Chapter 3 Matter and Energy Section 4 Thermal























- Slides: 32

Chapter 3: Matter and Energy Section 4: Thermal Energy

Learning Objectives �Convert between Fahrenheit, Celsius, and Kelvin temperature scales. �Relate energy, temperature change, and heat capacity.

Thermal Energy �The atoms and molecules that compose matter are in constant random motion they contain thermal energy �The temperature of a substance is a measure of its thermal energy.

Thermal Energy �The hotter an object, the greater the random motion of the atoms and molecules that compose it, and the higher its temperature.


Thermal Energy �Heat, which has units of energy, is the transfer or exchange of thermal energy caused by a temperature difference. when a piece of cold ice is dropped into a cup of warm water, heat (thermal energy) is transferred from the water to the ice.

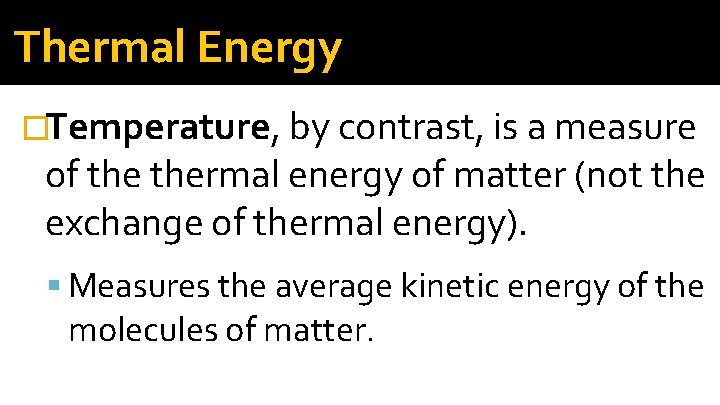
Thermal Energy �Temperature, by contrast, is a measure of thermal energy of matter (not the exchange of thermal energy). Measures the average kinetic energy of the molecules of matter.

Thermal Energy �Both cups of water are at the same temperature… Which has a higher average kinetic energy? Which contains more thermal energy?

Temperature Scales �The Fahrenheit scale was set according to the following standards 0 °F to the freezing point of a concentrated saltwater solution 96 °F to normal body temperature.

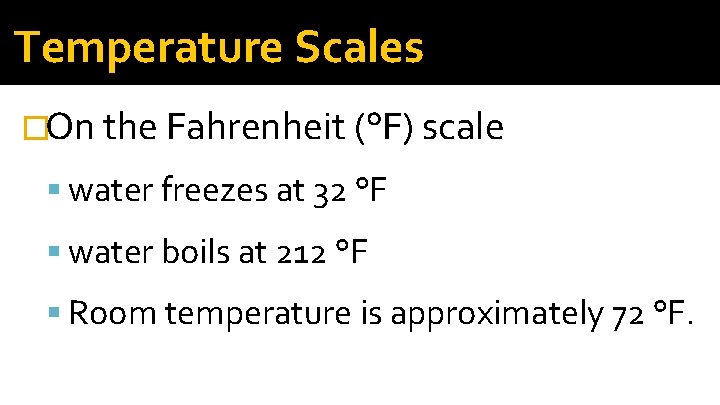
Temperature Scales �On the Fahrenheit (°F) scale water freezes at 32 °F water boils at 212 °F Room temperature is approximately 72 °F.

Temperature Scales �On the Celsius (°C) scale: water freezes at 0 °C water boils at 100 °C Room temperature is approximately 22 °C

Temperature Scales � The Kelvin (K) scale avoids negative temperatures by assigning 0 K to the coldest temperature possible, absolute zero. Absolute zero is the temperature at which molecular motion stops. � On the Kelvin (K) scale, water freezes at 273 K water boils at 373 K. Room temperature is approximately 295 K

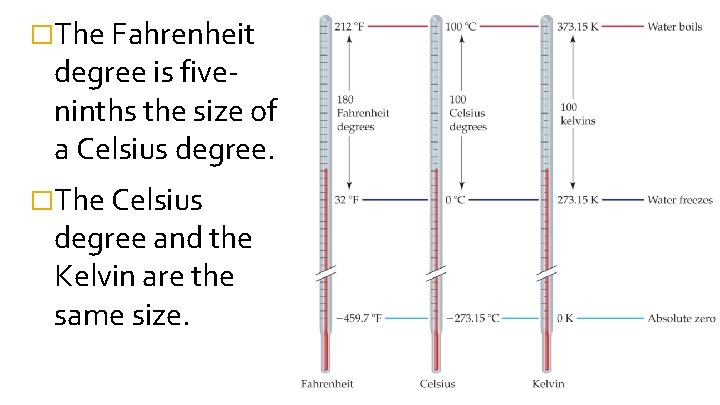
�The Fahrenheit degree is fiveninths the size of a Celsius degree. �The Celsius degree and the Kelvin are the same size.

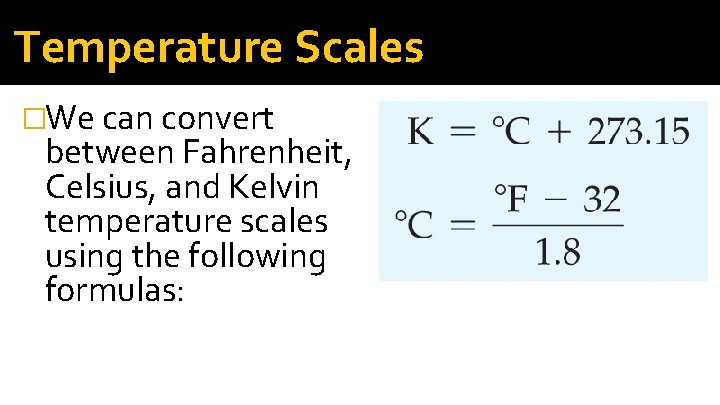
Temperature Scales �We can convert between Fahrenheit, Celsius, and Kelvin temperature scales using the following formulas:

Practice �Convert – 25 °C to kelvin.

Practice �Convert 358 K to Celsius.

Practice �Convert 55 °F to Celsius.

Practice �Convert 139 °C to Fahrenheit.

Practice �Convert 310 K to Fahrenheit.

Practice �Convert – 321 °F to kelvin.

Heat Capacity �Heat capacity: The quantity of heat (usually in joules) required to change the temperature of a given amount of the substance by 1 °C

Heat Capacity �Specific heat capacity: the amount of heat required to raise the temperature of 1 g of any substance by 1 o. C Specific heat capacity has units of joules per gram per degree Celsius, J/g °C

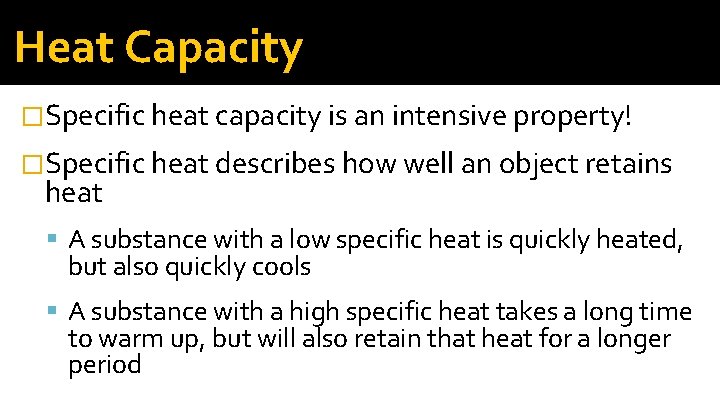
Heat Capacity �Specific heat capacity is an intensive property! �Specific heat describes how well an object retains heat A substance with a low specific heat is quickly heated, but also quickly cools A substance with a high specific heat takes a long time to warm up, but will also retain that heat for a longer period

Heat Capacity � Styrofoam is a very poor conductor of heat; it is a good insulator. It has a high specific heat. � Metals are good conductors of heat. They have low specific heats.


Check-in: � If you want to heat a metal plate to as high a temperature as possible for a given energy input, what metal should you use? (Assume all the plates have the same mass. ) a) copper b) iron c) aluminum d) it would make no difference

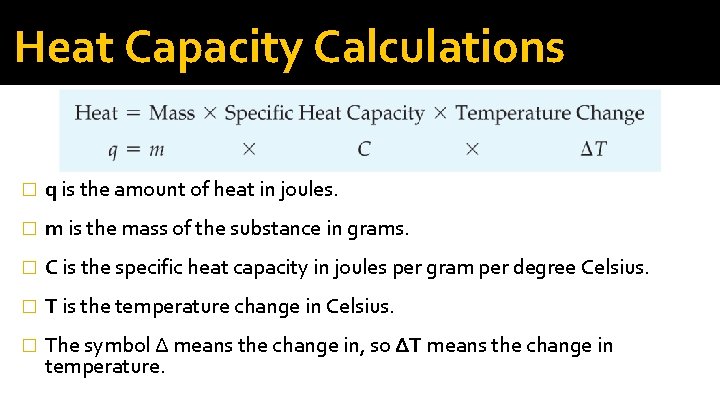
Heat Capacity Calculations � q is the amount of heat in joules. � m is the mass of the substance in grams. � C is the specific heat capacity in joules per gram per degree Celsius. � T is the temperature change in Celsius. � The symbol Δ means the change in, so ΔT means the change in temperature.

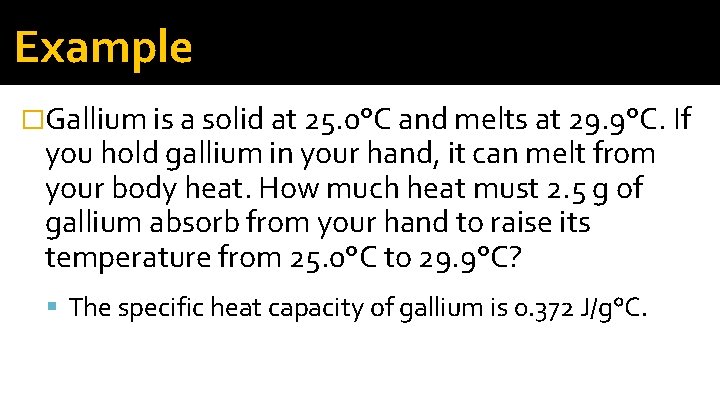
Example �Gallium is a solid at 25. 0°C and melts at 29. 9°C. If you hold gallium in your hand, it can melt from your body heat. How much heat must 2. 5 g of gallium absorb from your hand to raise its temperature from 25. 0°C to 29. 9°C? The specific heat capacity of gallium is 0. 372 J/g°C.

Practice �The temperature of a lead fishing weight rises from 26°C to 38°C as it absorbs 11. 3 J of heat. What is the mass of the fishing weight in grams?

Practice �A chemistry student finds a shiny rock that she suspects is gold. She determines that its mass is 14. 3 g. She then finds that the temperature of the rock rises from 25°C to 52°C upon absorption of 174 J of heat. Find the heat capacity of the rock and determine whether the value is consistent with the heat capacity of gold (which is listed in Table 3. 4).

Practice �A 328 g sample of water absorbs 5. 78 × 103 J of heat. Calculate the change in temperature for the water. If the water is initially at 25. 0°C, what is its final temperature?

Check-in: �The heat capacity of substance A is twice that of substance B. If samples of equal mass of the two substances absorb the same amount of heat, which substance undergoes the larger change in temperature?
 Chapter 5 thermal energy answer key
Chapter 5 thermal energy answer key Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Matter and thermal energy section 1
Matter and thermal energy section 1 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers The science duo physical and chemical changes
The science duo physical and chemical changes Thermal energy states of matter
Thermal energy states of matter Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Use of heat
Use of heat Section 1 composition of matter
Section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Section 1 composition of matter
Section 1 composition of matter Flow of energy vs flow of matter
Flow of energy vs flow of matter Thermal energy vs heat energy
Thermal energy vs heat energy Thermal energy and mass
Thermal energy and mass Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Chapter 12 study guide thermal energy
Chapter 12 study guide thermal energy Chapter 12 thermal energy answers
Chapter 12 thermal energy answers Chapter 5 thermal energy
Chapter 5 thermal energy Work and energy section 2 describing energy answer key
Work and energy section 2 describing energy answer key Whats gray matter
Whats gray matter Cerebral aqueduct
Cerebral aqueduct Gray matter and white matter
Gray matter and white matter Rhinencephalon
Rhinencephalon Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Section 4 review physical science
Section 4 review physical science Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 Chapter 2 principles of ecology answer key
Chapter 2 principles of ecology answer key What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Difference between heat and thermal energy
Difference between heat and thermal energy Difference between heat and thermal energy
Difference between heat and thermal energy Flannel shirt conductor or insulator
Flannel shirt conductor or insulator