by atoms as electrons move from higher-energy outer levels to lower -energy inner levels. The energy of an emitted photon is equal to the difference in energy between the two levels. Because an electron is restricted to discrete levels, only lights of distinct frequencies are emitted. – The move between levels is instantaneous! © 2013 Pearson Education, Inc.

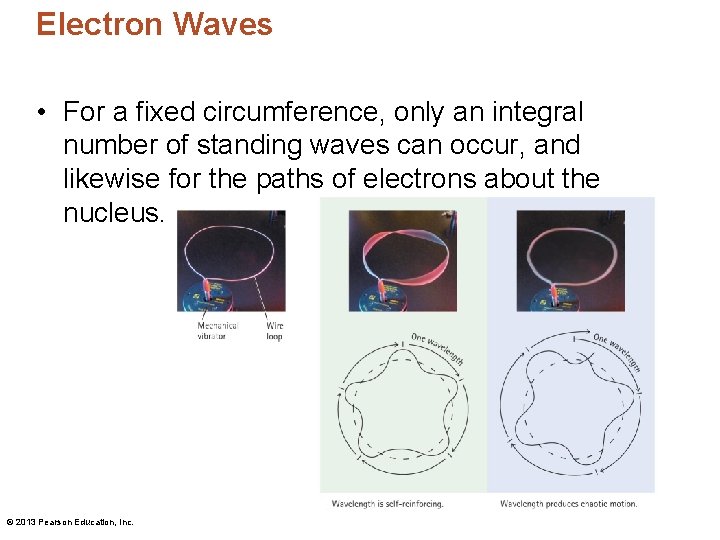
Electron Waves • An electron's wave nature explains why electrons in an atom are restricted to particular energy levels. The permitted energy levels are a natural consequence of standing electron waves closing in on themselves in a synchronized manner. • The orbit for n = 1 consists of a single wavelength, n = 2 of two wavelengths, and so on. © 2013 Pearson Education, Inc.

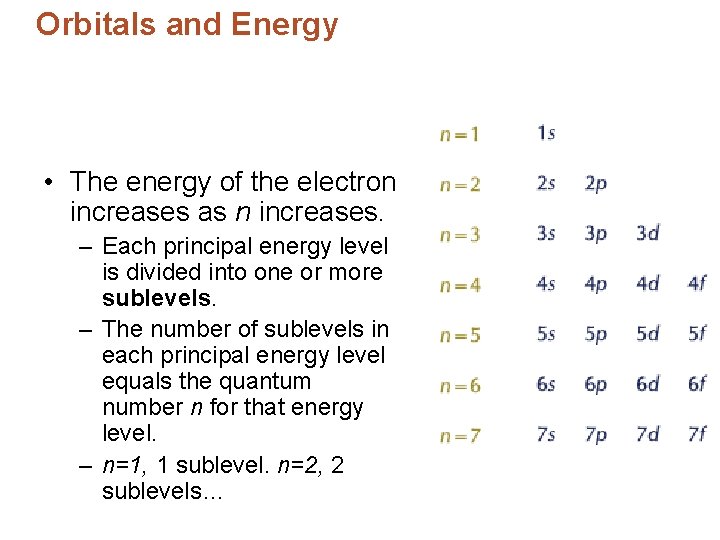
Orbitals and Energy • Bohr said that energies of electrons in atoms are quantized. – Electrons = energy levels • Principal energy levels – designated by the quantum number, n.

Orbitals and Energy • The energy of the electron increases as n increases. – Each principal energy level is divided into one or more sublevels. – The number of sublevels in each principal energy level equals the quantum number n for that energy level. – n=1, 1 sublevel. n=2, 2 sublevels…

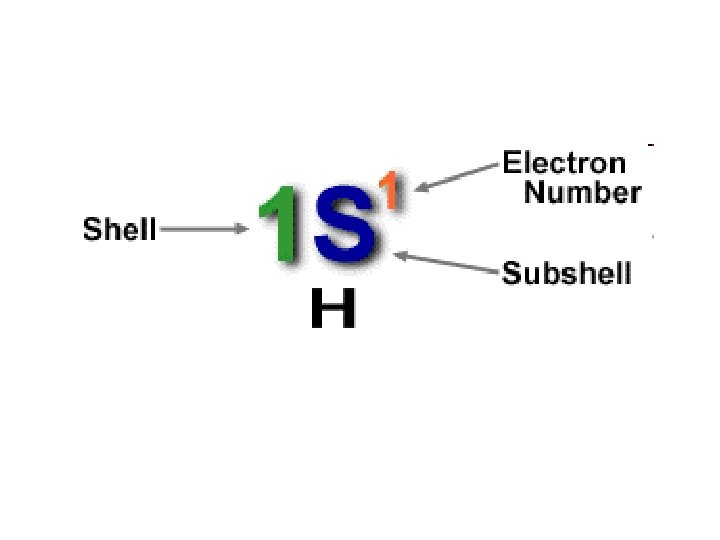
Orbitals and Energy • The sublevels are labeled with a number that is the value of n, and a letter (s, p, d, f, ) which identifies the sublevel. • *Each principal energy level (n=1, n=2. . ) consists of one or more sublevels – *Each sublevel consists of one or more orbitals. • * Orbital – a region in which an electron with a particular energy is likely to be found.


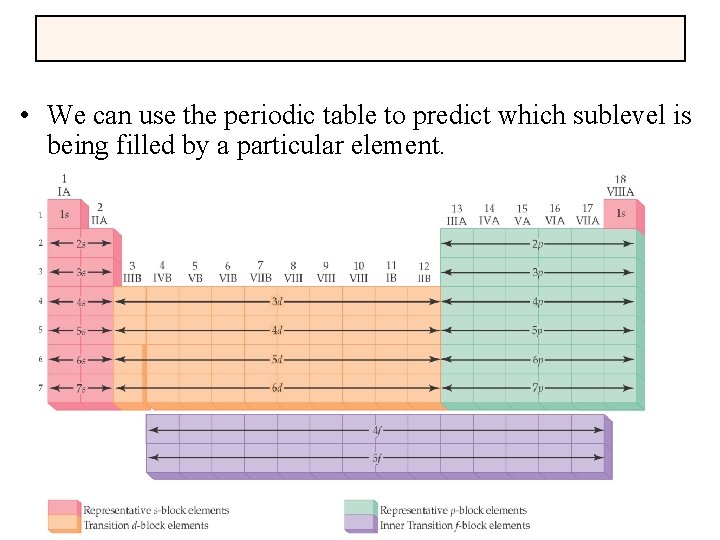
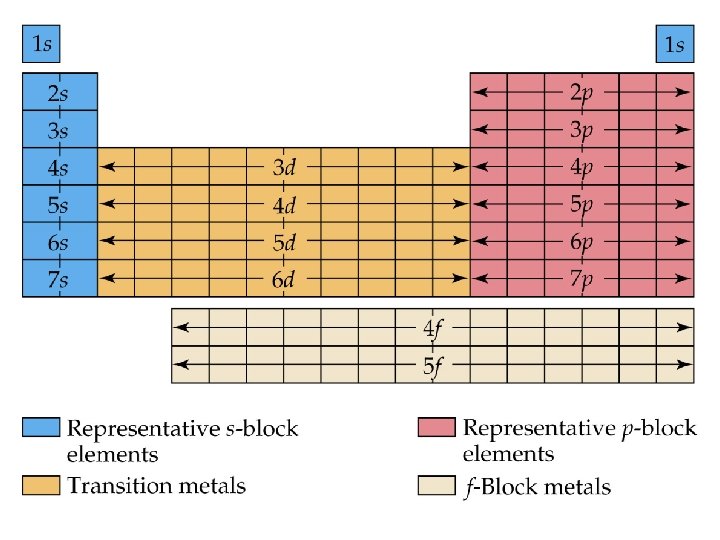
• We can use the periodic table to predict which sublevel is being filled by a particular element.



Electron Waves • Electrons or any particle, can show itself as a wave or as a particle, depending on how you examine it. • This is the wave-particle duality © 2013 Pearson Education, Inc.

Matter Waves • We can’t observe both the particle and wave properties of an electron by using the same experiment. – Depending on what property the experiment is designed to bring out then that’s what the electron is going to show.

Matter Waves • Scientists aren’t always concerned with duality. – When scientists study the motion of the space shuttle, they are concerned more with the shuttle as a particle than a wave. – In small particles like the electron however, scientists have to be concerned with both wave and particle properties.

Heisenberg’s Uncertainty Principle • Werner Heisenberg (1901 -1976) – proposed that the position and the momentum of a moving object cannot simultaneously be measured and known exactly. • Significant when dealing with electrons. – Only way to locate an electron is to strike it with a photon – because of its small mass measurement changes its position. – No way to observe or to measure the orbit of an electron in an atom. – Arrangement of electrons in atoms are discussed in terms of the probability of finding an electron in certain locations within the atom.

Heisenberg’s Uncertainty Principle • For us to see an object it must be hit by a photon of radiant energy • A photon hitting you or any object you can see has a negligible effect on it. • However, a collision between a photon and an electron results in a large change in the energy of the electron

Heisenberg’s Uncertainty Principle • When you “see” an electron using some sort of radiant energy as “illumination” you have found the exact position of the electron. • However, the collision between the photon and electron caused its velocity to change. • Therefore, we know the electrons position but not its velocity

Heisenberg’s Uncertainty Principle • On the other hand, if we measure an electron’s velocity, we will change the electron’s position

Heisenberg’s Uncertainty Principle • Heisenberg stated that there is always some uncertainty about the position and momentum of an electron • This became known as the Heisenberg Uncertainty Principle.

Heisenberg’s Uncertainty Principle • Computers can now calculate the probabilities of the location of the electron for thousands of points (places) in space.

Heisenberg’s Uncertainty Principle • There will be many points of equal probability. • If all points of highest probability are connected, a 3 D shape is formed. • This shape is just a mental model and does not exist.

Heisenberg’s Uncertainty Principle • Electrons are constantly in motion at high speeds. They look like a cloud. – Ex = like fan blades when a fan is turned on. • You can try to fit something in between the rotating fan blades but you will find that you can’t. • The fan blades are moving so fast that they are taking up all the space.

Heisenberg’s Uncertainty Principle • The volume occupied by an electron is somewhat vague. • This is why we refer to the electrons as an electron cloud rather than to a specific position.

Electron Waves • For a fixed circumference, only an integral number of standing waves can occur, and likewise for the paths of electrons about the nucleus. © 2013 Pearson Education, Inc.

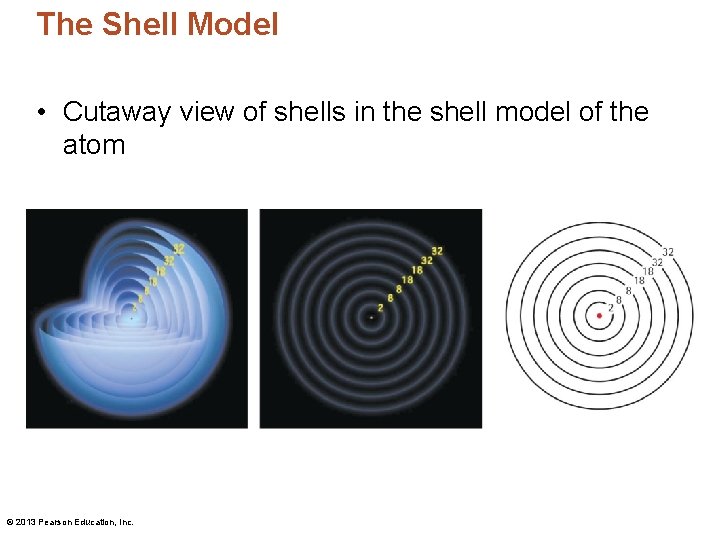
The Shell Model • Cutaway view of shells in the shell model of the atom © 2013 Pearson Education, Inc.

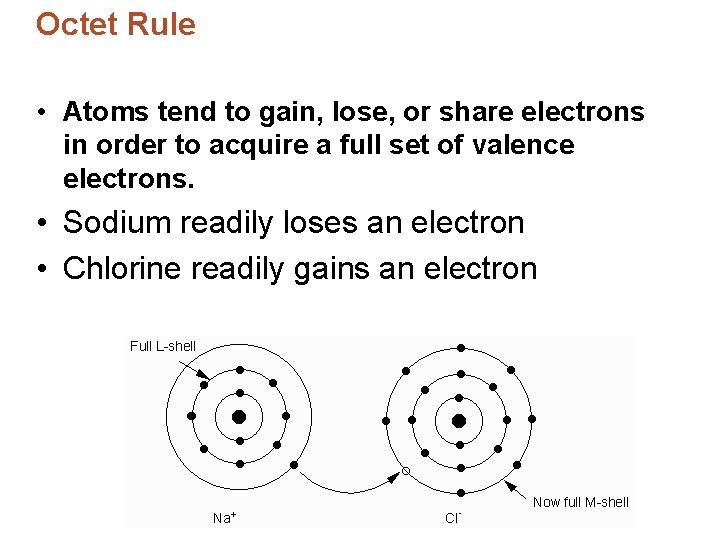
Octet Rule • Sodium chloride is much more stable than each element on its own. • Atoms are stable when their valence shell is full – 8 electrons. • Electron configurations: – Chlorine: [Ne]3 s 23 p 5 – Sodium: [Ne]3 s 1 • Chlorine has 7 valence electrons = needs to gain one • Sodium has 1 valence electrons = needs to lose one


Octet Rule • Atoms tend to gain, lose, or share electrons in order to acquire a full set of valence electrons. • Sodium readily loses an electron • Chlorine readily gains an electron

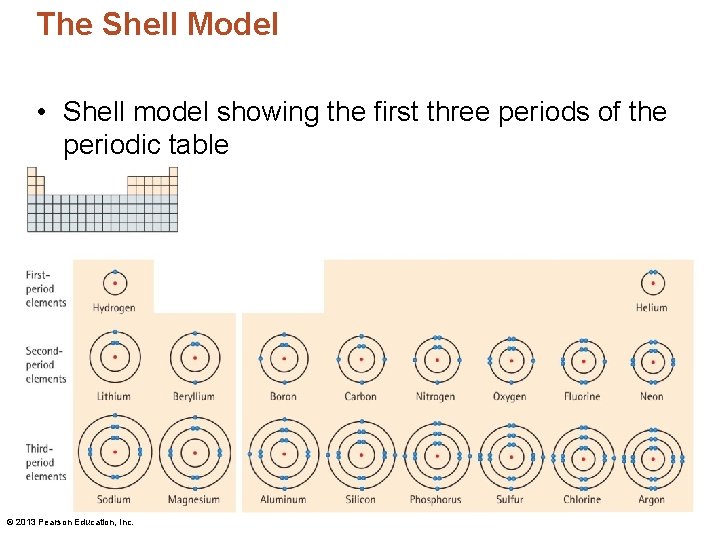
The Shell Model • Shell model showing the first three periods of the periodic table © 2013 Pearson Education, Inc.