Thermal Energy Temperature Whats todays temperature outside Depending














- Slides: 19

Thermal Energy

Temperature • What’s today’s temperature outside? • Depending on the weather, we wear different clothing and accessories. For example, when it’s cold, we wear? • But, does “hot” mean the same thing to you as it does to me? What about “cold”?

What is temperature? • In order to define temperature we need to do a quick review of atoms! • Atoms: the smallest particles that make up all matter. • Matter: everything around us (has mass and takes up space) • Solid, Liquid, & Gas: atoms move differently in each of these • Kinetic energy: energy that moves!

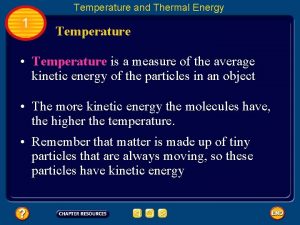
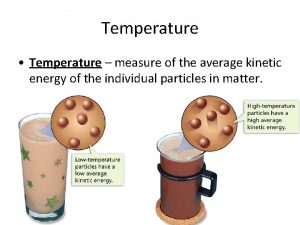
Temperature & Kinetic Energy • Note: Temperature – is a measure of the average kinetic energy of particles in an object. • Let’s make the connection: The higher the temperature of an object, the more it’s atoms and molecules have more _______ energy.

Measuring Temperature • Celsius- scale was devised by dividing the range of temperature between the freezing and boiling temperatures of pure water at standard atmospheric condition into 100 equal parts: freezing point of water is 0 C and boiling point is 100 C; bigger than F • Kelvin- temperature scale is an extension of the degree Celsius scale down to absolute zero • Fahrenheit- non-metric temperature scale was devised and evolved over time so that the freezing and boiling temperatures of water are whole numbers, but not round numbers as in the Celsius temperature scale; freezing point of water 32 F and boiling point is 212 F (divided into 180 degrees) – used mainly in the US.

Converting • From F to C: 5/9 x (F – 32) • From C to F: (9/5 x C) + 32 • From C to K: C + 273

Just Thermal! • Thermal energy: – Particles in matter are moving – They have energy because they are moving – Continual motion = Thermal energy – Can be transferred (all energy can)

Thermal in Action

Thermal Energy & Heat • Thermal Energy happens when? Let’s Review • We also know that thermal energy (all energy) can be transferred. • Let’s think about a cold glass of tea. It’s colder than my hand that’s holding it. The heat from my hand transfer to the glass…and the glass warms up. • Note: Heat – is the transfer of thermal energy from a substance at a higher temperature to a substance at a lower temperature.

Thermal Energy & Heat Continued • Note: Thermal energy moves from warmer objects to cooler ones. • Note: Water is unusual because it takes a large amount of thermal energy to raise its temperature; water’s temperature doesn’t change as much as surrounding air or land. • Note: Thermal energy can be transferred in 3 ways: Conduction, Convection & Radiation

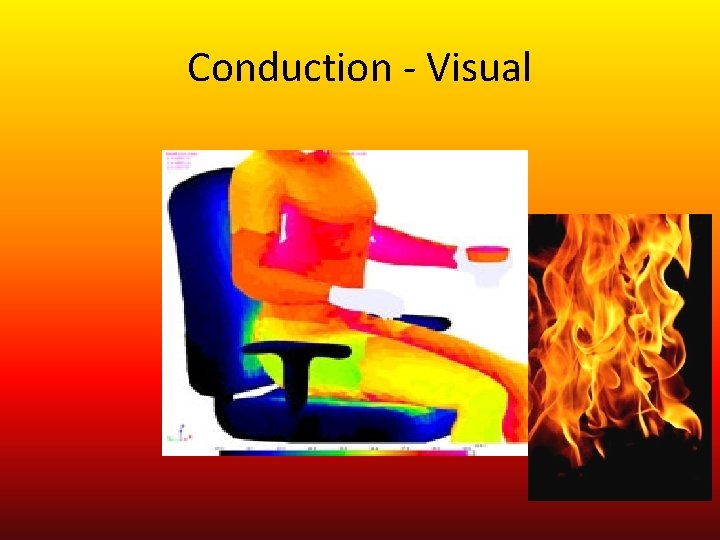
Conduction • Have you ever picked up a metal spoon from a boiling pot? How does it feel? • HOT!!!! But, how did it get so hot and why? • The answer is Conduction! • Note: Conduction - is the transfer of thermal energy by collisions between atoms; usually occurs in solids • As part of spoon in the boiling water becomes warmer, the atoms and molecules move faster…. increasing it’s temperature. These particles hit (collide) with the slower particles in the spoon, and thermal energy is transferred.

Conduction - Visual

Conduction in Action • Conduction

Good Conductor & Insulators • Thermal energy moves differently depending on the materials it’s moving to. • Good conductors – are materials in which thermal energy can transfer easily • What are some good conductors that you know of? • - Metals (are the most popular) • Insulators are different. These materials are NOT good conductors, so they are used as thermal insulators. • Insulators keep the heat in. (Ex. Sweaters, blankets, thermal underwear)

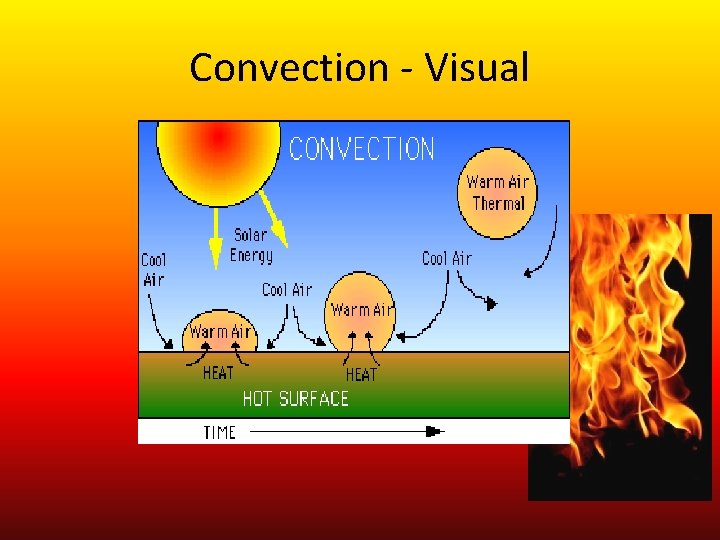
Convection • Thermal energy can also be transferred by particles that move from one place to another. • Note: Convection – transfers thermal energy when particles move between objects or areas that have different temperature. Convection 101 Convection in Weather

Convection - Visual

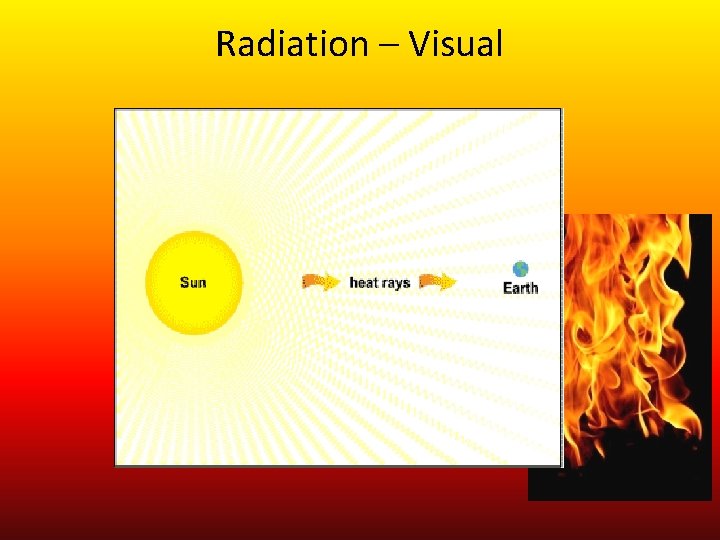
Radiation • • • Background: Comes from Radient Energy (sunlight) Radiation: Thermal energy that is transferred by waves. These waves can be visible (light waves) or invisible. The sun transfers energy to the Earth via radiation. When you stand by a fire the radiation of heat makes you feel warm. • A microwave cooks your food using microwave radiation to transfer energy to your food. Radiation

Radiation – Visual

We use all 3!
 Section 3 using thermal energy worksheet answer key
Section 3 using thermal energy worksheet answer key What is todays temperature
What is todays temperature Whats todays temperature
Whats todays temperature Heat thermal energy and temperature
Heat thermal energy and temperature Heat thermal energy and temperature
Heat thermal energy and temperature Thermal energy vs heat
Thermal energy vs heat Thermal energy vs heat energy
Thermal energy vs heat energy Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Thermal energy vs temperature
Thermal energy vs temperature Thermal energy vs. temperature
Thermal energy vs. temperature Q mct
Q mct Todays wordlw
Todays wordlw Thermal energy and mass
Thermal energy and mass Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Nims management characteristics
Nims management characteristics Thermal cycler temperature verification system
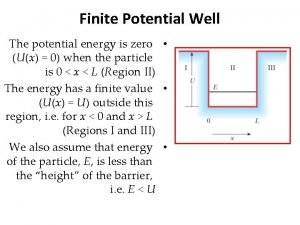
Thermal cycler temperature verification system Potential energy well
Potential energy well Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat?