Chapter 6 Thermal energy 6 1 Thermal Energy







- Slides: 30

Chapter 6 Thermal energy

6. 1 Thermal Energy, Heat and Temperature • Recall: • Kinetic Energy? • Potential Energy? • Thermal Energy?

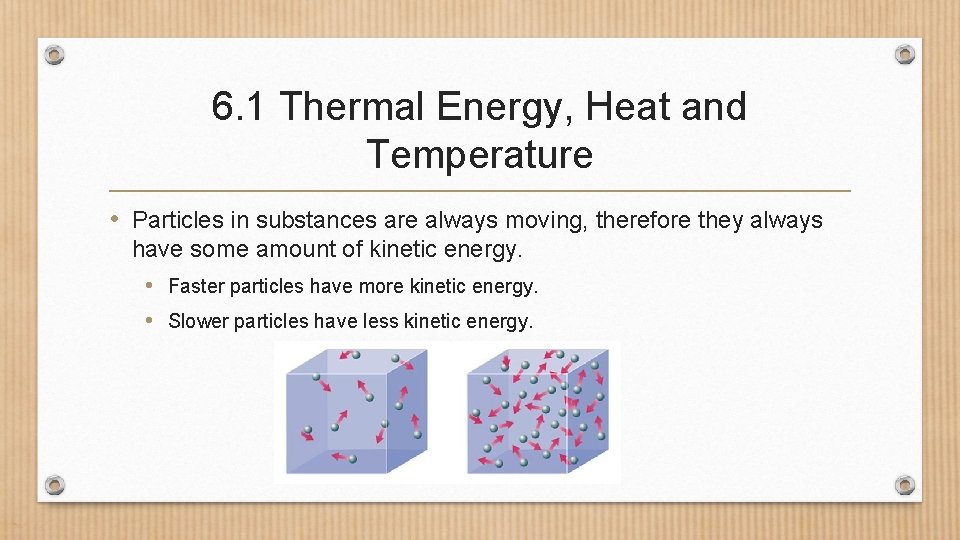
6. 1 Thermal Energy, Heat and Temperature • Particles in substances are always moving, therefore they always have some amount of kinetic energy. • Faster particles have more kinetic energy. • Slower particles have less kinetic energy.

6. 1 Thermal Energy, Heat and Temperature • Particles also have potential energy, due to the attraction of the particles to one another. • The greater the distance between the particles, the greater the potential energy. • Which state has greatest average potential energy; solid, liquid, or gas? Why? Gas which has greater distance between particles than both liquids and solids.

6. 1 Thermal Energy, Heat and Temperature • Thermal Energy- sum of the kinetic and potential energy of the particles that make up a material. • Describes the energy of solids, liquids and gases

6. 1 Thermal Energy, Heat and Temperature • Temperature is the average kinetic energy of the particles that make up a material. • Particles that are moving faster have more kinetic energy and higher temperature. • Inside a house vs. outside a house.

6. 1 Thermal Energy, Heat and Temperature • Temperature and thermal energy are related but not the same! • As ice melts, there is both water and ice at the same temperature however they do not have the same thermal energy. • Which has more thermal energy? Why? • The average distance between the particles in a liquid is greater than a solid therefore it has more potential and thermal energy.

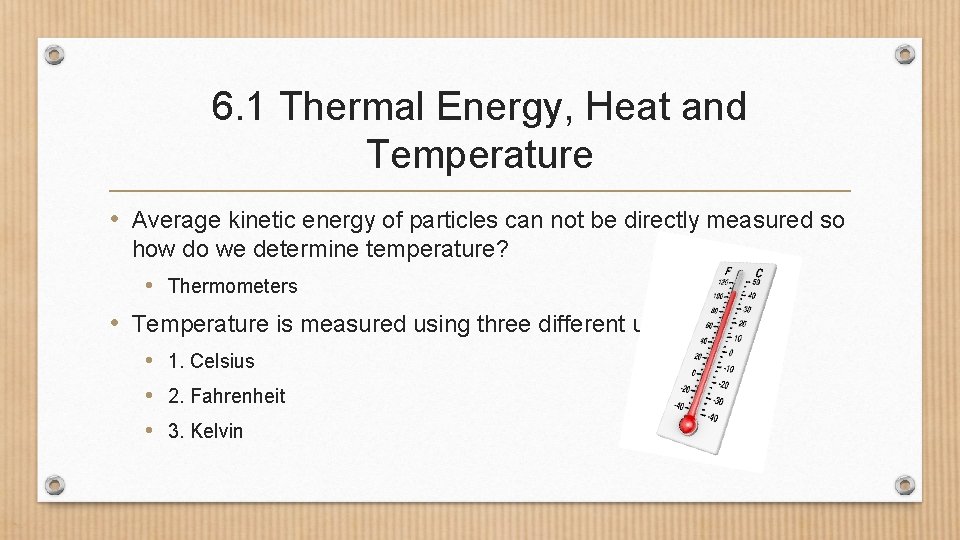
6. 1 Thermal Energy, Heat and Temperature • Average kinetic energy of particles can not be directly measured so how do we determine temperature? • Thermometers • Temperature is measured using three different units: • 1. Celsius • 2. Fahrenheit • 3. Kelvin

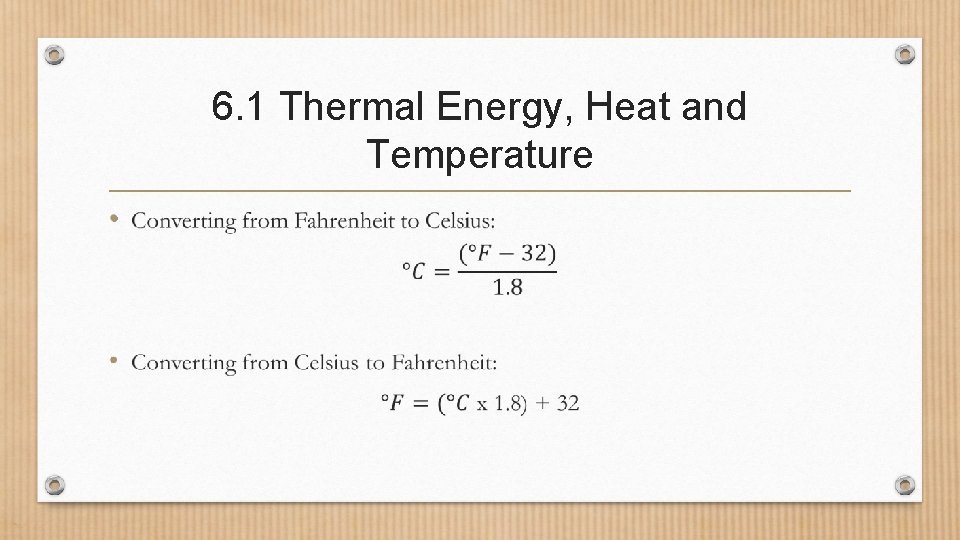
6. 1 Thermal Energy, Heat and Temperature •

6. 1 Thermal Energy, Heat and Temperature • Average room temperature is 37°C. What is this in Fahrenheit? • The average body temperature of a house cat is 101. 5 °F. What is this temperature in Celsius?

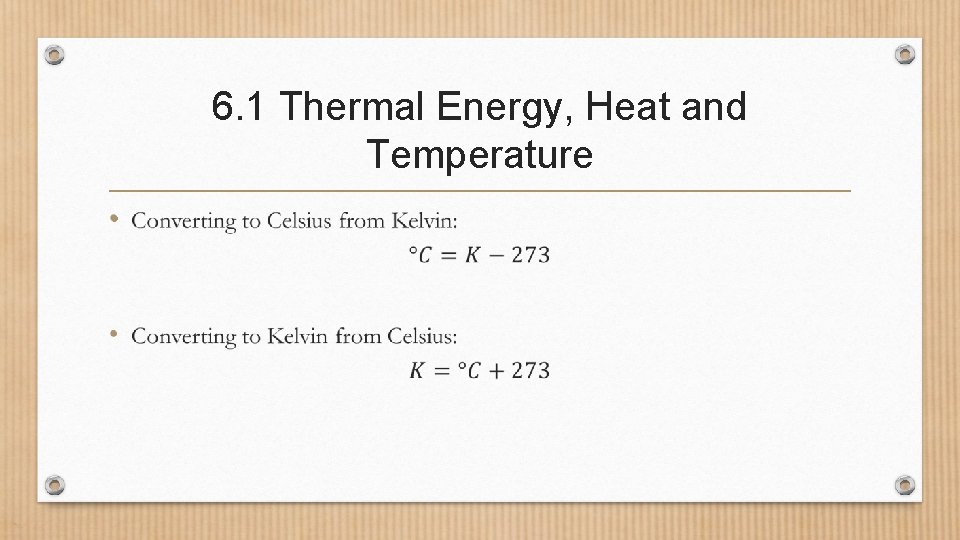
6. 1 Thermal Energy, Heat and Temperature •

6. 1 Thermal Energy, Heat and Temperature • The temperature of a boiling pot of water is 373 K, what is the temperature in Celsius? • On a cold winter day a thermometer reads -15°C, what is that temperature in Kelvin?

6. 1 Thermal Energy, Heat and Temperature • A cold glasses of lemonade has a temperature of -32°F, find that temperature in Kelvin. • The average temperature on Earth is about 57°F, what would be the temperature in Kelvin?

6. 1 Thermal Energy, Heat and Temperature • Heat- the movement of thermal energy from a warmer object to a cooler object. • The greater the temperature difference, the more heat transferred. • Heating continues until all objects are the same temperature.

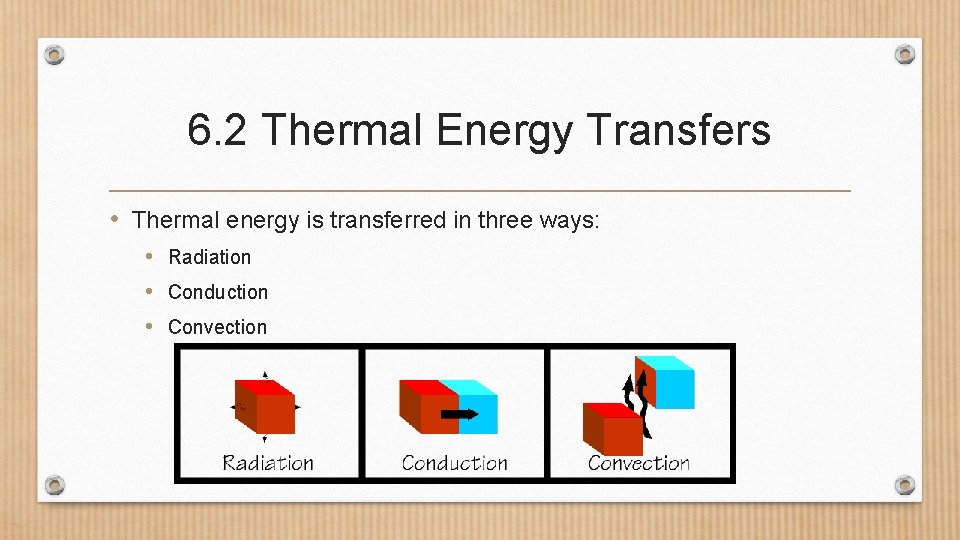
6. 2 Thermal Energy Transfers • Thermal energy is transferred in three ways: • Radiation • Conduction • Convection

6. 2 Thermal Energy Transfers • Radiation- transfer of thermal energy by electromagnetic waves. • All objects transfer some thermal energy by radiation. • Which transfers more thermal energy hot objects or cold objects?

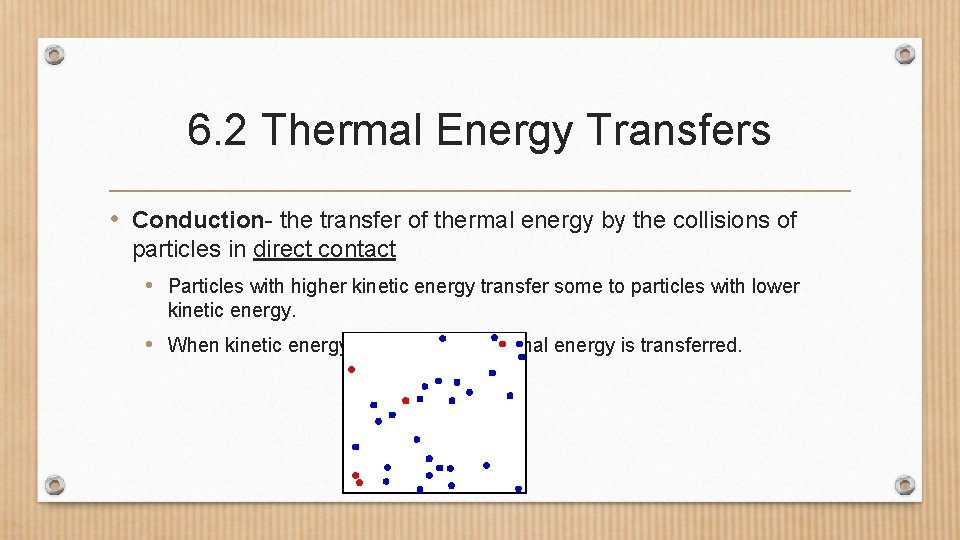
6. 2 Thermal Energy Transfers • Conduction- the transfer of thermal energy by the collisions of particles in direct contact • Particles with higher kinetic energy transfer some to particles with lower kinetic energy. • When kinetic energy is transferred, thermal energy is transferred.

6. 2 Thermal Energy Transfers • Thermal Conductors vs. Thermal Insulators • Conductors allow thermal energy to flow easily • Examples: copper, aluminum • Insulators resists the flow of thermal energy. • Examples: plastic, wood

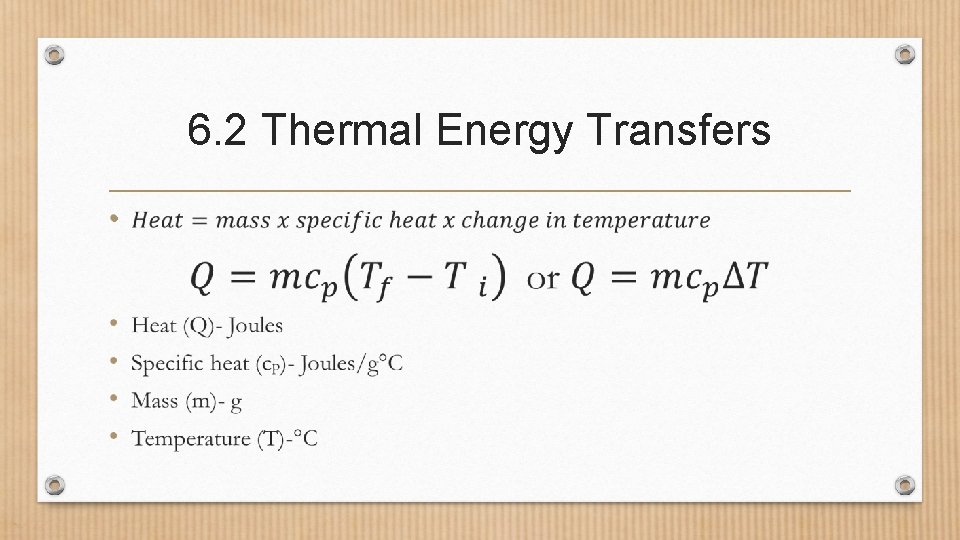
6. 2 Thermal Energy Transfers • All materials require a certain amount of energy to change it’s temperature. • This is known as a material’s Specific heat. • That is the amount of thermal energy required to increase the temperature of 1 kg of a material 1°C.

6. 2 Thermal Energy Transfers • Higher specific heat, more thermal energy required to increase the temperature. • Which has a higher specific heat a thermal conductor or insulator? Why? Thermal insulator because it resists the flow of thermal energy, requiring more to increase its temperature.

6. 2 Thermal Energy Transfers • Water has a very high specific heat meaning it requires a lot of thermal energy to increase the temperature. • Is that good or bad? • Prevents your body from overheating • Keeps pools and oceans cool in the summer • Good for cooling machinery

6. 2 Thermal Energy Transfers •

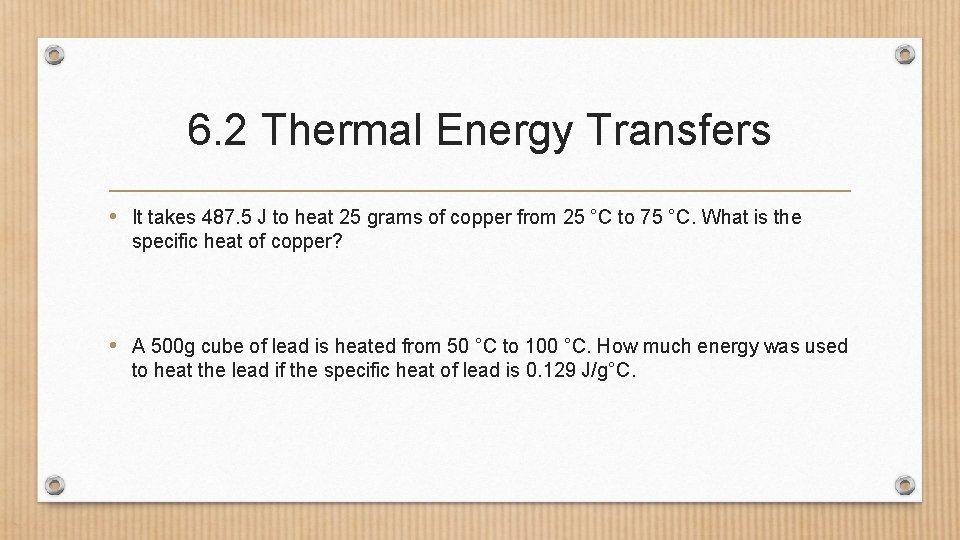
6. 2 Thermal Energy Transfers • It takes 487. 5 J to heat 25 grams of copper from 25 °C to 75 °C. What is the specific heat of copper? • A 500 g cube of lead is heated from 50 °C to 100 °C. How much energy was used to heat the lead if the specific heat of lead is 0. 129 J/g°C.

6. 2 Thermal Energy Transfers • A cook places a 150 g potato wrapped in aluminum foil (Cp=0. 902 J/g°C) in the oven and it is heated up with 700 J of energy. What was the change in temperature? • What is the mass of a ball of iron, if it is heated using 250 J of energy to go from 10°C to 100°C and has a specific heat of. 134 J/g°C.

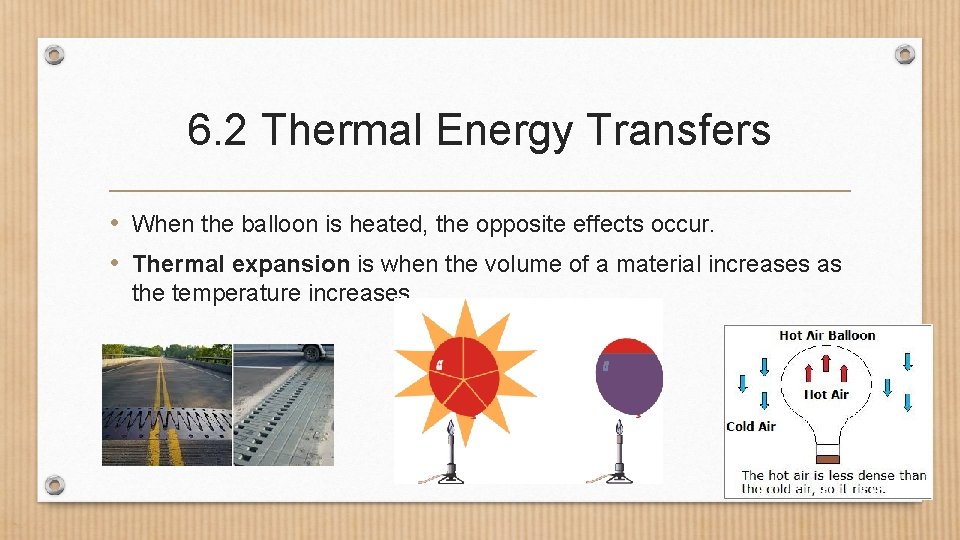
6. 2 Thermal Energy Transfers • What happens when you take a balloon outside on a cold day? • The balloon shrinks in size due to thermal contraction. • Thermal contraction is a decrease in an materials volume when the temperature decreases. https: //www. youtube. com/watch? v=QLTWt 9 r_NF 0

6. 2 Thermal Energy Transfers • When the balloon is heated, the opposite effects occur. • Thermal expansion is when the volume of a material increases as the temperature increases.

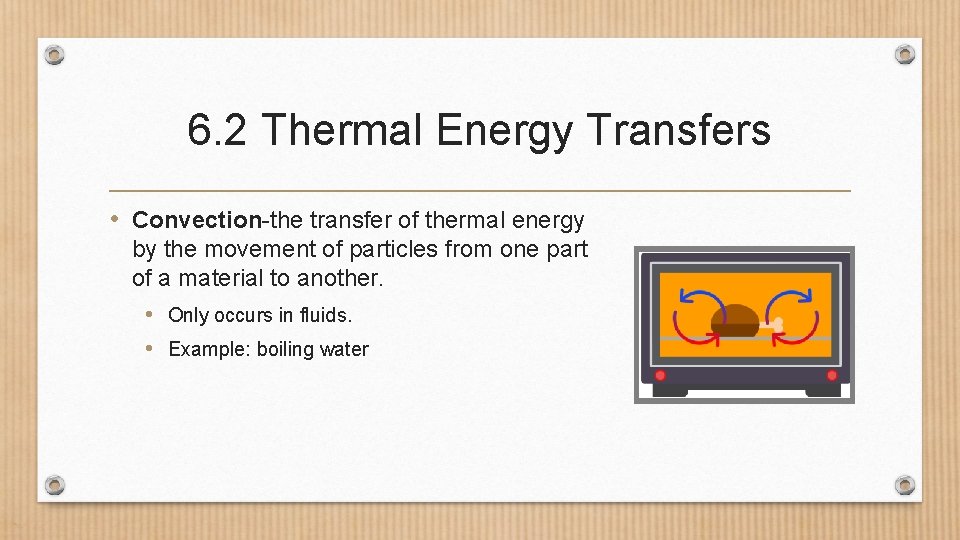
6. 2 Thermal Energy Transfers • Convection-the transfer of thermal energy by the movement of particles from one part of a material to another. • Only occurs in fluids. • Example: boiling water

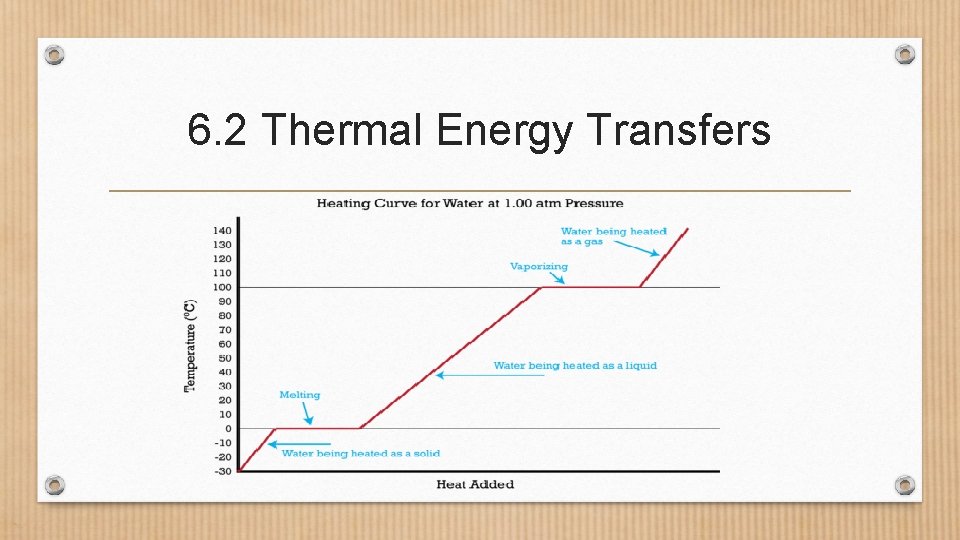
6. 2 Thermal Energy Transfers • Heating Curve- a graph that shows how the temperature of a substance changes as heat is added. • X-axis: Heat • Y-axis: Temperature (°C) • Horizontal lines represent a change in state

6. 2 Thermal Energy Transfers

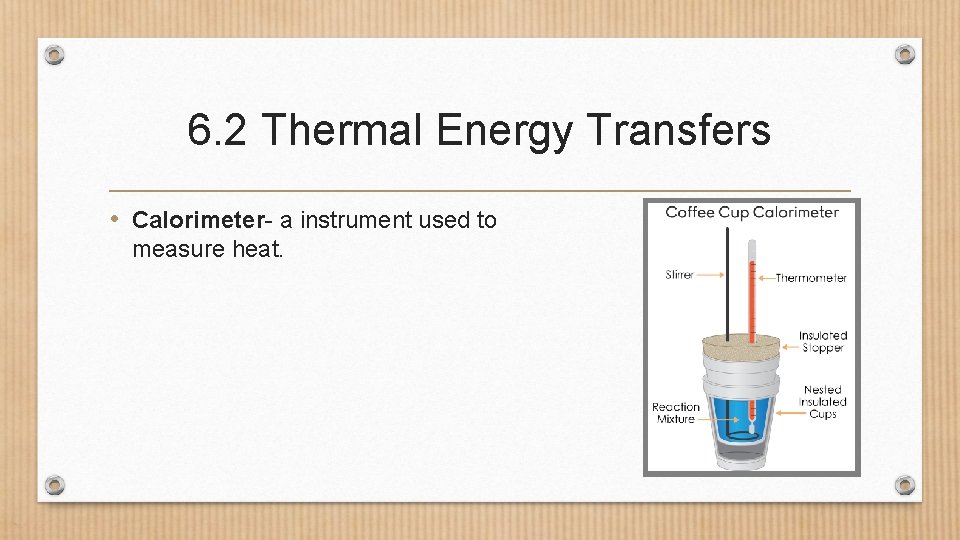
6. 2 Thermal Energy Transfers • Calorimeter- a instrument used to measure heat.
 Chapter 5 thermal energy answer key
Chapter 5 thermal energy answer key How are thermal energy and temperature different
How are thermal energy and temperature different Mass and thermal energy
Mass and thermal energy Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Chapter 12 study guide thermal energy
Chapter 12 study guide thermal energy Chapter 12 thermal energy answers
Chapter 12 thermal energy answers Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat Chapter 5 thermal energy
Chapter 5 thermal energy Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Which is the best surface for reflecting heat radiation
Which is the best surface for reflecting heat radiation Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Difference between heat and thermal energy
Difference between heat and thermal energy Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Thermal energy depends on
Thermal energy depends on Whats thermal energy
Whats thermal energy Thermal energy definition
Thermal energy definition Difference between heat and thermal energy
Difference between heat and thermal energy Heat vs thermal energy
Heat vs thermal energy How to measure heat energy
How to measure heat energy Heat transfer types
Heat transfer types Thermal energy in states of matter
Thermal energy in states of matter Heat thermal energy and temperature
Heat thermal energy and temperature Kinetic energy formula
Kinetic energy formula Heat transfer jeopardy
Heat transfer jeopardy What is specific heat capacity
What is specific heat capacity Thermal energy formula
Thermal energy formula Define thermal energy
Define thermal energy Types of thermal energy transfers
Types of thermal energy transfers Example of heat energy
Example of heat energy