Chapter 12 Lecture Conceptual Integrated Science Second Edition






- Slides: 21

Chapter 12 Lecture Conceptual Integrated Science Second Edition Chemical Bonds and Mixtures © 2013 Pearson Education, Inc.

This lecture will help you understand: • • • Electron-Dot Structures The Formation of Ions Ionic Bonds Metallic Bonds Covalent Bonds Polar Covalent Bonds Molecular Polarity Molecular Attractions Most Materials Are Mixtures The Chemist's Classification of Matter Solutions Solubility © 2013 Pearson Education, Inc.

Electron-Dot Structures • Atoms bond together through their electrons. To learn about bonding, therefore, we need to know something about how the electrons in an atom are organized. • Electrons behave as though they are contained within seven concentric shells. © 2013 Pearson Education, Inc.

Electron-Dot Structures • The numbers indicate the maximum number of electrons each shell may contain. Note: • This is a "conceptual model" and not a representation of what an atom "looks like. " • Rather, it helps us to understand how the electrons in atoms behave. © 2013 Pearson Education, Inc.

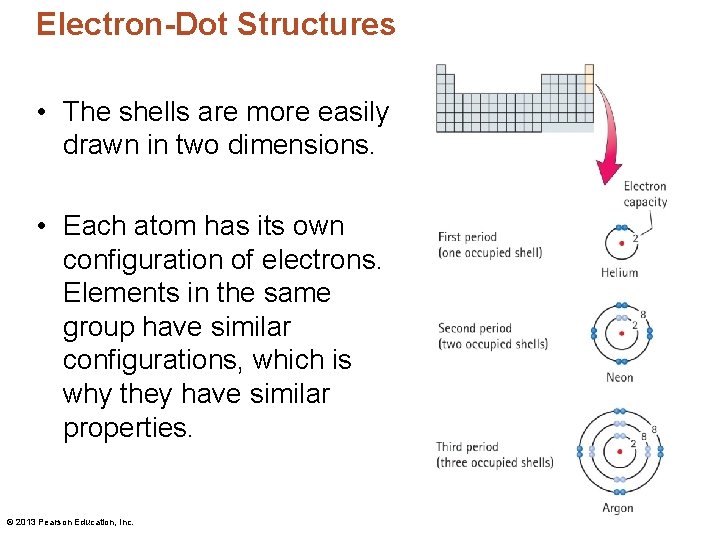
Electron-Dot Structures • The shells are more easily drawn in two dimensions. • Each atom has its own configuration of electrons. Elements in the same group have similar configurations, which is why they have similar properties. © 2013 Pearson Education, Inc.

© 2013 Pearson Education, Inc.

Bonding • Diversity of matter comes from atom interaction. • Atoms composed of particles. • Electrons play an important role in linking atoms together. – Chemical bonds • Ionic bonding • Covalent bonding

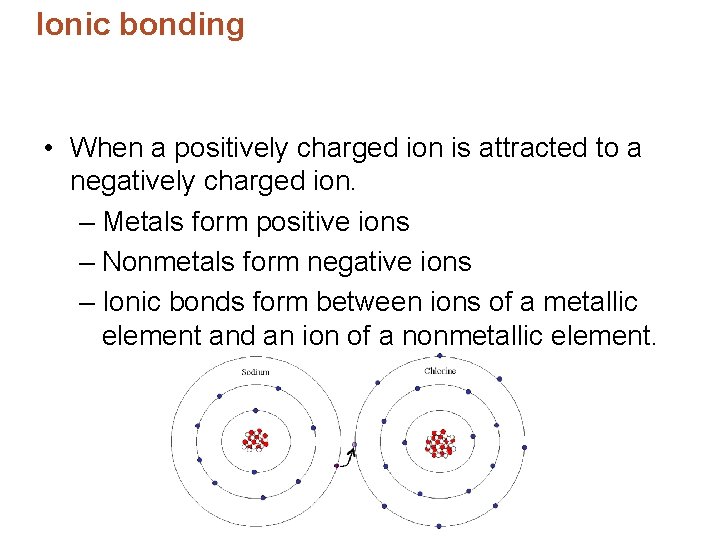
Ionic bonding • When a positively charged ion is attracted to a negatively charged ion. – Metals form positive ions – Nonmetals form negative ions – Ionic bonds form between ions of a metallic element and an ion of a nonmetallic element.

Ionic bonding • Ionic compound – a compound made up entirely of ions. • Ionic compound contain: – Cations – positive ions – Anions – negative ions • Ionic compounds are neutral. Electric charge is balanced between cations and anions.


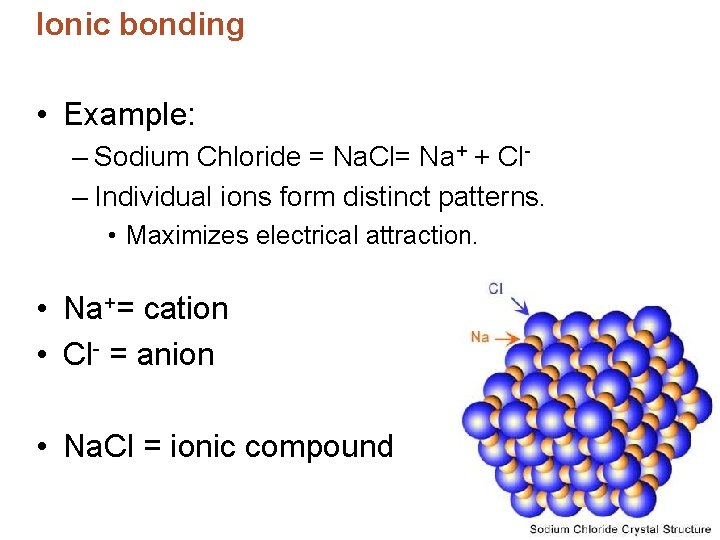
Ionic bonding • Example: – Sodium Chloride = Na. Cl= Na+ + Cl– Individual ions form distinct patterns. • Maximizes electrical attraction. • Na+= cation • Cl- = anion • Na. Cl = ionic compound

Ionic Compounds • Have high melting points. – Strong bonds • Brittle structure – Breaks in specific directions. • Water soluble – Conduct electricity in a dissolved state.

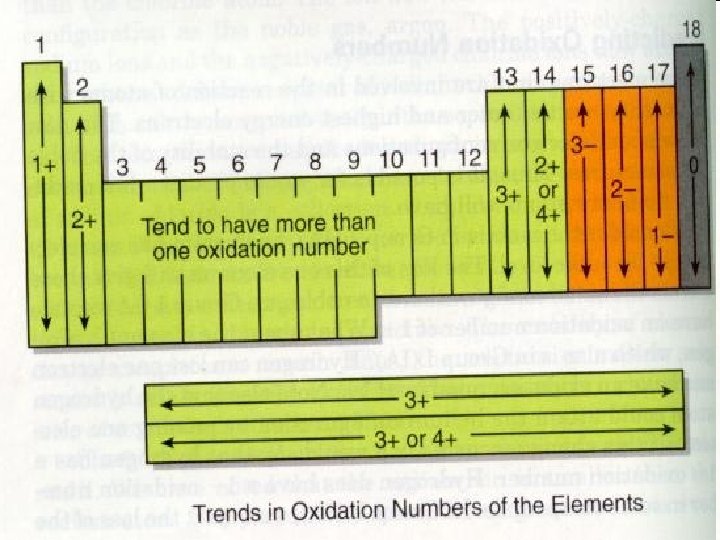
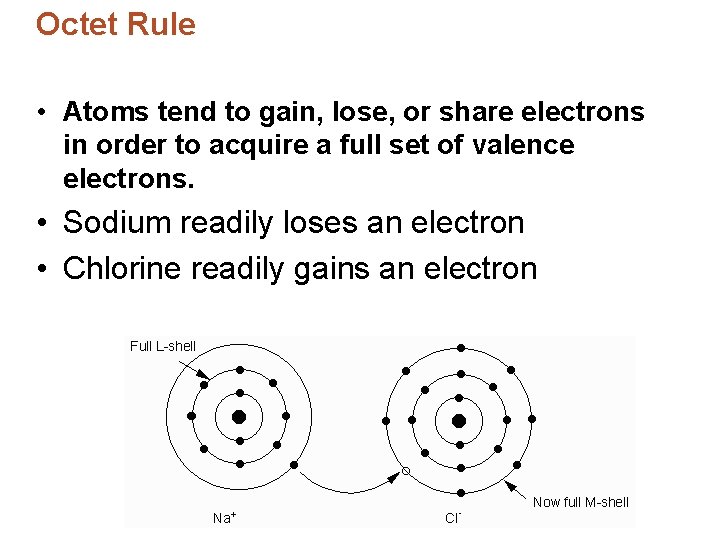
Octet Rule • Sodium chloride is much more stable than each element on its own. • Atoms are stable when their valence shell is full – 8 electrons. • Electron configurations: – Chlorine: [Ne]3 s 23 p 5 – Sodium: [Ne]3 s 1 • Chlorine has 7 valence electrons = needs to gain one • Sodium has 1 valence electrons = needs to lose one


Octet Rule • Atoms tend to gain, lose, or share electrons in order to acquire a full set of valence electrons. • Sodium readily loses an electron • Chlorine readily gains an electron

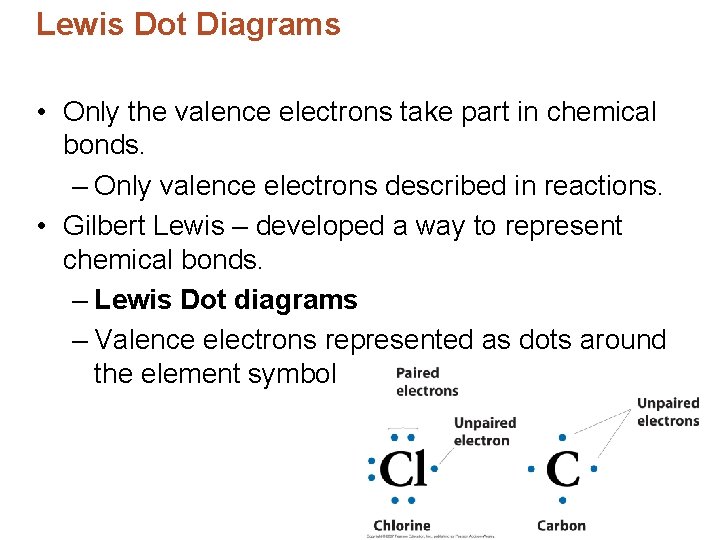
Lewis Dot Diagrams • Only the valence electrons take part in chemical bonds. – Only valence electrons described in reactions. • Gilbert Lewis – developed a way to represent chemical bonds. – Lewis Dot diagrams – Valence electrons represented as dots around the element symbol

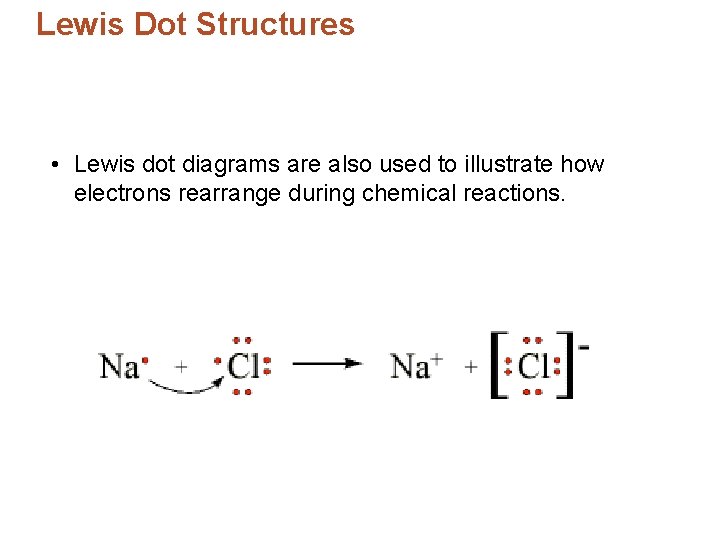
Lewis Dot Structures • Lewis dot diagrams are also used to illustrate how electrons rearrange during chemical reactions.

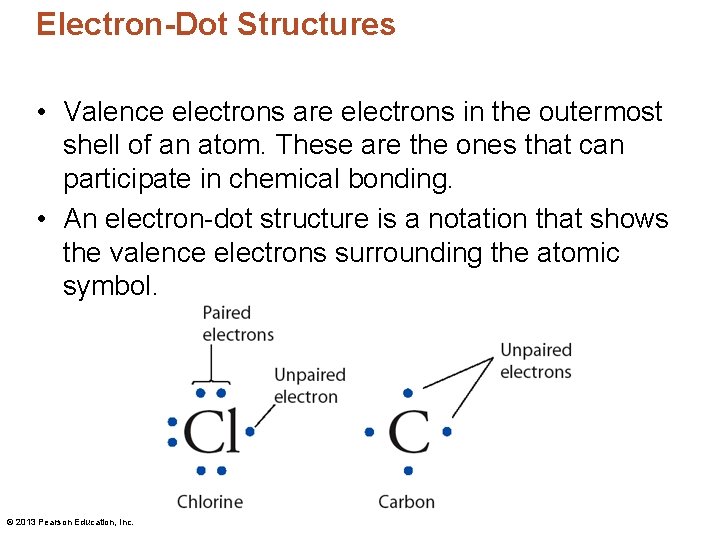
Electron-Dot Structures • Valence electrons are electrons in the outermost shell of an atom. These are the ones that can participate in chemical bonding. • An electron-dot structure is a notation that shows the valence electrons surrounding the atomic symbol. © 2013 Pearson Education, Inc.

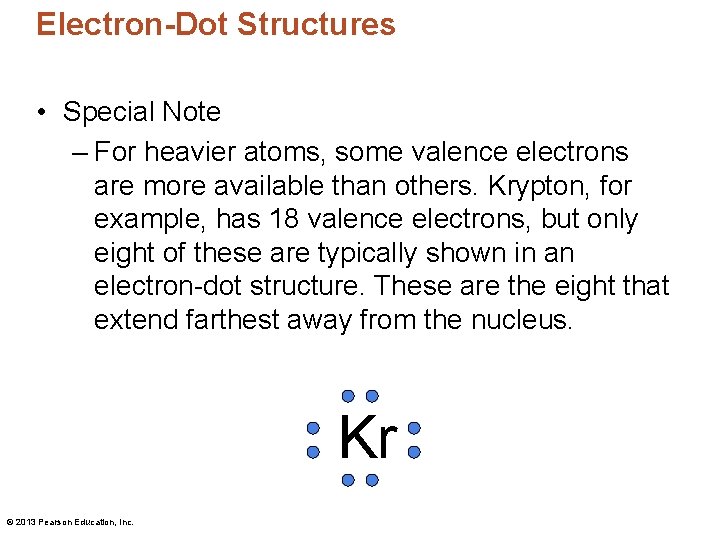
Electron-Dot Structures • Special Note – For heavier atoms, some valence electrons are more available than others. Krypton, for example, has 18 valence electrons, but only eight of these are typically shown in an electron-dot structure. These are the eight that extend farthest away from the nucleus. Kr © 2013 Pearson Education, Inc.

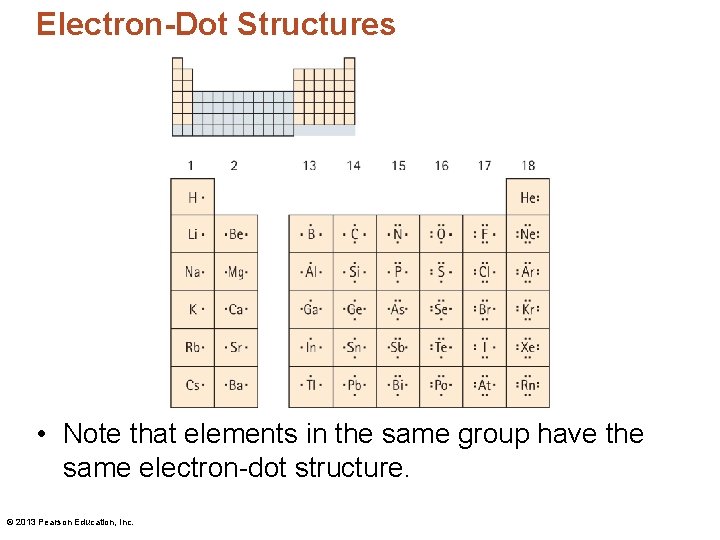
Electron-Dot Structures • Note that elements in the same group have the same electron-dot structure. © 2013 Pearson Education, Inc.

Electron-Dot Structures CHECK YOUR NEIGHBOR Sodium, Na, atomic number 11, has only one valence electron. Upon losing this electron, what other atom in the periodic table does the sodium resemble? A. B. C. D. neon, Ne, atomic number 10 magnesium, Mg, atomic number 12 lithium, Li, atomic number 3 Sodium can resemble only sodium. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.