Thermal Analysis Techniques Thermal Analysis Thermal Gravimetric Analysis


- Slides: 17

Thermal Analysis Techniques

Thermal Analysis • Thermal Gravimetric Analysis (TGA) Measure change in weight during heating or cooling • Differential Thermal Analysis (DTA)They are use for thermal investigation where thermal change can be observed and characterised. • Differential Scanning Calorimetry (DSC)Measure heat absorbed or liberated during heating or cooling • Thermomechanical Analysis (TMA)Measure change in dimensions during heating or cooling

THERMOGAVIMETRIC ANALYSIS (TGA)

GENERAL PRINCIPLES INVOLVED IN THERMOGRAVIMETRY • PRINCIPLE : Thermogravimetry is a technique in which a change in the weight of a substance is recorded as a function of temperature or time. • Instrument: Instrument used for thermogravimetry is “Thermobalance”. Data recorded in form of curve known as ‘Thermogram’.

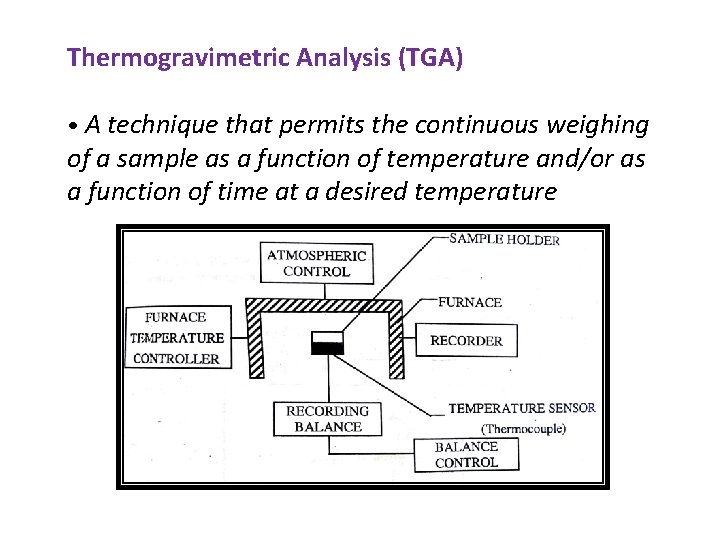
Thermogravimetric Analysis (TGA) • A technique that permits the continuous weighing of a sample as a function of temperature and/or as a function of time at a desired temperature

The Various componants of thermobalance 1) Sample Holder: The shape, size and material of the sample holder have an important effect on the shape of the TG curve. 2)Recording Balance: It is the most important componant of a thermobalance. It must fulfill the following requirements. • Its accuracy , reproducibility, sensitivity and capacity should be similar to those of an analytical balance. • It should have rapid response to weight changes. • Simple to operate and versatile.

3) Furnace and Furnace temperature controllar: The choice of the Furnace heating element upon the temperature range being studied. 4) Temperature sensor(Thermocouple): Measurement of temperature is done by a use of a thermocouple. 5) Recorder: i) Time based potentiometric stripchart recorder ii) X-Y recorder

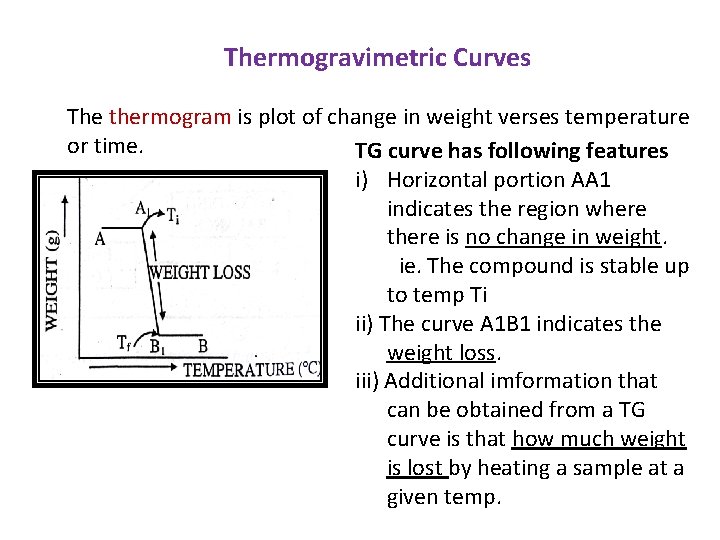
Thermogravimetric Curves The thermogram is plot of change in weight verses temperature or time. TG curve has following features i) Horizontal portion AA 1 indicates the region where there is no change in weight. ie. The compound is stable up to temp Ti ii) The curve A 1 B 1 indicates the weight loss. iii) Additional imformation that can be obtained from a TG curve is that how much weight is lost by heating a sample at a given temp.

Factors affecting thermogravimetric curve i) Instrumental factors ii) Characteristic of the sample i) Instrumental (Thermobalance) factors a) Heating rate: § If substance is heated at faster rate, it decomposes at a higher temp. § If substance is heated at slower rate, it decomposes at a lower temp. b) Furnace atmosphere: § The atmosphere in the furnace affects the nature of the TG curve.

ii)Characteristic of the sample a) Weight of the sample: A smaller weiht of the sample gives better results than a larger weight which causes deviation from the linear curve of weight loss. b) Partical size of the sample: The partical sizs of the sample is small, decomposition takes place at lower temp. c) Heat of a reaction: The heat of a reaction (heat evolved or absorbed) makes the temp. of the sample and furnace different. This affects the nature of the TG curve. d) Compactness of the Sample : A compressed sample will decompose at a higher temp. than loose sample. e) Source of the sample: If the sample is derived from two different sources , its decomposition temp. is different.

Applications of TGA 1) The determination of purity and thermal stability of both primary and secondary standards used in volumetric analysis. 2) Determination of correct drying temp. 3) Determination of composition of complex mixtures. 4) Determination of suitable ignition temp. 5) Direct application to analytical problems.

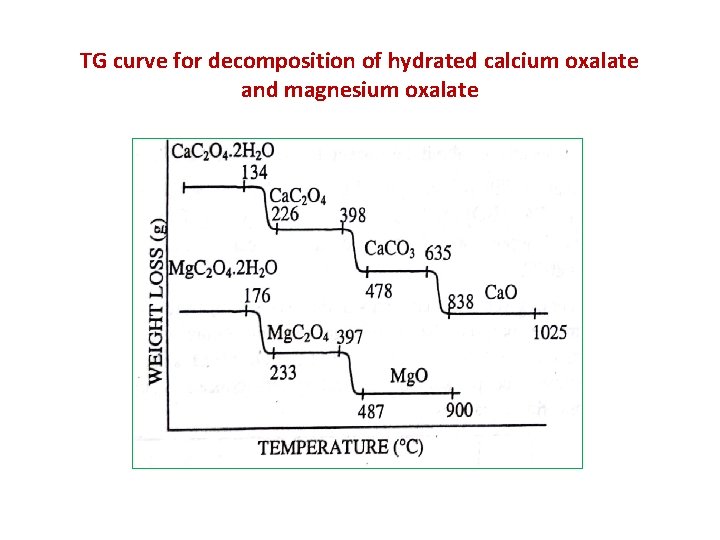
TG curve for decomposition of hydrated calcium oxalate and magnesium oxalate

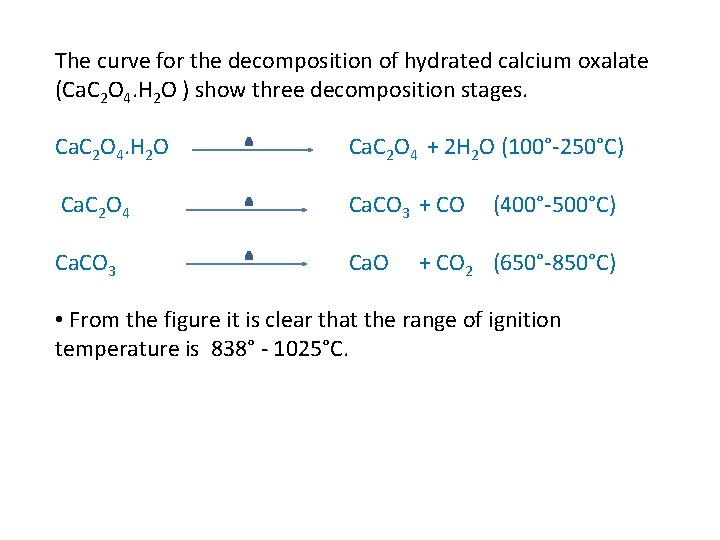
The curve for the decomposition of hydrated calcium oxalate (Ca. C 2 O 4. H 2 O ) show three decomposition stages. Ca. C 2 O 4. H 2 O Ca. C 2 O 4 + 2 H 2 O (100°-250°C) Ca. C 2 O 4 Ca. CO 3 + CO Ca. CO 3 Ca. O (400°-500°C) + CO 2 (650°-850°C) • From the figure it is clear that the range of ignition temperature is 838° - 1025°C.

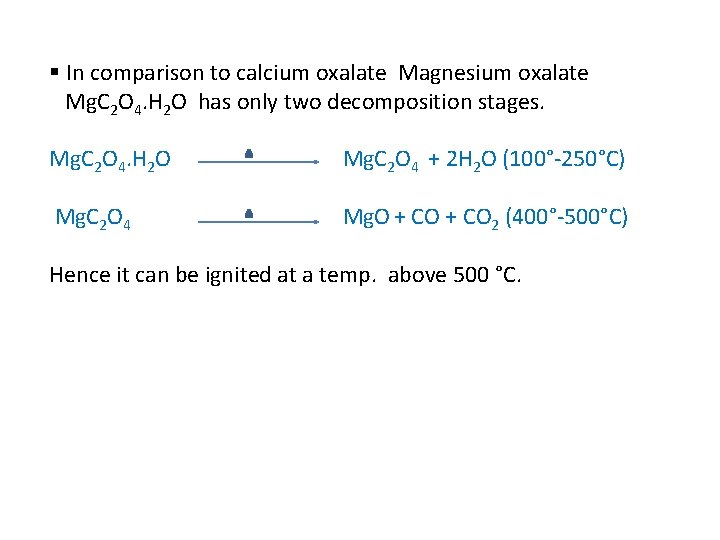
§ In comparison to calcium oxalate Magnesium oxalate Mg. C 2 O 4. H 2 O has only two decomposition stages. Mg. C 2 O 4. H 2 O Mg. C 2 O 4 + 2 H 2 O (100°-250°C) Mg. C 2 O 4 Mg. O + CO 2 (400°-500°C) Hence it can be ignited at a temp. above 500 °C.

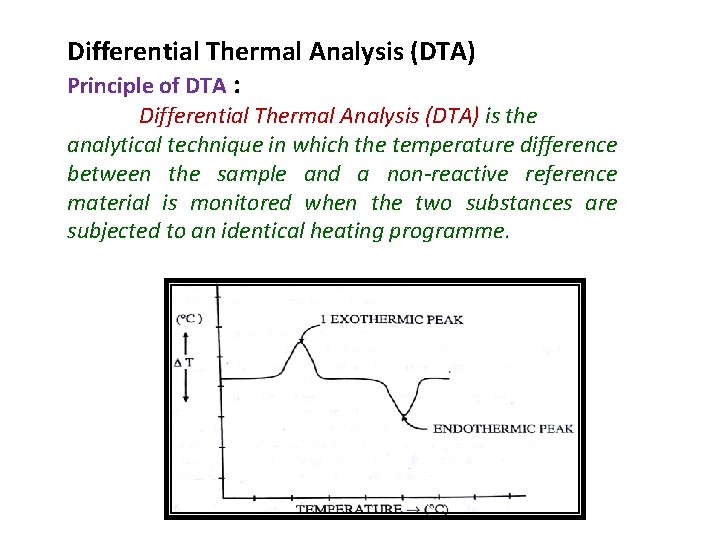
Differential Thermal Analysis (DTA) Principle of DTA : Differential Thermal Analysis (DTA) is the analytical technique in which the temperature difference between the sample and a non-reactive reference material is monitored when the two substances are subjected to an identical heating programme.

Applications of DTA 1) DTA provides a good deal of information about the temperatures of transitions. 2) It is used to determine specific heat of substances. 3) It is used to detect energy changes occuring during melting and polymorphic transitions. 4) It is used to determine directly melting points of a substance and hence check the purity of a compound.
