Thermal Physics Chapter 10 Thermal Physics Thermal physics













- Slides: 24

Thermal Physics Chapter 10

Thermal Physics • • Thermal physics looks at temperature, heat, and internal energy Heat and temperature are not the same thing although we use them interchangeably in our everyday language

Thermometer • A device calibrated to measure the temperature (not heat) of an object

Zeroth Law of Thermodynamics • • AKA the Law of Equilibrium If objects A and B are separately in thermal equilibrium with a third object C, then A and B are in thermal equilibrium with each other.

Temperature • Defn: the property that determines whether or not an object is in thermal equilibrium with other objects

Temperature • • Temperature is a measure of the intensity of heat or how hot a system is regardless of size. Kelvin is the official metric unit To convert degrees Celsius to Kelvin by adding 273. To convert Kelvin to degrees Celsius subtract 273.

Thermal Expansion • • Defn: as temperature increases, volume increases Ex. Useful in building designs, concrete highways, and bridges

Expressing Thermal Expansion • If thermal expansion of an object is sufficiently small compared with the object’s initial dimensions, then the change in any dimension is proportional to the first power of the temperature change.

Defining the Variables • • • L 0 initial length along some direction at some temperature DL increase in length DT change in temperature a = coefficient of linear expansion for a given material and has untis of 0 C-1 DL = a. L 0 DT

Area Expansion • • • g= coefficient of area expansion A = area DA = A-A 0 = g. A 0 DT

Coefficient of Volume Expansion • • b= coefficient of volume expansion DV = b. V 0 DT

Application • • Why would a glass break if it hot liquid is poured into it too quickly? You have a metallic lid stuck on a glass jar. Describe how you would loosen it without any tools.

Kinetic Molecular Theory of Gases 1. 2. 3. 4. 5. A gas consists of small particles (atoms/molecules) that move randomly with rapid velocities The attractive forces between particles of a gas can be neglected The actual volume occupied by a gas molecule is extremely small compared to the volume that gas occupies. The average kinetic energy of a gas molecule is proportional to Kelvin temperature Gas particles are in constant motion, moving rapidly in straight paths.

• Properties of a gas Pressure: k. Pa, atm, mm of Hg, torr – Conversion 760 mm Hg = 760 torr = 1 atm= 101. 3 k. Pa • Volume: L, m. L or cm 3 – Conversions 1000 m. L = 1 L – 1 m. L = 1 cm • Temperature: 0 C or K – Conversions 0 C + 273 = K or 0 C = K -273

• • • Boyle’s Law: Pressure and volume are inversely proportional As pressure increases volume decreases As pressure decreases volume increases Pressure and Volume units must be the same on both sides P 1 V 1 = P 2 V 2

Charles’ Law: • • • Temperature and Volume are directly proportional As temperature increases volume increases As temperature decreases volume decreases Temperature must be in Kelvin (add 273) Volume units must be consistent on both sides V 1 / T 1 = V 2/T 2

Gay-Lussac’s Law: • • • Pressure and Temperature are directly proportional As pressure increases temperature increases As pressure decreases temperature decreases Pressure units must be consistent on both sides Temperature units must be in Kelvin (add 273) P 1/T 1 = P 2/T 2

Combined Gas Law : P 1 V 1 = P 2 V 2 T 1 T 2 • Pressure and volume and temperature vary according to this equation when all three change • Temperature must be in Kelvin

The Mole and Avagadro’s Law • • Avagadro’s Law: V 1 / n 1 = V 2/n 2 n = number of moles, moles are large quantities of very small objects like molecules of a gas STP = Standard Temperature and Pressure 00 C or 273 K and 1 atm Molar volume: 22. 4 L

Ideal Gas Law: (Eqn. of State) • • PV = n. RT R is the universal gas constant and varies depending on which unit is used for measuring pressure R = 0. 0821 L x atm. /mol or if using k. Pa R = 8. 31 J/mol x K

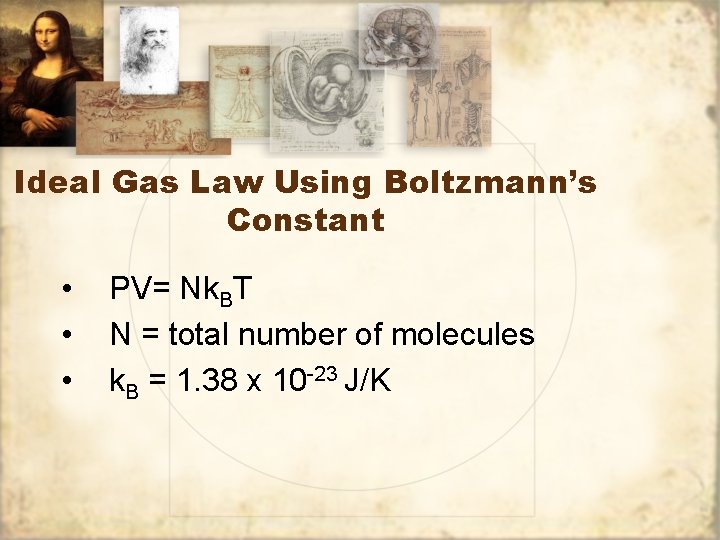
Ideal Gas Law Using Boltzmann’s Constant • • • PV= Nk. BT N = total number of molecules k. B = 1. 38 x 10 -23 J/K

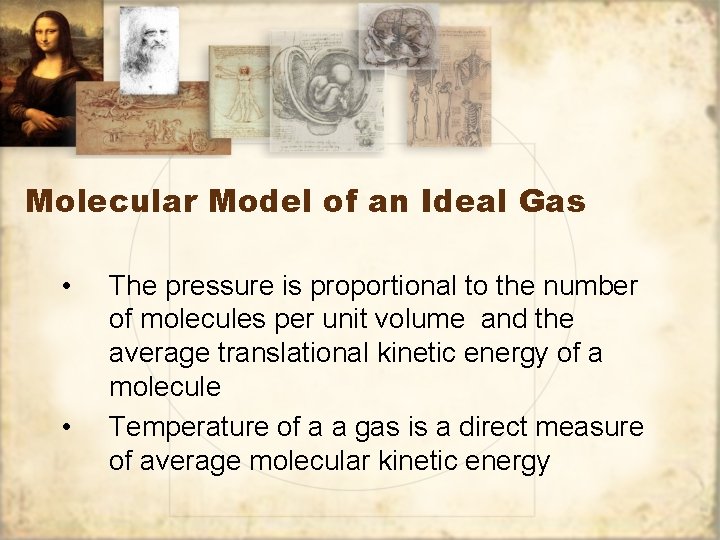
Molecular Model of an Ideal Gas • • The pressure is proportional to the number of molecules per unit volume and the average translational kinetic energy of a molecule Temperature of a a gas is a direct measure of average molecular kinetic energy

Internal Energy, U, for a monatomic gas • • U = 3/2(n. RT) Again, temperature must be in Kelvin

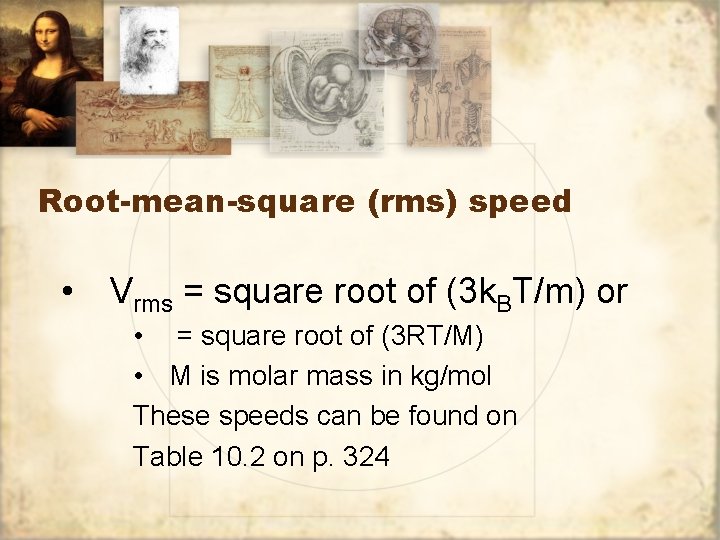
Root-mean-square (rms) speed • Vrms = square root of (3 k. BT/m) or • = square root of (3 RT/M) • M is molar mass in kg/mol These speeds can be found on Table 10. 2 on p. 324