Chapter 14 Lecture Conceptual Integrated Science Second Edition













- Slides: 67

Chapter 14 Lecture Conceptual Integrated Science Second Edition Organic Compounds © 2013 Pearson Education, Inc.

This lecture will help you understand: • • Organic Chemistry Hydrocarbons Unsaturated Hydrocarbons Functional Groups Alcohols, Phenols, and Ethers Amines and Alkaloids Carbonyl Compounds Polymers © 2013 Pearson Education, Inc.

Organic Chemistry • Organic chemistry is the branch of chemistry that involves the study of carbon-containing chemical compounds. • An organic compound is a carbon-containing chemical compound. More than 13 million organic compounds are known. © 2013 Pearson Education, Inc.

Organic Chemistry • Carbon atoms connect with one another through strong and stable covalent bonds. © 2013 Pearson Education, Inc.

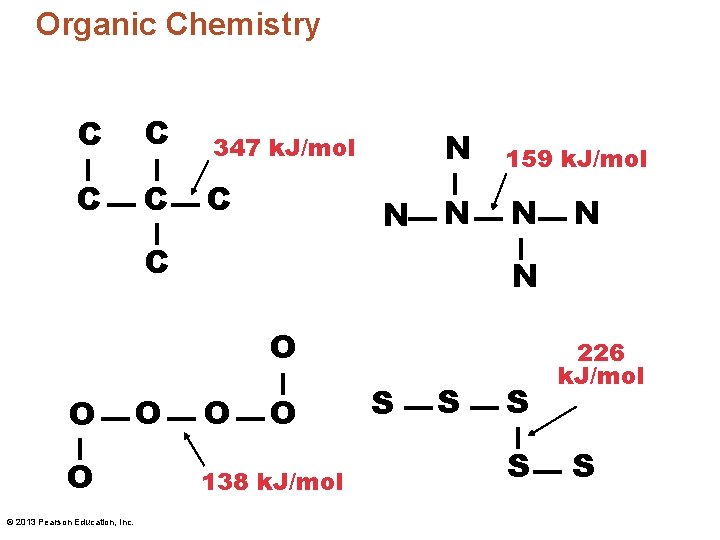
Organic Chemistry C C 347 k. J/mol C C C N N 159 k. J/mol N N C N O O O © 2013 Pearson Education, Inc. O N O O 138 k. J/mol S S 226 k. J/mol S

Organic Chemistry • Carbon atoms also readily form bonds with many other types of atoms. This provides for a nearly infinite number of different kinds of organic compounds. © 2013 Pearson Education, Inc.

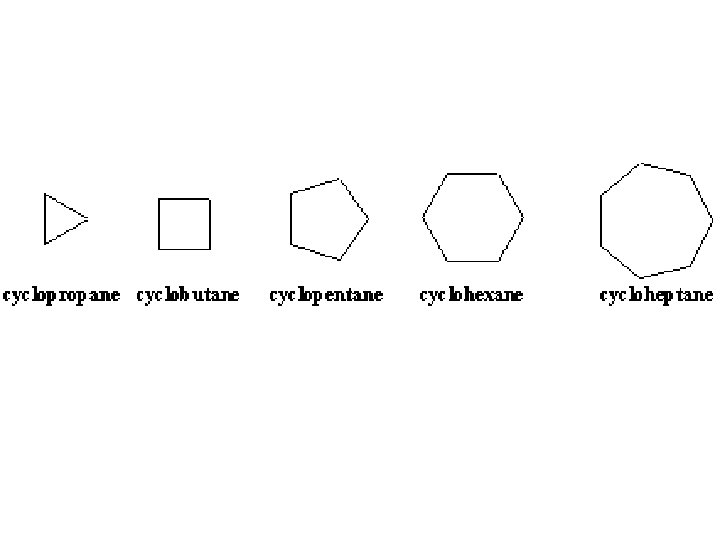
Hydrocarbons • A hydrocarbon is a chemical compound that contains only hydrogen and carbon. © 2013 Pearson Education, Inc.

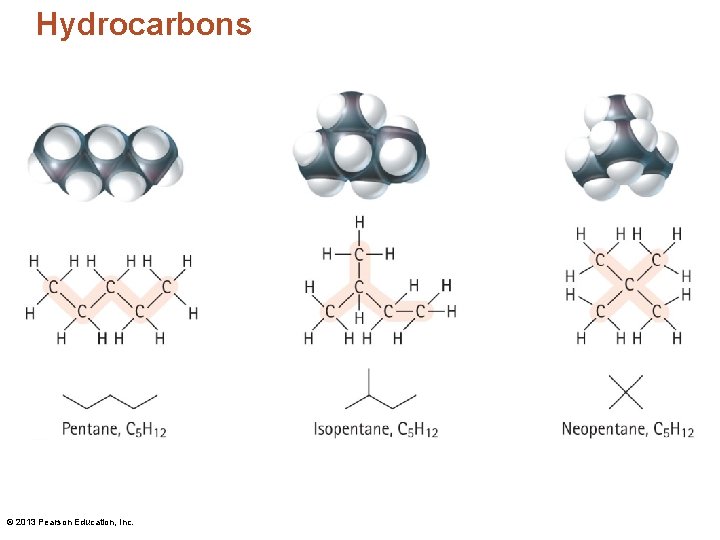
Hydrocarbons © 2013 Pearson Education, Inc.

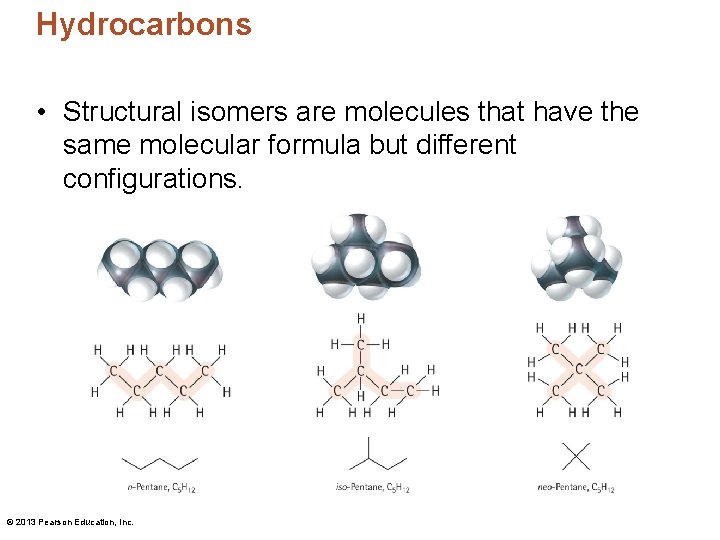
Hydrocarbons • Structural isomers are molecules that have the same molecular formula but different configurations. © 2013 Pearson Education, Inc.

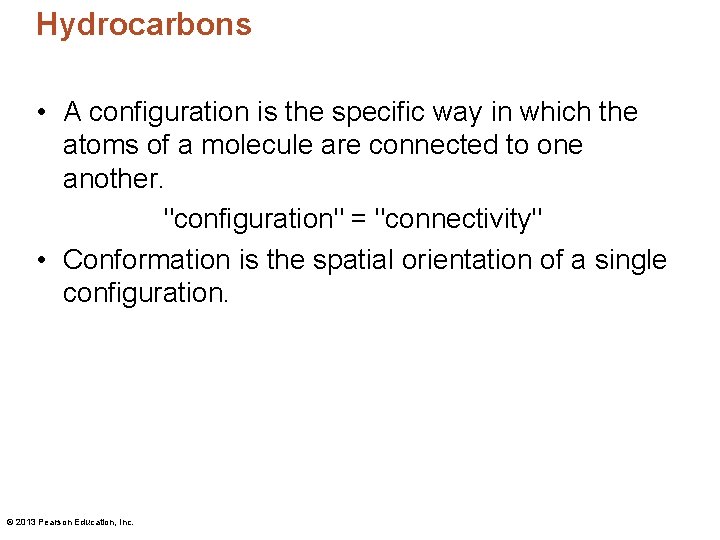
Hydrocarbons • A configuration is the specific way in which the atoms of a molecule are connected to one another. "configuration" = "connectivity" • Conformation is the spatial orientation of a single configuration. © 2013 Pearson Education, Inc.

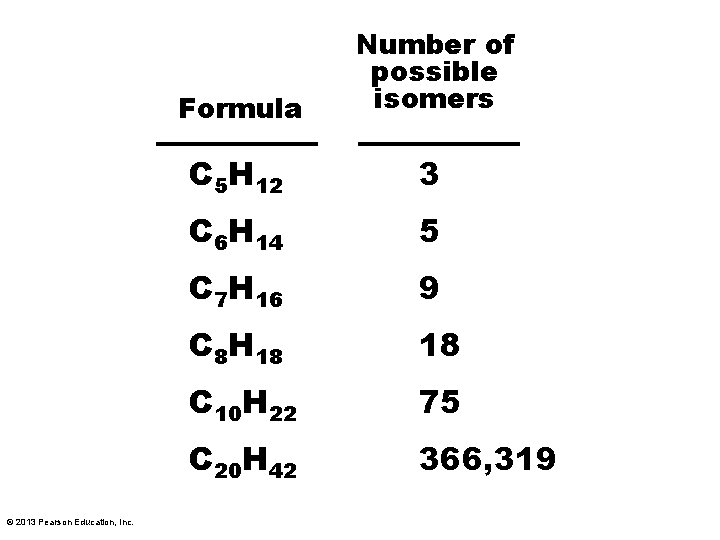
© 2013 Pearson Education, Inc. Formula Number of possible isomers C 5 H 12 3 C 6 H 14 5 C 7 H 16 9 C 8 H 18 18 C 10 H 22 75 C 20 H 42 366, 319

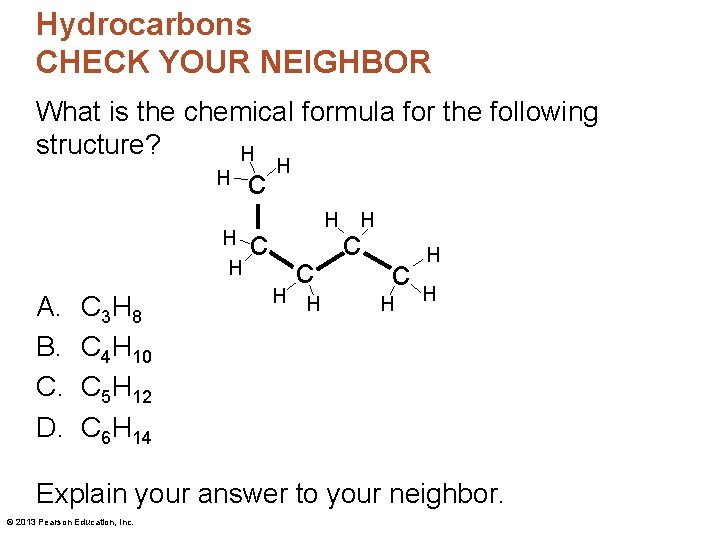
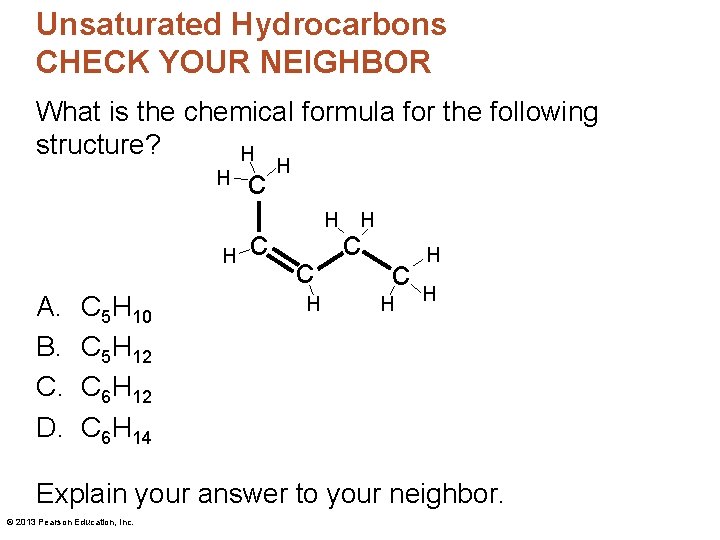
Hydrocarbons CHECK YOUR NEIGHBOR What is the chemical formula for the following structure? H H A. B. C. D. C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 c c H H H Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

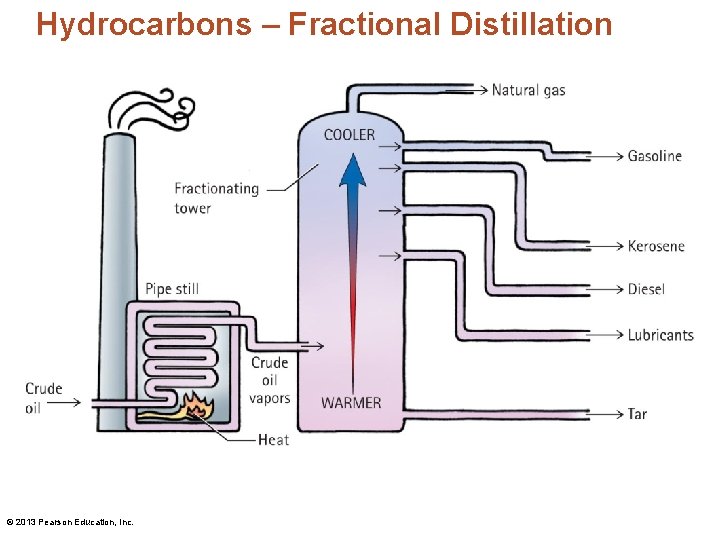
Hydrocarbons – Fractional Distillation © 2013 Pearson Education, Inc.

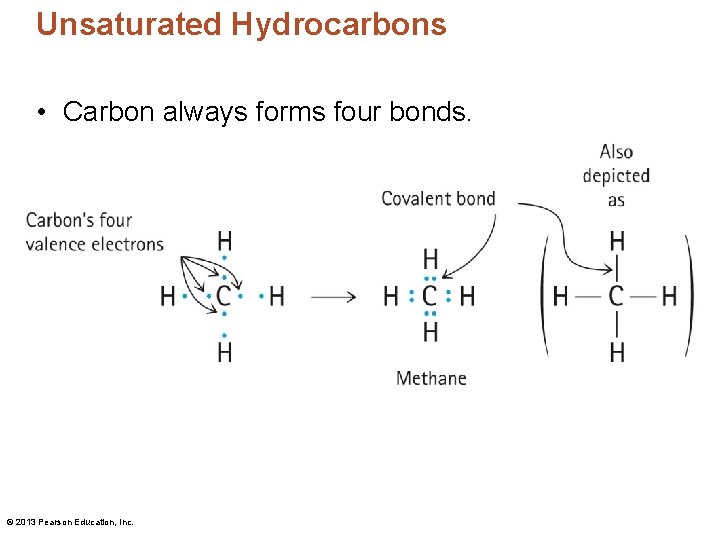
Unsaturated Hydrocarbons • Carbon always forms four bonds. © 2013 Pearson Education, Inc.

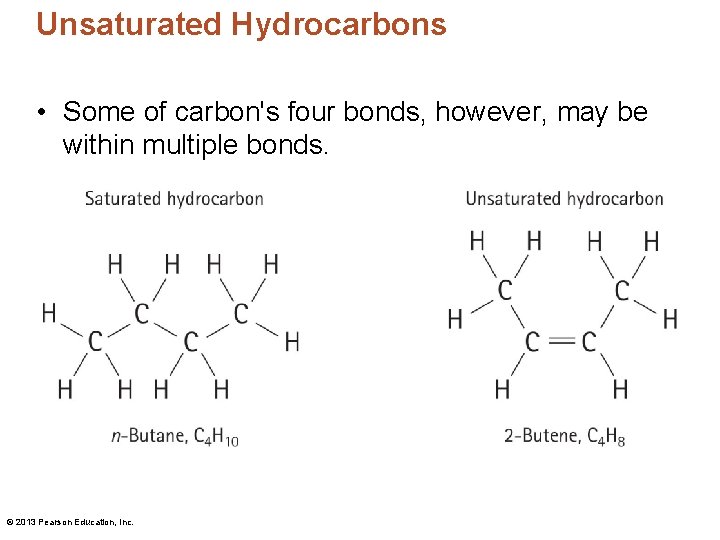
Unsaturated Hydrocarbons • Some of carbon's four bonds, however, may be within multiple bonds. © 2013 Pearson Education, Inc.

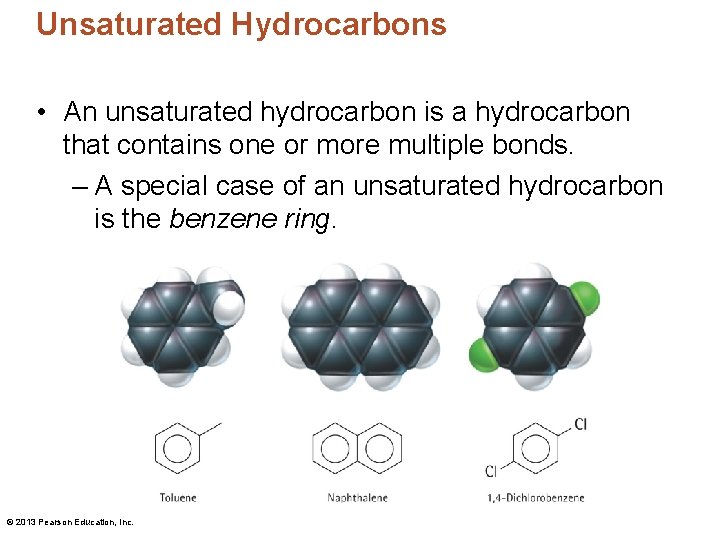
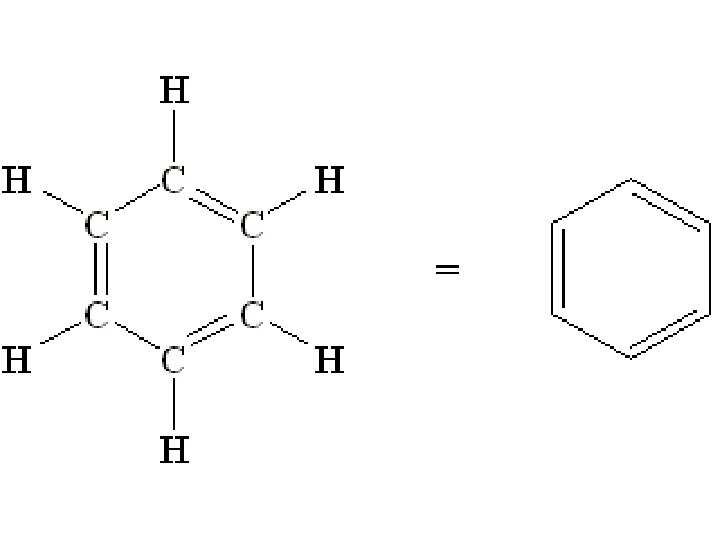
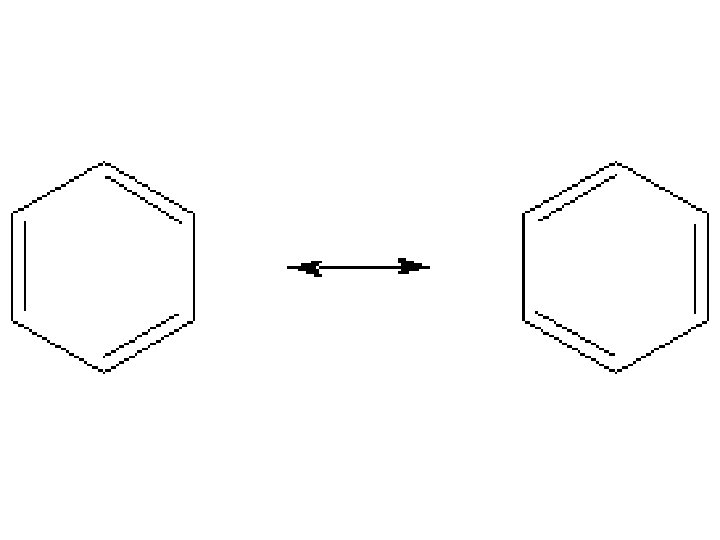
Unsaturated Hydrocarbons • An unsaturated hydrocarbon is a hydrocarbon that contains one or more multiple bonds. – A special case of an unsaturated hydrocarbon is the benzene ring. © 2013 Pearson Education, Inc.

Unsaturated Hydrocarbons CHECK YOUR NEIGHBOR What is the chemical formula for the following structure? H H H A. B. C. D. C 5 H 10 C 5 H 12 C 6 H 14 c c H H c H c H H H Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

• Compounds having only single bonds between carbon atoms are said to be saturated compounds. • The ending –ane is used to name saturated compounds (single bonds) • For compounds containing a carbon-carbon double bond, the suffix –ene is used • For compounds containing a carbon-carbon triple bond, the suffix -yne is used








• The existence of two or more substance with the same molecular formula, but different structures, is called isomerism • Theses structures are called isomers

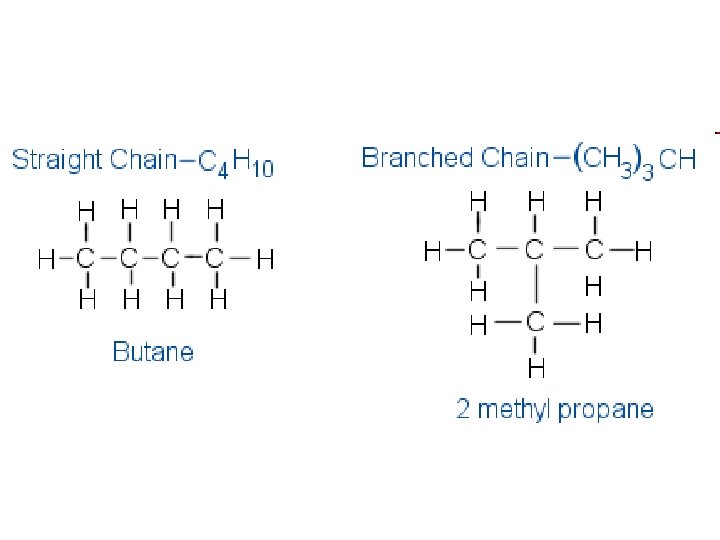
• Consider butane with the formula C 4 H 10 • There are 2 structures that can be written for this formula. – 1. Butane – 2. methylpropane – These are examples of structural isomers or skeleton isomers, since it is the carbon chain that is altered



• A second type of isomerism is positional isomerism. • This is when 2 compounds that differ only in the position of something such as a double bond or an atom other than hydrogen and carbon.

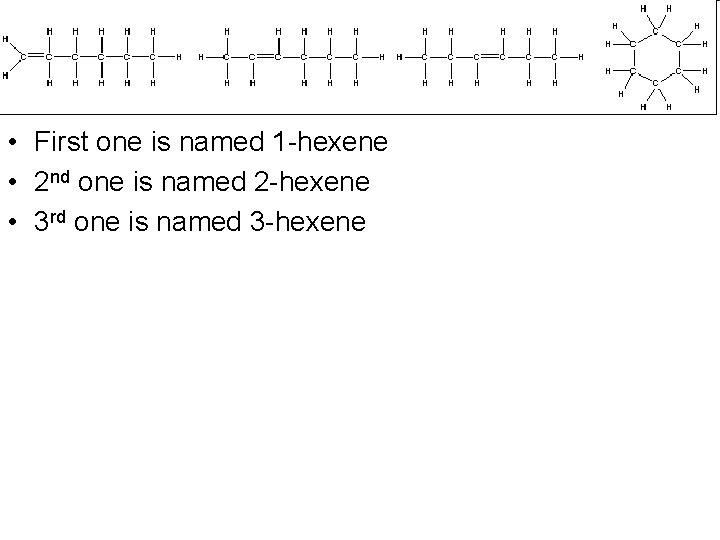
• The first 3 are all named hexene • We name isomers such as these by specifying on which carbon atom the double bond begins • So you start numbering from the end, giving the double bond the lowest number • Name the first 3 compounds above.

• First one is named 1 -hexene • 2 nd one is named 2 -hexene • 3 rd one is named 3 -hexene

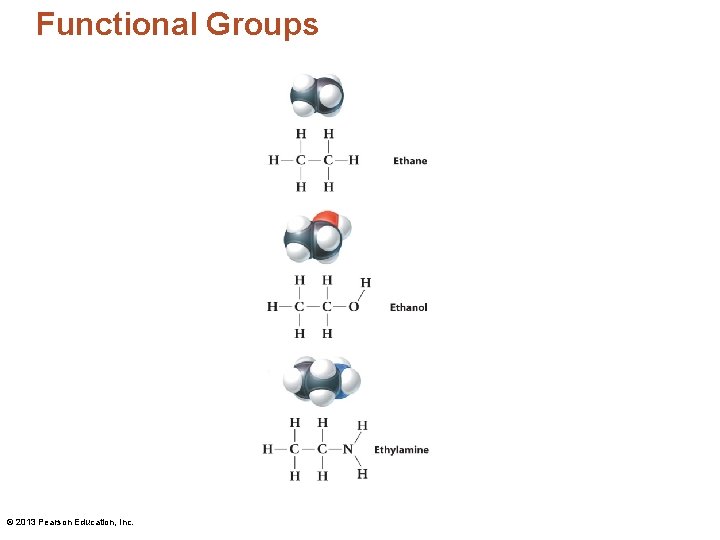
Functional Groups • A heteroatom is any atom other than hydrogen or carbon in an organic molecule. • A functional group is a combination of carbon, hydrogen, and heteroatoms that behave as a single unit. – Organic molecules are classified by the functional groups they contain. © 2013 Pearson Education, Inc.

Functional Groups © 2013 Pearson Education, Inc.

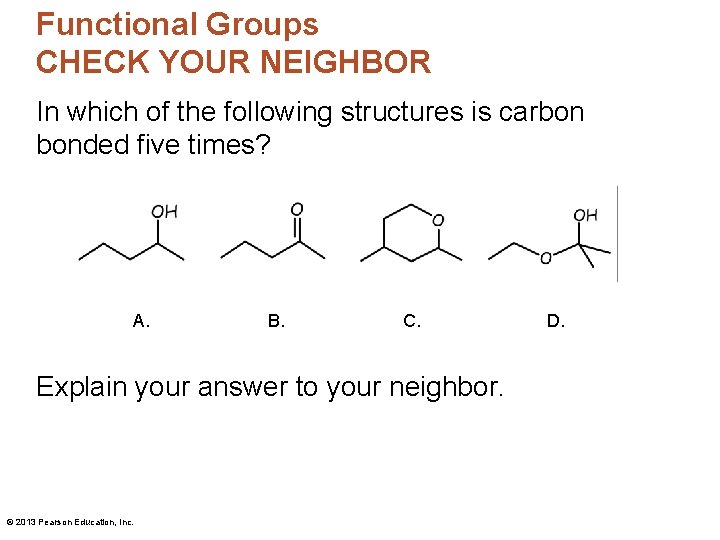
Functional Groups CHECK YOUR NEIGHBOR In which of the following structures is carbon bonded five times? A. B. C. Explain your answer to your neighbor. © 2013 Pearson Education, Inc. D.

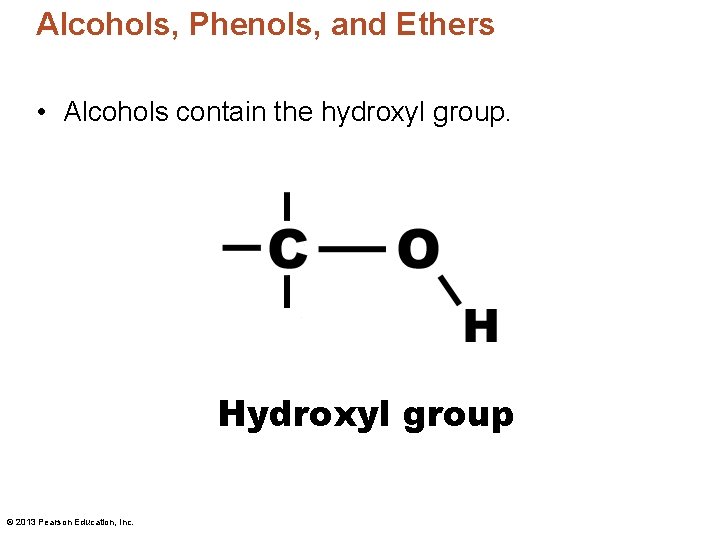
Alcohols, Phenols, and Ethers • Alcohols contain the hydroxyl group. Hydroxyl group © 2013 Pearson Education, Inc.

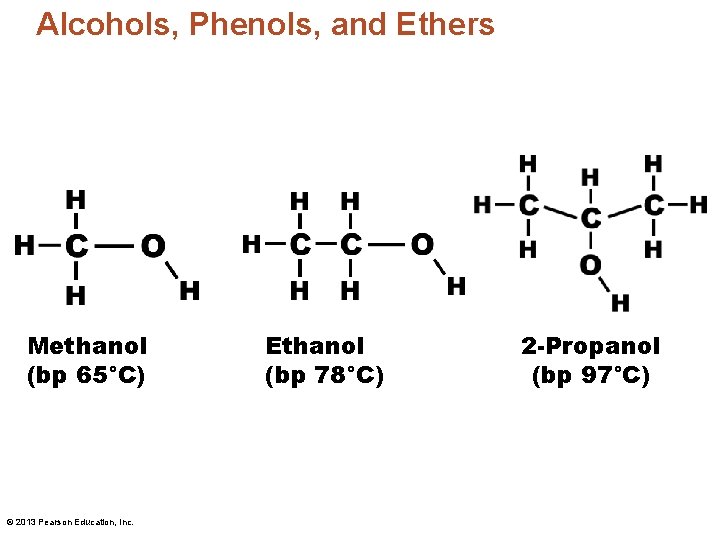
Alcohols, Phenols, and Ethers Methanol (bp 65°C) © 2013 Pearson Education, Inc. Ethanol (bp 78°C) 2 -Propanol (bp 97°C)

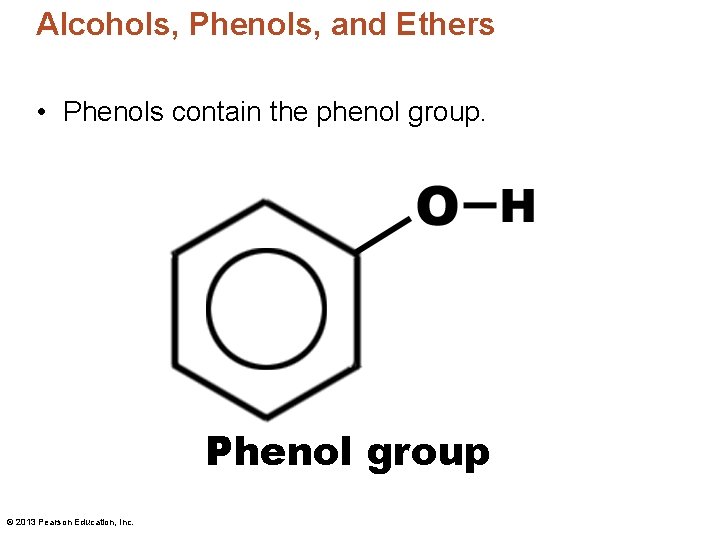
Alcohols, Phenols, and Ethers • Phenols contain the phenol group. Phenol group © 2013 Pearson Education, Inc.

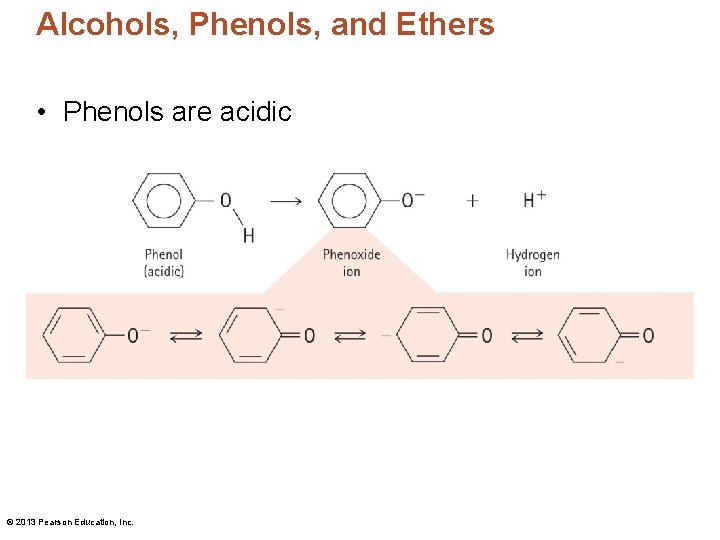
Alcohols, Phenols, and Ethers • Phenols are acidic © 2013 Pearson Education, Inc.

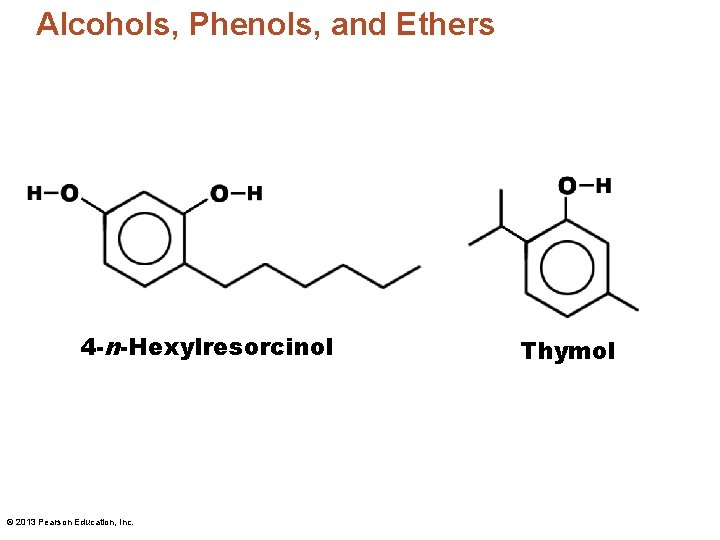
Alcohols, Phenols, and Ethers 4 -n-Hexylresorcinol © 2013 Pearson Education, Inc. Thymol

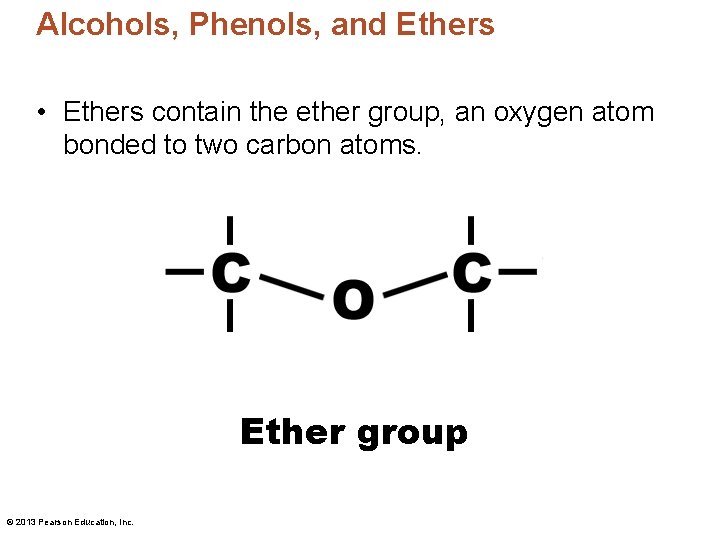
Alcohols, Phenols, and Ethers • Ethers contain the ether group, an oxygen atom bonded to two carbon atoms. Ether group © 2013 Pearson Education, Inc.

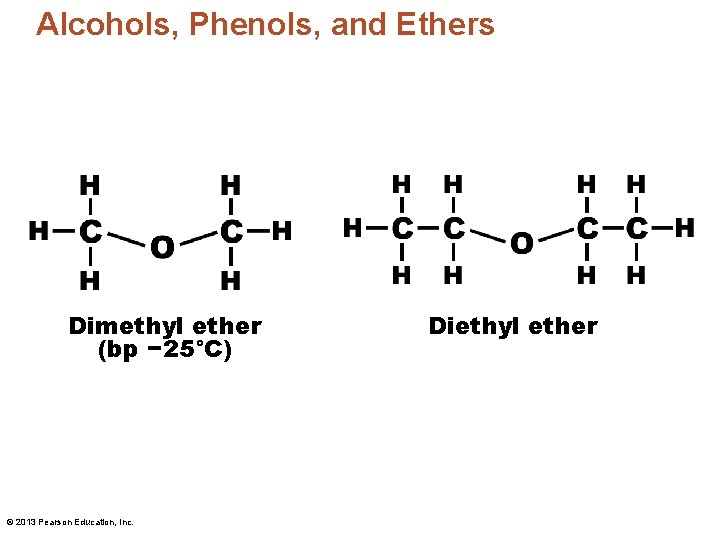
Alcohols, Phenols, and Ethers Dimethyl ether (bp − 25°C) © 2013 Pearson Education, Inc. Diethyl ether

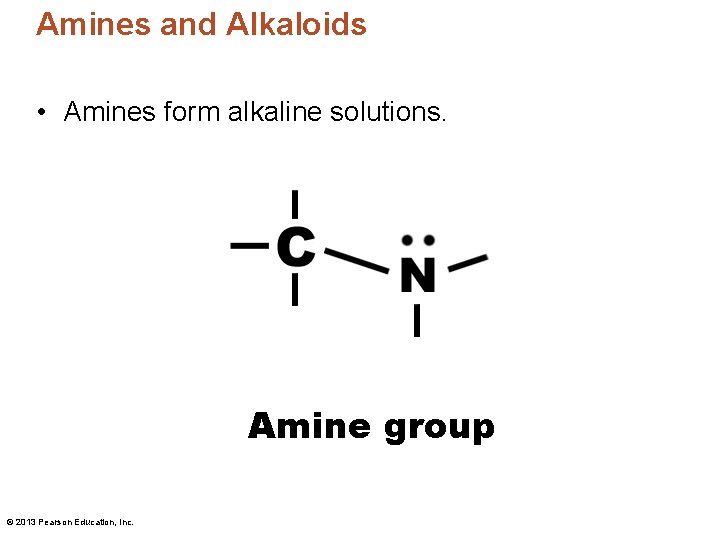
Amines and Alkaloids • Amines form alkaline solutions. Amine group © 2013 Pearson Education, Inc.

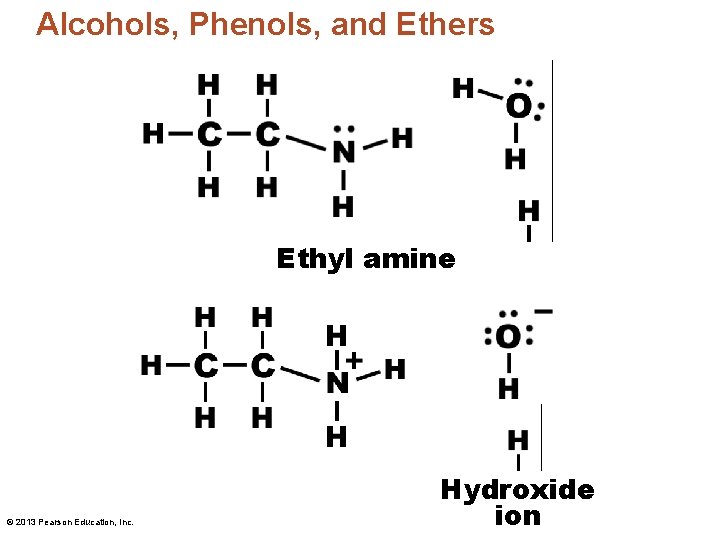
Alcohols, Phenols, and Ethers Ethyl amine © 2013 Pearson Education, Inc. Hydroxide ion

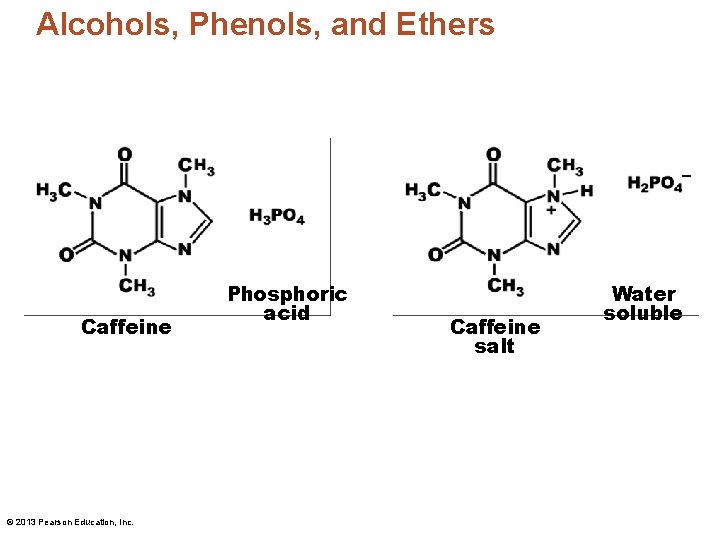
Alcohols, Phenols, and Ethers Caffeine © 2013 Pearson Education, Inc. Phosphoric acid Caffeine salt Water soluble

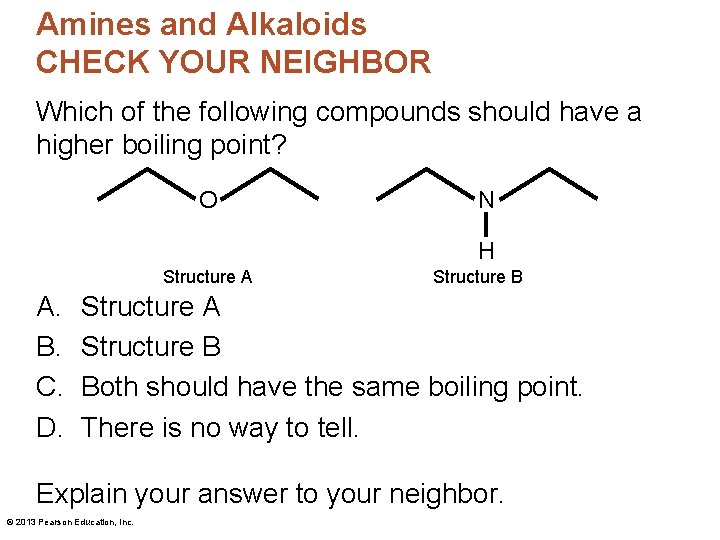
Amines and Alkaloids CHECK YOUR NEIGHBOR Which of the following compounds should have a higher boiling point? O N H Structure A A. B. C. D. Structure B Structure A Structure B Both should have the same boiling point. There is no way to tell. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

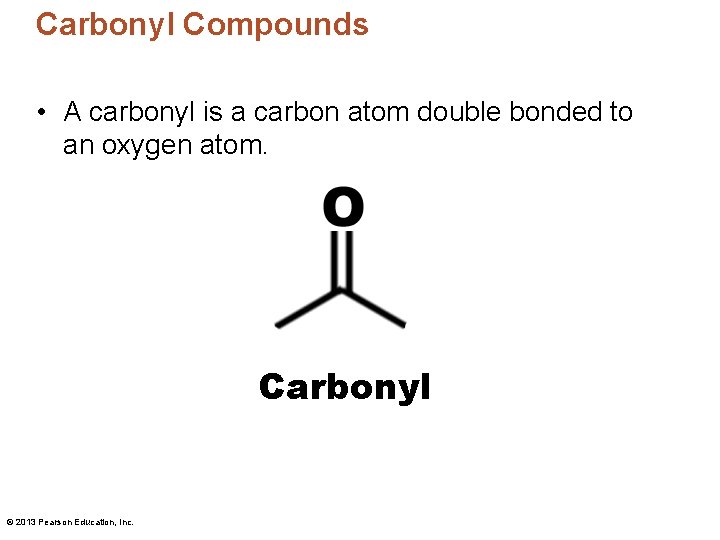
Carbonyl Compounds • A carbonyl is a carbon atom double bonded to an oxygen atom. Carbonyl © 2013 Pearson Education, Inc.

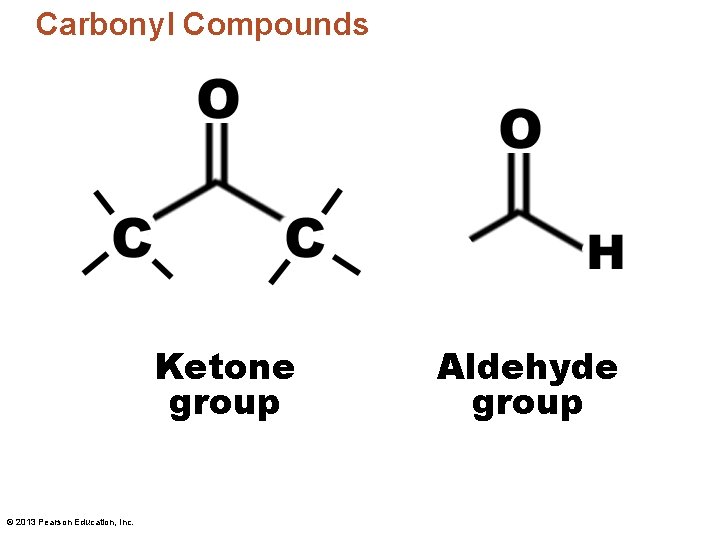
Carbonyl Compounds Ketone group © 2013 Pearson Education, Inc. Aldehyde group

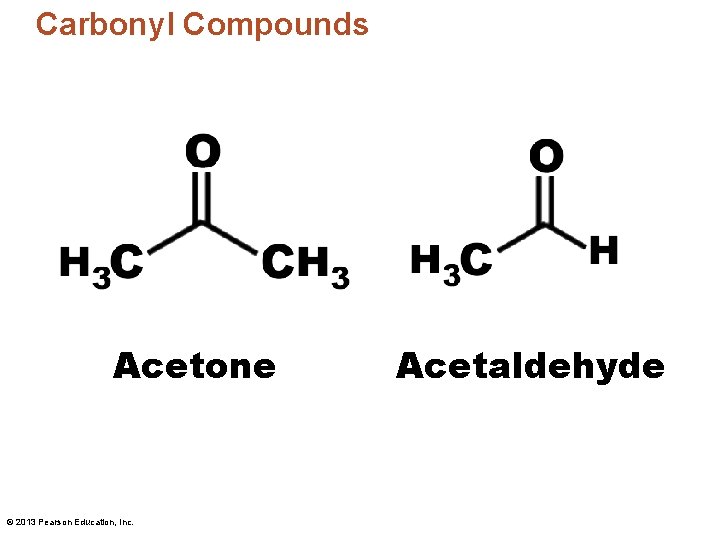
Carbonyl Compounds Acetone © 2013 Pearson Education, Inc. Acetaldehyde

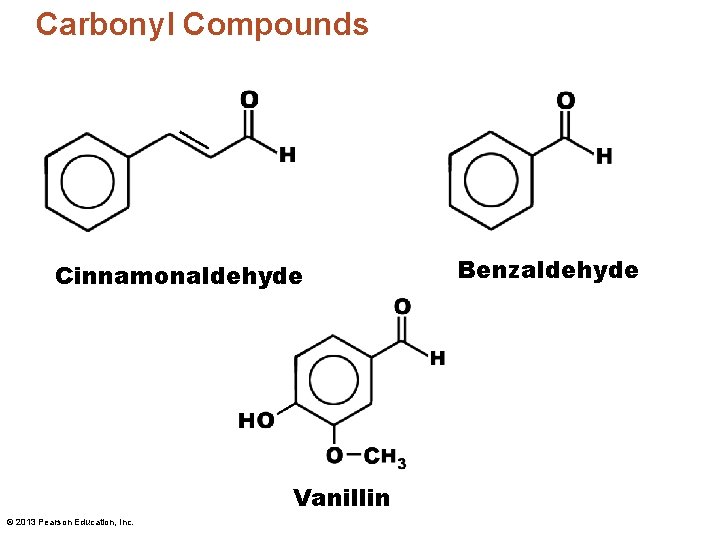
Carbonyl Compounds Cinnamonaldehyde Vanillin © 2013 Pearson Education, Inc. Benzaldehyde

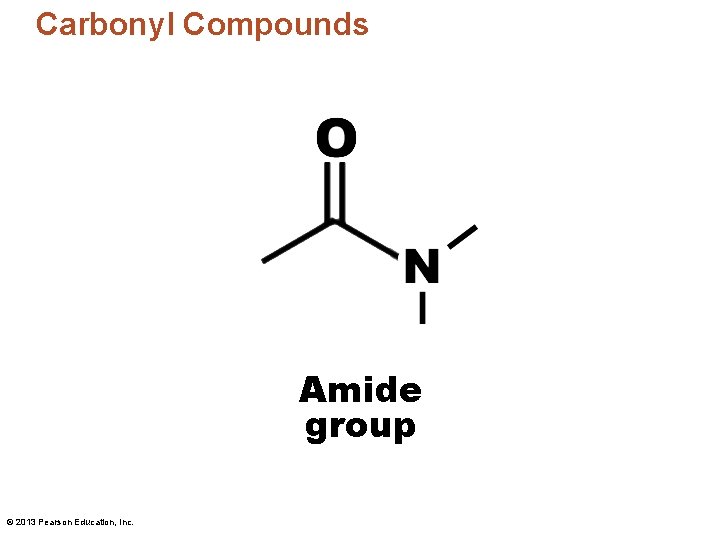
Carbonyl Compounds Amide group © 2013 Pearson Education, Inc.

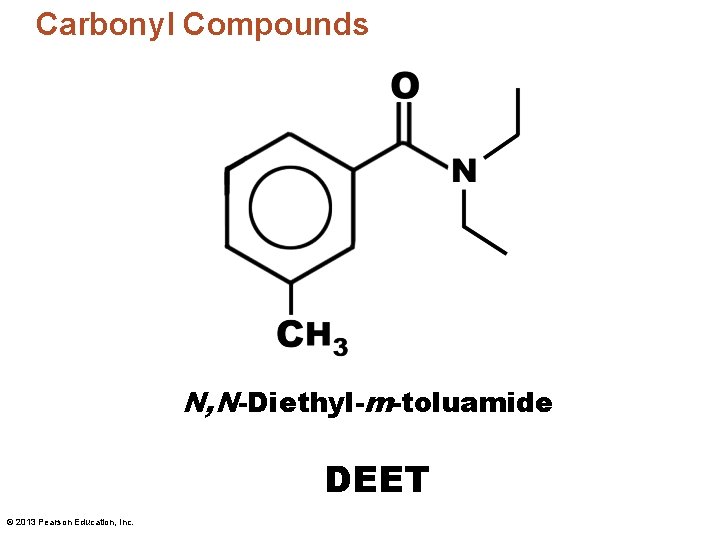
Carbonyl Compounds N, N-Diethyl-m-toluamide DEET © 2013 Pearson Education, Inc.

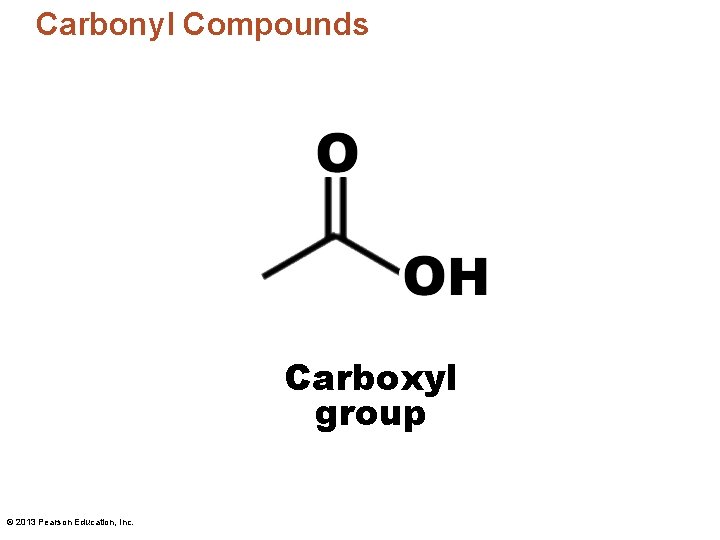
Carbonyl Compounds Carboxyl group © 2013 Pearson Education, Inc.

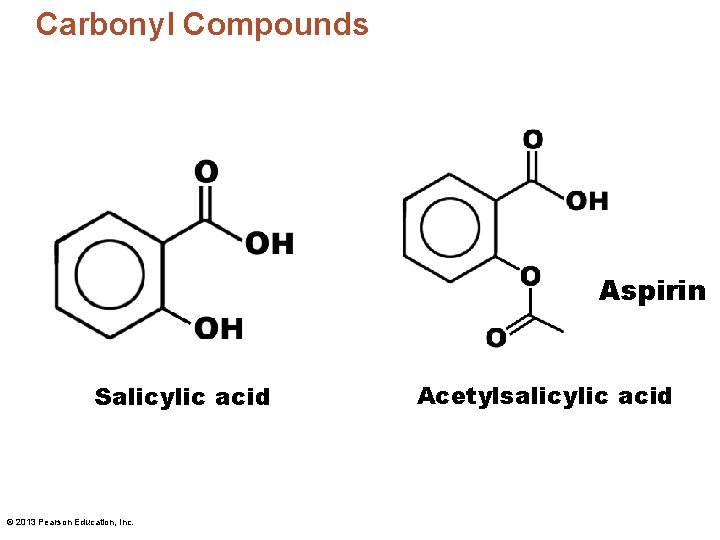
Carbonyl Compounds Aspirin Salicylic acid © 2013 Pearson Education, Inc. Acetylsalicylic acid

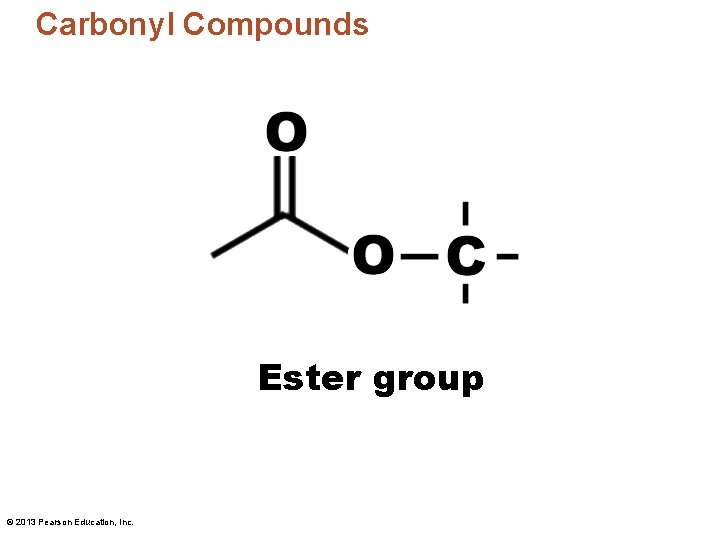
Carbonyl Compounds Ester group © 2013 Pearson Education, Inc.

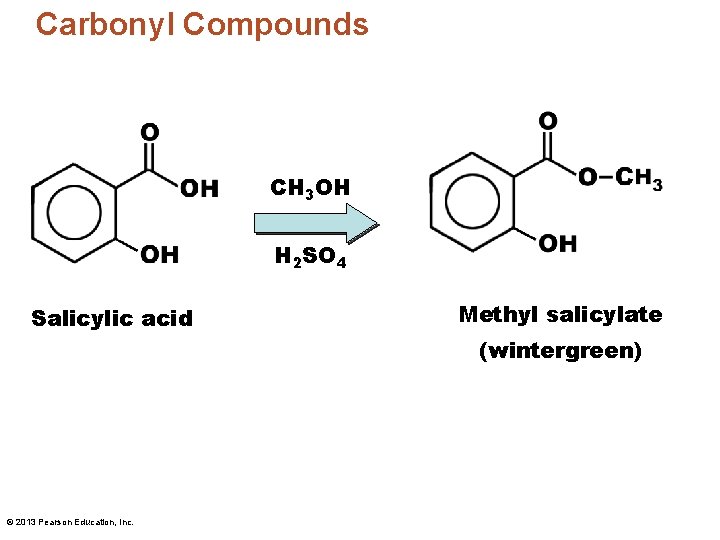
Carbonyl Compounds CH 3 OH H 2 SO 4 Salicylic acid Methyl salicylate (wintergreen) © 2013 Pearson Education, Inc.

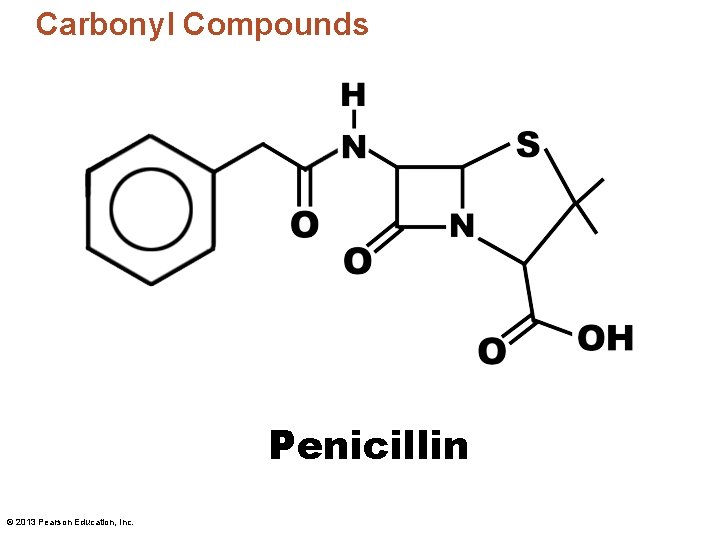
Carbonyl Compounds Penicillin © 2013 Pearson Education, Inc.

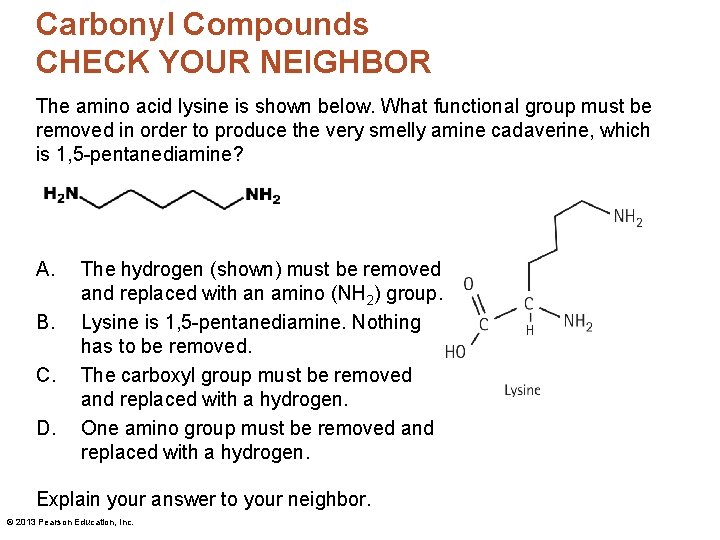
Carbonyl Compounds CHECK YOUR NEIGHBOR The amino acid lysine is shown below. What functional group must be removed in order to produce the very smelly amine cadaverine, which is 1, 5 -pentanediamine? A. B. C. D. The hydrogen (shown) must be removed and replaced with an amino (NH 2) group. Lysine is 1, 5 -pentanediamine. Nothing has to be removed. The carboxyl group must be removed and replaced with a hydrogen. One amino group must be removed and replaced with a hydrogen. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

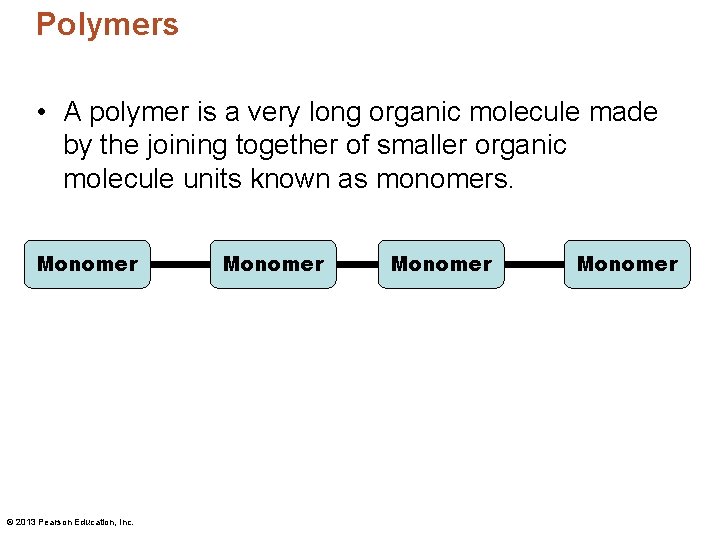
Polymers • A polymer is a very long organic molecule made by the joining together of smaller organic molecule units known as monomers. Monomer © 2013 Pearson Education, Inc. Monomer

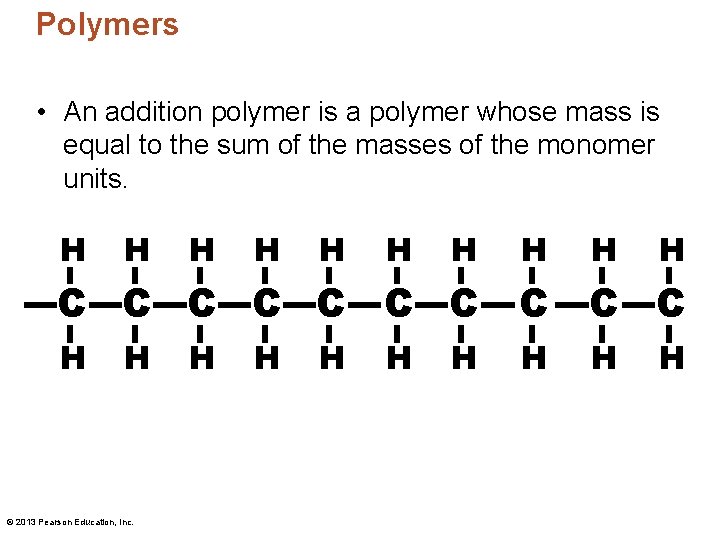
Polymers • An addition polymer is a polymer whose mass is equal to the sum of the masses of the monomer units. H H H H H C C C C C H H © 2013 Pearson Education, Inc. H H H H

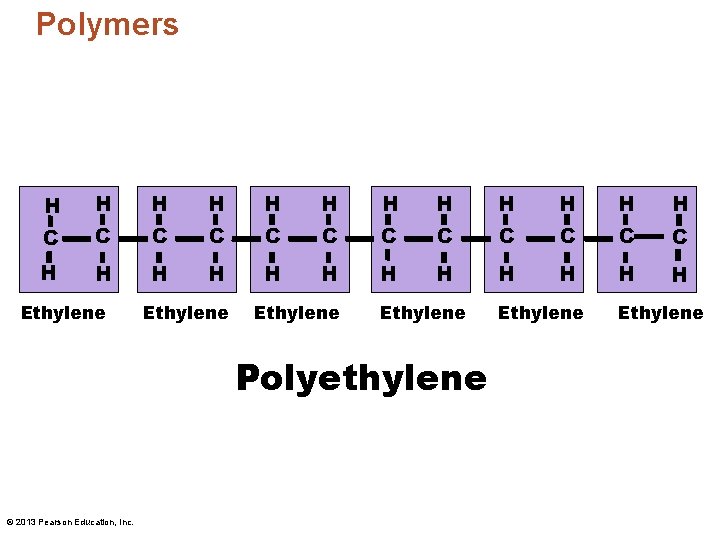
Polymers H H H C C C H H H Ethylene Polyethylene © 2013 Pearson Education, Inc. Ethylene

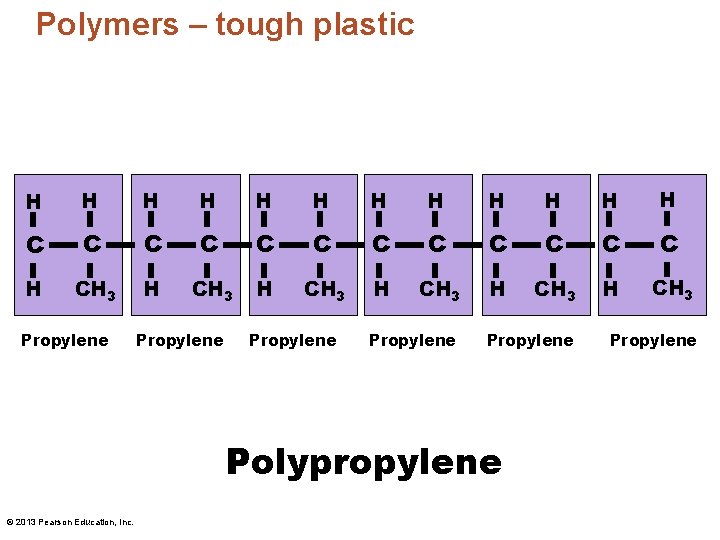
Polymers – tough plastic H H H C C C H CH 3 H CH 3 Propylene Propylene Polypropylene © 2013 Pearson Education, Inc. Propylene

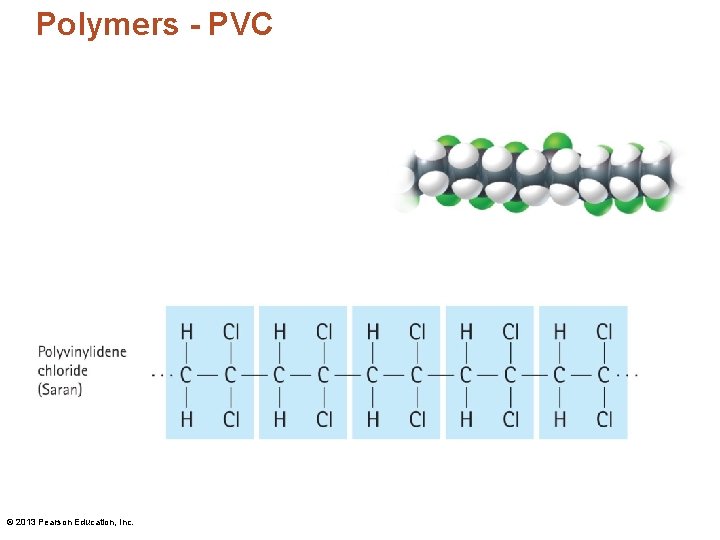
Polymers - PVC © 2013 Pearson Education, Inc.

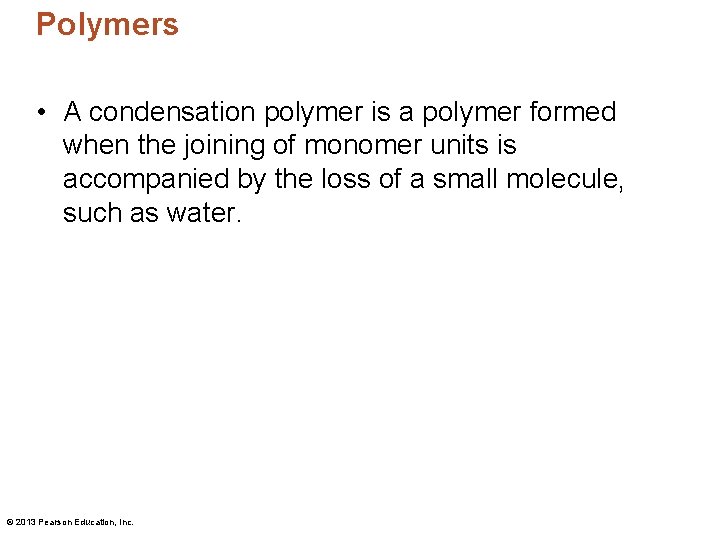
Polymers • A condensation polymer is a polymer formed when the joining of monomer units is accompanied by the loss of a small molecule, such as water. © 2013 Pearson Education, Inc.

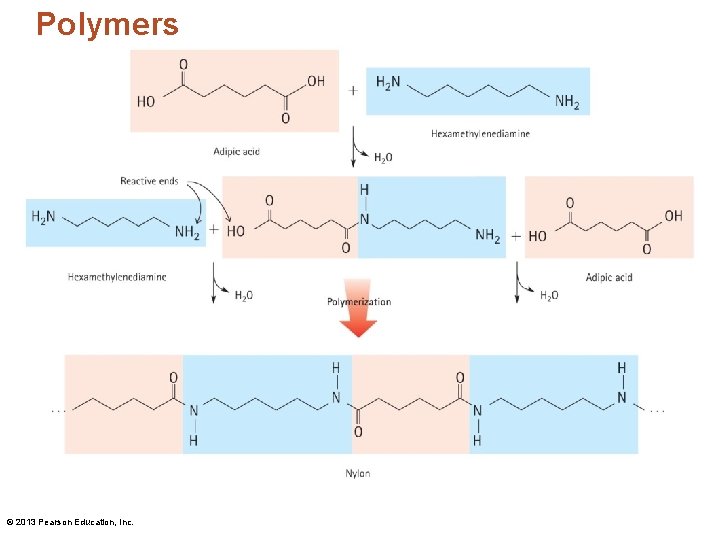
Polymers © 2013 Pearson Education, Inc.

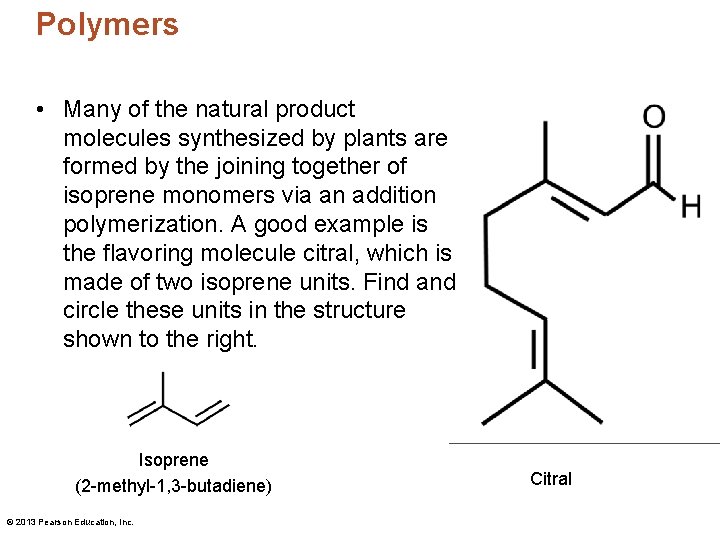
Polymers • Many of the natural product molecules synthesized by plants are formed by the joining together of isoprene monomers via an addition polymerization. A good example is the flavoring molecule citral, which is made of two isoprene units. Find and circle these units in the structure shown to the right. Isoprene (2 -methyl-1, 3 -butadiene) © 2013 Pearson Education, Inc. Citral

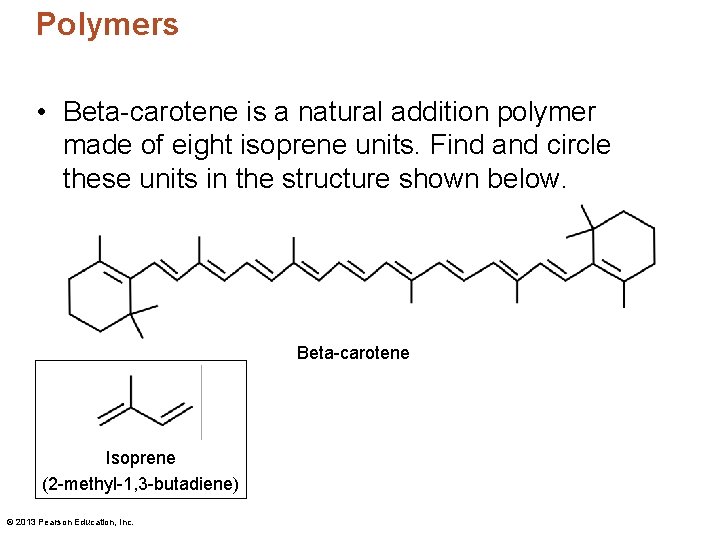
Polymers • Beta-carotene is a natural addition polymer made of eight isoprene units. Find and circle these units in the structure shown below. Beta-carotene Isoprene (2 -methyl-1, 3 -butadiene) © 2013 Pearson Education, Inc.