Ch 4 Chemical Reactions Solution Stoichiometry Ch 4





- Slides: 33

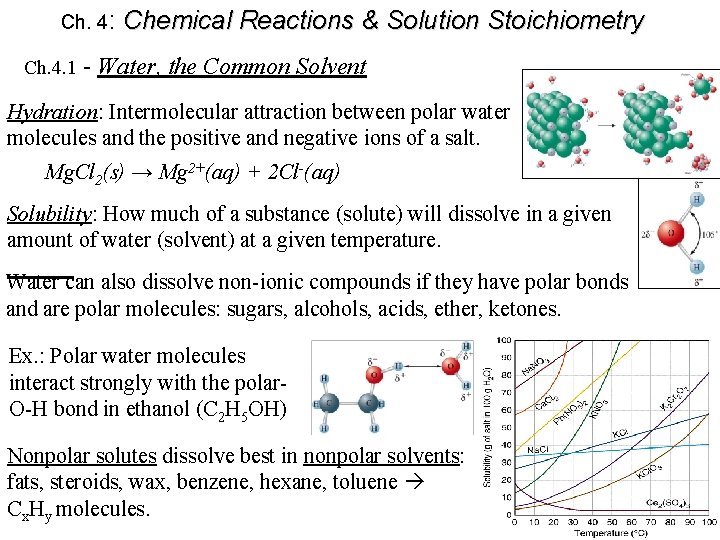
Ch. 4: Chemical Reactions & Solution Stoichiometry Ch. 4. 1 - Water, the Common Solvent Hydration: Intermolecular attraction between polar water molecules and the positive and negative ions of a salt. Mg. Cl 2(s) → Mg 2+(aq) + 2 Cl-(aq) Solubility: How much of a substance (solute) will dissolve in a given amount of water (solvent) at a given temperature. Water can also dissolve non-ionic compounds if they have polar bonds and are polar molecules: sugars, alcohols, acids, ether, ketones. Ex. : Polar water molecules interact strongly with the polar. O-H bond in ethanol (C 2 H 5 OH) Nonpolar solutes dissolve best in nonpolar solvents: fats, steroids, wax, benzene, hexane, toluene Cx. Hy molecules.

Ch. 4. 2: Nature of Aqueous Solutions - Electrolytes • Electrolyte – a substance whose aqueous solution conducts electricity. • Strong Electrolyte : compound completely dissociates in water (100% ionization) and allows a strong electric current to flow. • Ex. : soluble salts, strong acids (HCl, HBr, HI, H 2 SO 4, HNO 3, HCl. O 4) and strong bases (Na. OH, KOH, Ca(OH)2, Sr(OH)2, Ba(OH)2). • Weak Electrolyte : compound that only dissociates in water to a small degree (< 5% ionization) and allows only a small amount of current to flow. Ex. : weak acids: acetic acid (HC 2 H 3 O 2) , organic acids: citric acid, malic acid (apples), butyric acid (rancid butter), amino acids (proteins), ascorbic acid (Vitamin C); weak bases (NH 3). • Non-Electrolytes : compounds do not dissociate into ions and no current flows. Ex. : alcohols, sugars, soluble molecules.

Reactions of Acids and Bases: Strong and Weak Acids • Strong acids are strong electrolytes; completely ionized in water. In water: HCl(aq) → H+(aq) + Cl–(aq) No HCl in solution, only H+ and Cl– ions. • Weak acids are weak electrolytes. Only some of the dissolved molecules dissociate; the rest remain intact as molecules. Acetic acid in water: CH 3 COOH (aq) ⇄ H+(aq) + CH 3 COO-(aq) ⇄ : state of equilibrium where the rxn. occurs in both directions. For acetic acid, only about 1% of the CH 3 COOH molecules dissociates into ions. Only a few H+ and CH 3 COO– ions in solution. Most CH 3 COOH molecules remain intact. Some acids have more than one ionizable hydrogen atom. They ionize in “steps” (more in Chapter 15 on Acids & Bases). H 2 SO 4 (aq) → H+ (aq) + HSO 4– (aq) → H+ (aq) + SO 42– (aq)

Strong and Weak Bases • Strong bases: Most are ionic hydroxides (Group IA and IIA, though some IIA hydroxides aren’t very soluble). • Weak bases: Like weak acids, they ionize partially. Ionization process is different. • Weak bases form OH– by accepting H+ from water … NH 3 + H 2 O → NH 4+ + OH– CH 3 NH 2 + H 2 O → CH 3 NH 3+ + methylamine Most of the weak base remains in the molecular form. OH– methylammonium ion Just a little OH– forms. • NH 3 , under some conditions, can also act as a weak acid (amphoteric subst. ) 2 Li + 2 NH 3 → 2 Li. NH 2 + H 2

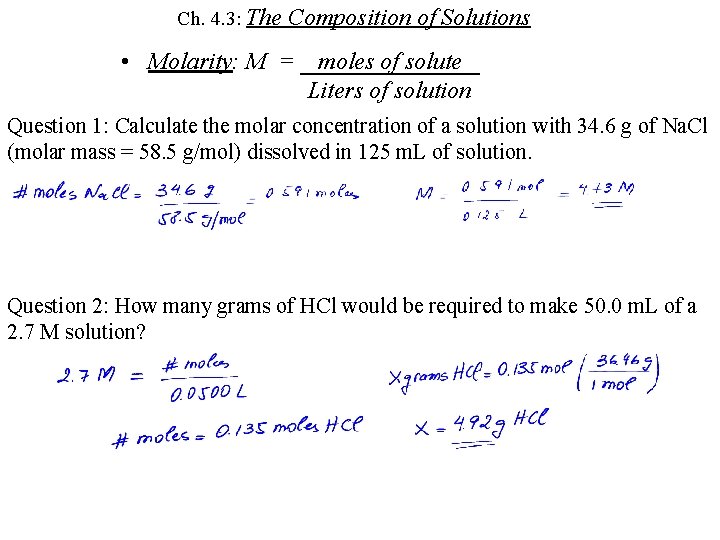
Ch. 4. 3: The Composition of Solutions • Molarity: M = moles of solute Liters of solution Question 1: Calculate the molar concentration of a solution with 34. 6 g of Na. Cl (molar mass = 58. 5 g/mol) dissolved in 125 m. L of solution. Question 2: How many grams of HCl would be required to make 50. 0 m. L of a 2. 7 M solution?

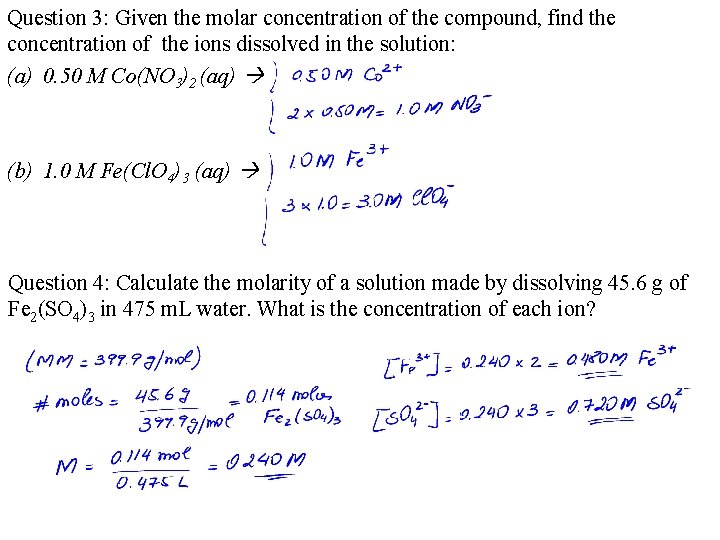
Question 3: Given the molar concentration of the compound, find the concentration of the ions dissolved in the solution: (a) 0. 50 M Co(NO 3)2 (aq) (b) 1. 0 M Fe(Cl. O 4)3 (aq) Question 4: Calculate the molarity of a solution made by dissolving 45. 6 g of Fe 2(SO 4)3 in 475 m. L water. What is the concentration of each ion?

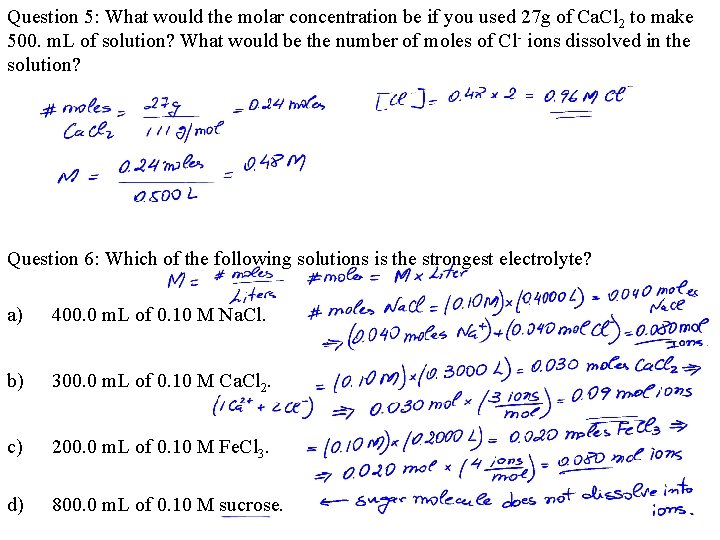
Question 5: What would the molar concentration be if you used 27 g of Ca. Cl 2 to make 500. m. L of solution? What would be the number of moles of Cl- ions dissolved in the solution? Question 6: Which of the following solutions is the strongest electrolyte? a) 400. 0 m. L of 0. 10 M Na. Cl. b) 300. 0 m. L of 0. 10 M Ca. Cl 2. c) 200. 0 m. L of 0. 10 M Fe. Cl 3. d) 800. 0 m. L of 0. 10 M sucrose.

Question 7: Which of the following solutions contains the greatest number of dissolved ions? (Assume complete solubility for all salts. ) a) One mole of potassium chloride dissolved in 1. 0 L of water. b) One mole of sodium phosphate dissolved in 1. 0 L of water. c) One mole of iron(II) nitrate dissolved in 1. 0 L of water. d) One mole of sodium hydroxide dissolved in 1. 0 L of water. e) At least two of the above solutions have an equally great number of ions.

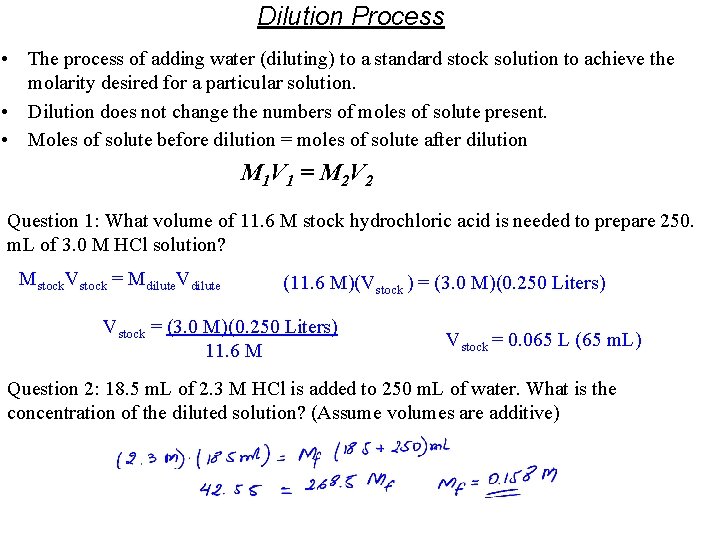
Dilution Process • The process of adding water (diluting) to a standard stock solution to achieve the molarity desired for a particular solution. • Dilution does not change the numbers of moles of solute present. • Moles of solute before dilution = moles of solute after dilution M 1 V 1 = M 2 V 2 Question 1: What volume of 11. 6 M stock hydrochloric acid is needed to prepare 250. m. L of 3. 0 M HCl solution? Mstock. Vstock = Mdilute. Vdilute (11. 6 M)(Vstock ) = (3. 0 M)(0. 250 Liters) Vstock = (3. 0 M)(0. 250 Liters) 11. 6 M Vstock = 0. 065 L (65 m. L) Question 2: 18. 5 m. L of 2. 3 M HCl is added to 250 m. L of water. What is the concentration of the diluted solution? (Assume volumes are additive)

Q. 3: You need to make 225 m. L of 0. 05 M H 2 SO 4 solution. How much of a 3. 0 M stock solution should you use? How much water must you add to the stock solution to obtain the desired concentration of the diluted solution? Q. 4: You have a 4. 0 M stock solution of K 3 PO 4. (a) Describe how to make 1. 0 L of a 0. 75 M solution. (b) What is the concentration of the ions in the final, diluted solution?

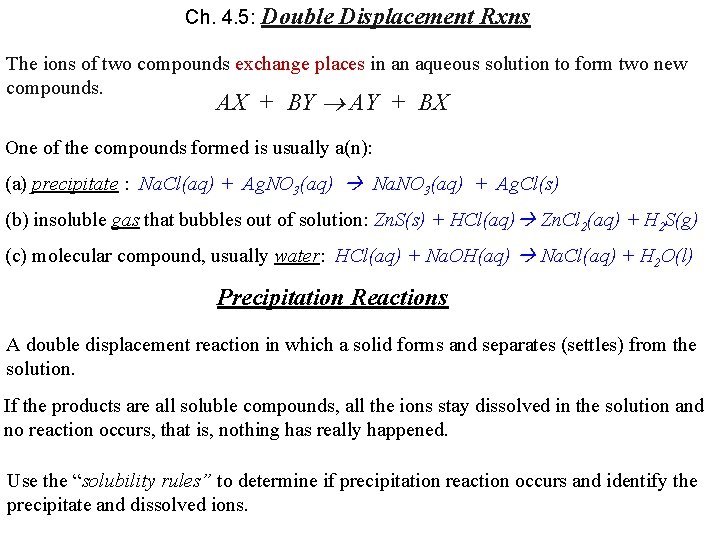
Ch. 4. 5: Double Displacement Rxns The ions of two compounds exchange places in an aqueous solution to form two new compounds. AX + BY AY + BX One of the compounds formed is usually a(n): (a) precipitate : Na. Cl(aq) + Ag. NO 3(aq) Na. NO 3(aq) + Ag. Cl(s) (b) insoluble gas that bubbles out of solution: Zn. S(s) + HCl(aq) Zn. Cl 2(aq) + H 2 S(g) (c) molecular compound, usually water: HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) Precipitation Reactions A double displacement reaction in which a solid forms and separates (settles) from the solution. If the products are all soluble compounds, all the ions stay dissolved in the solution and no reaction occurs, that is, nothing has really happened. Use the “solubility rules” to determine if precipitation reaction occurs and identify the precipitate and dissolved ions.

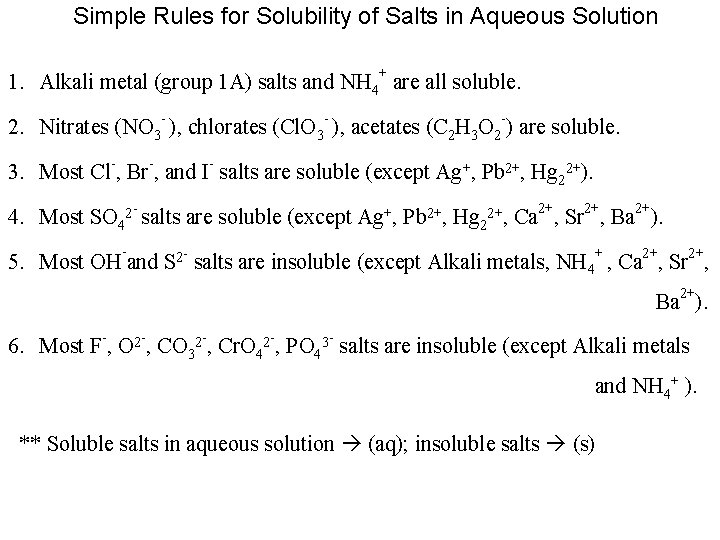
Simple Rules for Solubility of Salts in Aqueous Solution 1. Alkali metal (group 1 A) salts and NH 4+ are all soluble. - - 2. Nitrates (NO 3 ), chlorates (Cl. O 3 ), acetates (C 2 H 3 O 2 -) are soluble. 3. Most Cl-, Br-, and I- salts are soluble (except Ag+, Pb 2+, Hg 22+). 4. Most SO 42 - salts are soluble (except Ag+, Pb 2+, Hg 22+, Ca 2+, Sr 2+, Ba 2+). - 5. Most OH and S 2 - salts are insoluble (except Alkali metals, NH 4+ , Ca 2+, Sr 2+, Ba 2+). 6. Most F-, O 2 -, CO 32 -, Cr. O 42 -, PO 43 - salts are insoluble (except Alkali metals and NH 4+ ). ** Soluble salts in aqueous solution (aq); insoluble salts (s)

Double replacement forming a precipitate… (Rxn. #1) lead(II) nitrate + potassium iodide potassium nitrate + lead(II) iodide Molecular Equation (ME) Pb(NO 3)2(aq) + 2 KI(aq) 2 KNO 3(aq) + Pb. I 2(s) Complete Ionic Equation (CIE) Pb 2+(aq) +2 NO 3 -(aq) + 2 K+(aq) +2 I-(aq) 2 K+(aq) + 2 NO 3 - (aq) + Pb. I 2(s) Spectator Ions: NO 3 - & K+ Net Ionic Equation (NIE) excludes the spectator ions Pb 2+(aq) + 2 I-(aq) Pb. I 2(s)

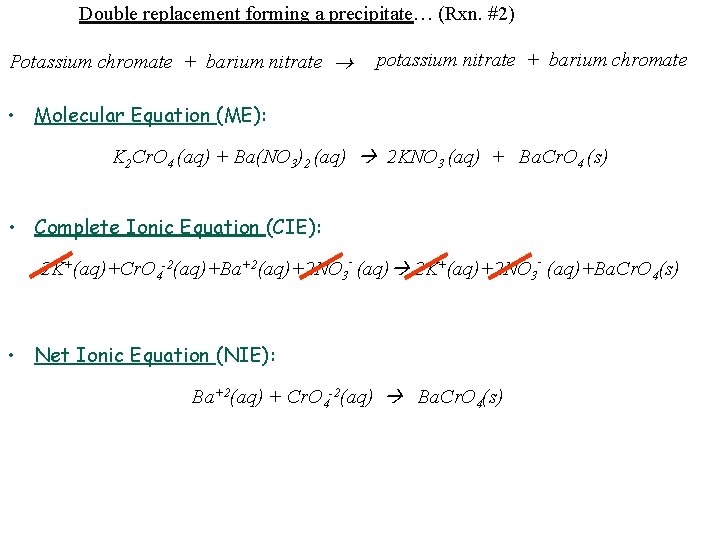
Double replacement forming a precipitate… (Rxn. #2) Potassium chromate + barium nitrate potassium nitrate + barium chromate • Molecular Equation (ME): K 2 Cr. O 4 (aq) + Ba(NO 3)2 (aq) 2 KNO 3 (aq) + Ba. Cr. O 4 (s) • Complete Ionic Equation (CIE): 2 K+(aq)+Cr. O 4 -2(aq)+Ba+2(aq)+2 NO 3 - (aq) 2 K+(aq)+2 NO 3 - (aq)+Ba. Cr. O 4(s) • Net Ionic Equation (NIE): Ba+2(aq) + Cr. O 4 -2(aq) Ba. Cr. O 4(s)

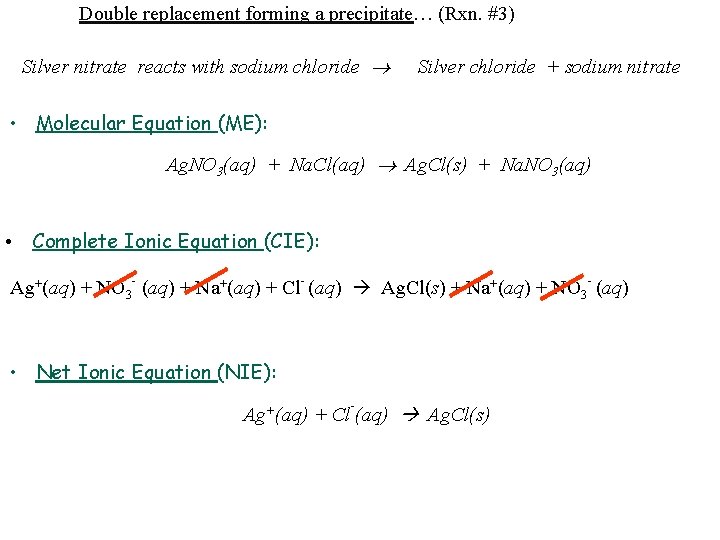
Double replacement forming a precipitate… (Rxn. #3) Silver nitrate reacts with sodium chloride Silver chloride + sodium nitrate • Molecular Equation (ME): Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) • Complete Ionic Equation (CIE): Ag+(aq) + NO 3 - (aq) + Na+(aq) + Cl- (aq) Ag. Cl(s) + Na+(aq) + NO 3 - (aq) • Net Ionic Equation (NIE): - Ag+(aq) + Cl (aq) Ag. Cl(s)

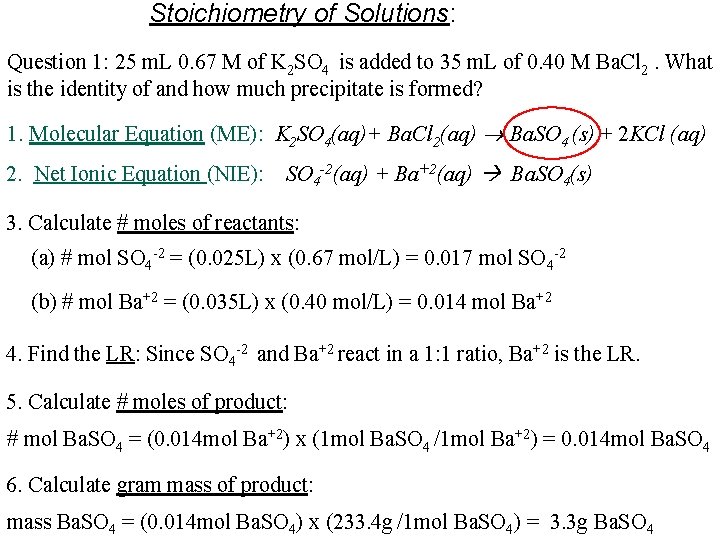
Stoichiometry of Solutions: Question 1: 25 m. L 0. 67 M of K 2 SO 4 is added to 35 m. L of 0. 40 M Ba. Cl 2. What is the identity of and how much precipitate is formed? 1. Molecular Equation (ME): K 2 SO 4(aq)+ Ba. Cl 2(aq) Ba. SO 4 (s) + 2 KCl (aq) 2. Net Ionic Equation (NIE): SO 4 -2(aq) + Ba+2(aq) Ba. SO 4(s) 3. Calculate # moles of reactants: (a) # mol SO 4 -2 = (0. 025 L) x (0. 67 mol/L) = 0. 017 mol SO 4 -2 (b) # mol Ba+2 = (0. 035 L) x (0. 40 mol/L) = 0. 014 mol Ba+2 4. Find the LR: Since SO 4 -2 and Ba+2 react in a 1: 1 ratio, Ba+2 is the LR. 5. Calculate # moles of product: # mol Ba. SO 4 = (0. 014 mol Ba+2) x (1 mol Ba. SO 4 /1 mol Ba+2) = 0. 014 mol Ba. SO 4 6. Calculate gram mass of product: mass Ba. SO 4 = (0. 014 mol Ba. SO 4) x (233. 4 g /1 mol Ba. SO 4) = 3. 3 g Ba. SO 4

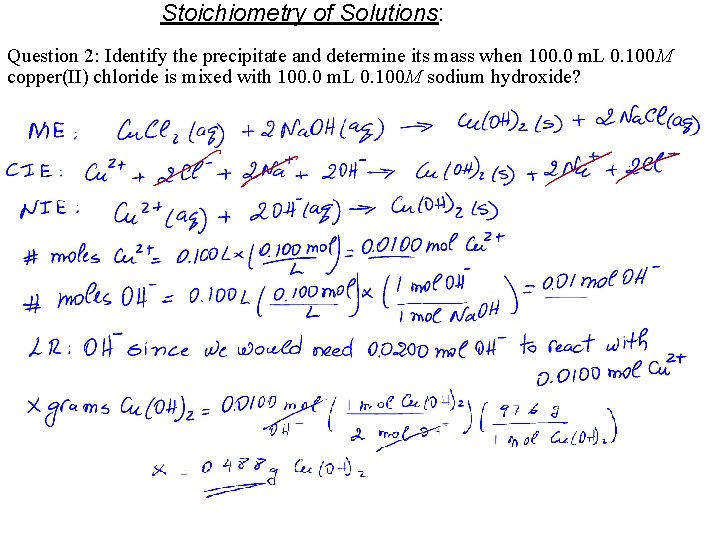
Stoichiometry of Solutions: Question 2: Identify the precipitate and determine its mass when 100. 0 m. L 0. 100 M copper(II) chloride is mixed with 100. 0 m. L 0. 100 M sodium hydroxide?

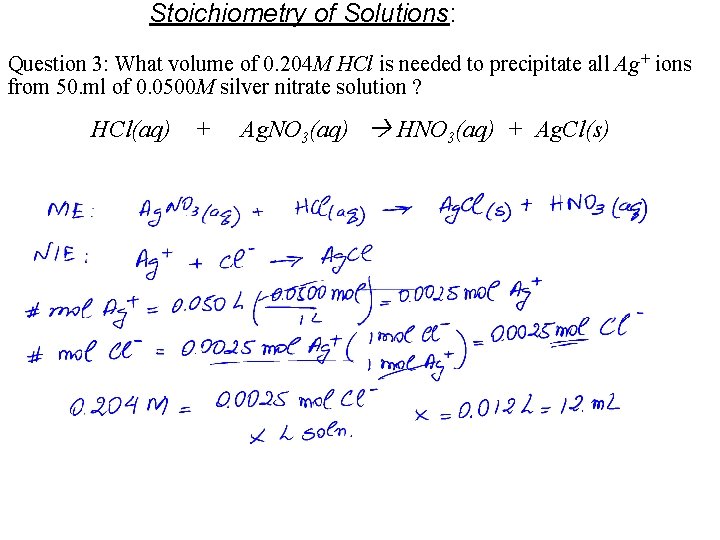
Stoichiometry of Solutions: Question 3: What volume of 0. 204 M HCl is needed to precipitate all Ag+ ions from 50. ml of 0. 0500 M silver nitrate solution ? HCl(aq) + Ag. NO 3(aq) HNO 3(aq) + Ag. Cl(s)

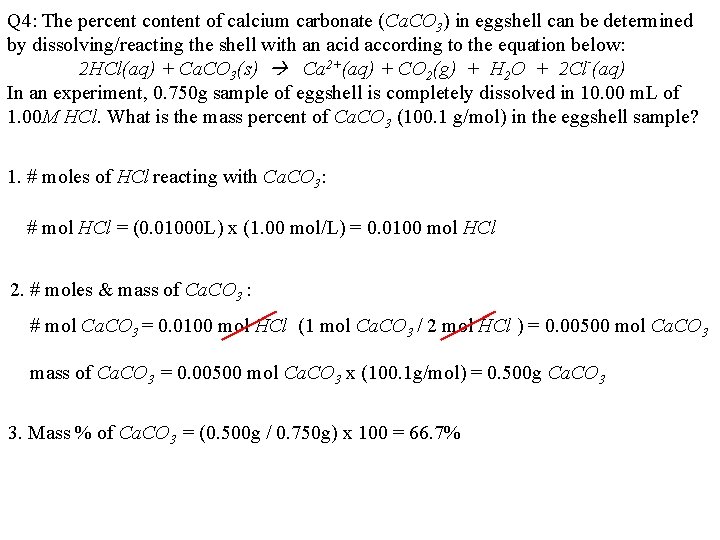
Q 4: The percent content of calcium carbonate (Ca. CO 3) in eggshell can be determined by dissolving/reacting the shell with an acid according to the equation below: 2 HCl(aq) + Ca. CO 3(s) Ca 2+(aq) + CO 2(g) + H 2 O + 2 Cl-(aq) In an experiment, 0. 750 g sample of eggshell is completely dissolved in 10. 00 m. L of 1. 00 M HCl. What is the mass percent of Ca. CO 3 (100. 1 g/mol) in the eggshell sample? 1. # moles of HCl reacting with Ca. CO 3: # mol HCl = (0. 01000 L) x (1. 00 mol/L) = 0. 0100 mol HCl 2. # moles & mass of Ca. CO 3 : # mol Ca. CO 3 = 0. 0100 mol HCl (1 mol Ca. CO 3 / 2 mol HCl ) = 0. 00500 mol Ca. CO 3 mass of Ca. CO 3 = 0. 00500 mol Ca. CO 3 x (100. 1 g/mol) = 0. 500 g Ca. CO 3 3. Mass % of Ca. CO 3 = (0. 500 g / 0. 750 g) x 100 = 66. 7%

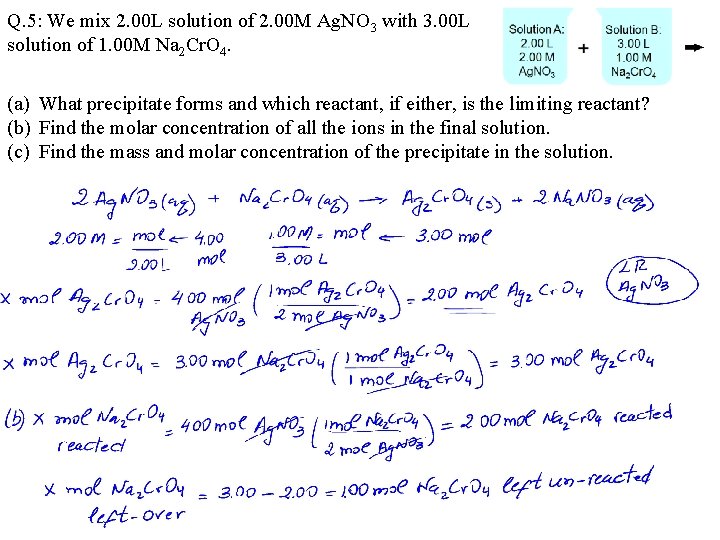
Q. 5: We mix 2. 00 L solution of 2. 00 M Ag. NO 3 with 3. 00 L solution of 1. 00 M Na 2 Cr. O 4. (a) What precipitate forms and which reactant, if either, is the limiting reactant? (b) Find the molar concentration of all the ions in the final solution. (c) Find the mass and molar concentration of the precipitate in the solution.

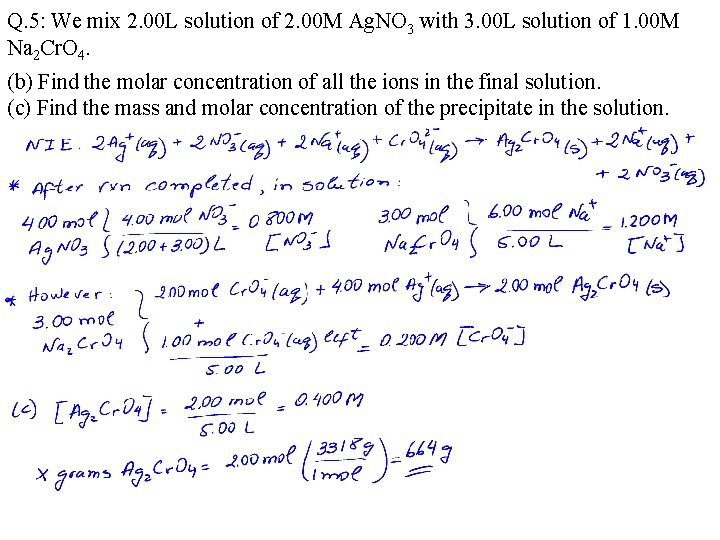
Q. 5: We mix 2. 00 L solution of 2. 00 M Ag. NO 3 with 3. 00 L solution of 1. 00 M Na 2 Cr. O 4. (b) Find the molar concentration of all the ions in the final solution. (c) Find the mass and molar concentration of the precipitate in the solution.

Ch. 4. 8: Acid-Base Acids: Reactions (Brønsted -Lowry) • Proton (H+) donors • p. H < 7; Sour taste • p. H indicators: blue litmus turns red; methyl orange turns red • React with active metals, producing H 2 and a salt • Neutralize bases producing a salt and H 2 O. Bases: • • Proton (H+) acceptors; – Often releasing (OH ) in the solution; p. H > 7; Bitter taste p. H indicators: red litmus turns blue; phenolphthalein turns pink • Solutions of bases feel slippery • Neutralize acids producing a salt and H 2 O.

Ch. 4. 8: Acid-Base Reactions (Brønsted-Lowry) (A) For a strong acid and strong base neutralization reaction: HCl(aq) + KOH(aq) KCl (aq) + H 2 O(l) • Complete Ionic Equation: – – – H+(aq) + Cl (aq) + K+(aq) + OH (aq) K+(aq) + Cl (aq) + H 2 O(l) – • Net Ionic Equation: H+(aq) + OH (aq) H 2 O(l) (B) For a weak acid and strong base reaction: HC 2 H 3 O 2 (aq) + KOH(aq) KC 2 H 3 O 2 (aq) + H 2 O(l) • Complete Ionic Equation: H 2 O(l) HC 2 H 3 O 2 (aq) + K+(aq) – – + OH (aq) K+(aq) – + C 2 H 3 O 2 (aq) + – • NIE: HC 2 H 3 O 2 (aq) + OH (aq) C 2 H 3 O 2 (aq) + H 2 O(l) – ** OH ions in solution react with any weak acid, bonding with H+ ions and forming H 2 O(l).

Ch. 4. 8: Acid-Base Reactions (Brønsted-Lowry) (C) For a strong acid and weak base neutralization reaction: H 2 SO 4 (aq) + 2 NH 3 (aq) (NH 4)2 SO 4(aq) • Complete Ionic Equation: 2 - 2 - 2 H+(aq) + SO 4 (aq) + 2 NH 3 (aq) 2 NH 4+(aq) + SO 4 (aq) • Net Ionic Equation: 2 H+(aq) + 2 NH 3 (aq) 2 NH 4+(aq)

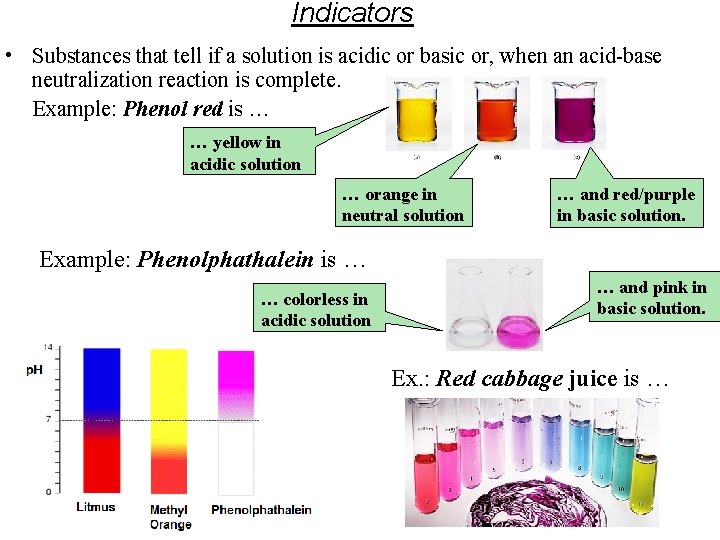
Indicators • Substances that tell if a solution is acidic or basic or, when an acid-base neutralization reaction is complete. Example: Phenol red is … … yellow in acidic solution … orange in neutral solution … and red/purple in basic solution. Example: Phenolphathalein is … … colorless in acidic solution … and pink in basic solution. Ex. : Red cabbage juice is …

Ch. 4. 8: Acid-Base Neutralization Reactions Question 1: What volume of 0. 100 M HCl solution is needed to neutralize 25. 0 m. L of 0. 350 M Na. OH? 1. Molecular Equation (ME): HCl(aq)+ Na. OH(aq) Na. Cl (aq) + H 2 O(l) 2. Net Ionic Equation (NIE): H+(aq) – + OH (aq) H 2 O(l) 3. Calculate # moles of reactants: # mol OH- = (0. 025 L) x (0. 350 mol/L) = 0. 00875 mol OH- (Na. OH) 4. Calculate # moles of H+ (HCl) needed to react with Na. OH: # mol H+ = (0. 000875 mol OH-) x (1 mol H+ /mol OH-) = 0. 00875 mol H+ (HCl) 5. Calculate volume of 0. 100 M HCL solution: Volume HCl = (0. 00875 mol HCl) / (0. 100 mol/Liter) = 0. 0875 L = 87. 5 m. L

Ch. 4. 8: Acid-Base Neutralization Reactions Q 2: 28. 0 m. L of 0. 250 M HNO 3 and 53. 0 m. L of 0. 320 M KOH are mixed. Calculate the amount of water formed in the resulting solution. What is the – concentration of H+ or OH ions in excess after the reaction is complete? 1. Molecular Equation (ME): HNO 3 (aq)+ KOH(aq) KNO 3 (aq) + H 2 O(l) 2. Net Ionic Equation (NIE): H+(aq) – + OH (aq) H 2 O(l) 3. Calculate # moles of reactants: # mol H+ = (0. 028 L) x (0. 250 mol/L) = 0. 00700 mol H+ # mol OH- = (0. 053 L) x (0. 320 mol/L) = 0. 0170 mol OH- 4. Find the LR: Since H+ and OH- react in a 1: 1 ratio, H+ is the LR. 5. Mass of H 2 O: 0. 00700 mol H+ 0. 00700 mol H 2 O mass of H 2 O = (0. 00700 mol H 2 O) x (18. 02 g /1 mol H 2 O ) = 0. 126 g H 2 O

Ch. 4. 8: Acid-Base Neutralization Reactions Q 2: 28. 0 m. L of 0. 250 M HNO 3 and 53. 0 m. L of 0. 320 M KOH are mixed. Calculate the amount of water formed in the resulting solution. What is the – concentration of H+ or OH ions in excess after the reaction is complete? 6. Moles of OH- in excess: (a) 0. 00700 mol H+ 0. 00700 mol OH- (b) moles of OH- in excess = 0. 0170 – 0. 00700 = 0. 0100 mol OH- 7. Molar Concentration of OH- : Solution Volume = 28. 0 m. L + 53. 0 m. L = 81. 0 m. L (0. 0810 L) M OH- = (0. 0100 mol OH-) / (0. 0810 L) = 0. 123 M OH-

Titration (Volumetric Analysis) • A solution of known molar concentration (titrant) is gradually mixed and reacts with a given volume of a solution whose concentration is to be determined (analyte). • The volume of titrant added is carefully and precisely measured. • A color indicator , initially added to the analyte soln. , tells when all the analyte has reacted and the reaction is complete. • Titrant – solution of known concentration (standard) used in the titration • Analyte – substance being analyzed for its concentration. • Equivalence point – enough titrant added to react completely with the given volume of the analyte. • Endpoint – the indicator changes color showing the equivalence point has been reached.

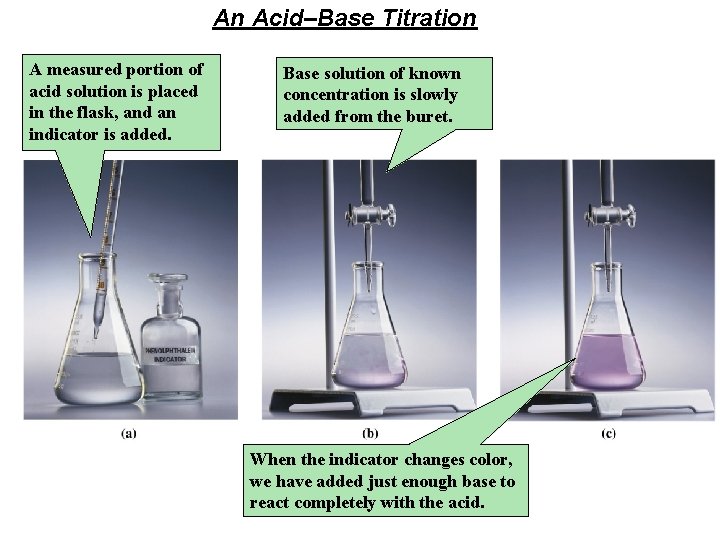
An Acid–Base Titration A measured portion of acid solution is placed in the flask, and an indicator is added. Base solution of known concentration is slowly added from the buret. When the indicator changes color, we have added just enough base to react completely with the acid.

Question 1: When 22. 0 m. L of hydrochloric acid of unknown concentration is titrated with 0. 2150 M Na. OH, the end-point is reached when 22. 40 m. L of the base has been added. What is the molar concentration of the acid? 1. Molecular Equation (ME): HCl(aq)+ Na. OH(aq) Na. Cl (aq) + H 2 O(l) – 2. Net Ionic Equation (NIE): H+(aq) + OH (aq) H 2 O(l) 3. # moles of titrant (OH-) added to reach the equivalence point: # mol OH- = (0. 02240 L) x (0. 2150 mol/L) = 0. 004816 mol OH- (Na. OH) 4. # moles of H+ (HCl) reacting with OH- (Na. OH): # mol H+ = # mol OH- = 0. 004816 mol H+ (HCl) 5. Molar Concentrationolume of the HCl solution: MHCl = (0. 004816 mol HCl) / (0. 0220 Liter) = 0. 219 M

Question 2: A titration of an aqueous solution with 0. 3518 g benzoic acid (HC 7 H 5 O 2 ) (122. 1 g/1 mol) required 10. 59 m. L of 0. 1546 M Na. OH to reach the end point for complete neutralization. Calculate the mass percent of HC 7 H 5 O 2 in the original sample. 1. ME: HC 7 H 5 O 2(aq) + Na. OH - (aq) Na. C 7 H 5 O 2 (aq) + H 2 O(l) 2. NIE: HC 7 H 5 O 2(aq) + OH - (aq) C 7 H 5 O 2 - (aq) + H 2 O(l) 3. # moles of OH- reacting with HC 7 H 5 O 2: # mol OH- = (0. 01059 L) x (0. 1546 mol/L) = 0. 001637 mol OH- 4. # moles & mass of HC 7 H 5 O 2 : # mol HC 7 H 5 O 2 = # mol OH- = 0. 001637 mol mass of HC 7 H 5 O 2= 0. 001637 mol x (122. 1 g/1 mol) = 0. 1999 g 5. Mass % of HC 7 H 5 O 2: (0. 1999 g / 0. 3518 g) x 100 = 56. 82%

Titration –Precipitation Rxn. (Volumetric Analysis) Q 3. : It takes 37. 50 m. L of 0. 152 M sodium chromate to titrate 25. 00 m. L solution of silver nitrate. Calculate the molarity of the silver nitrate solution. 1. ME: Na 2 Cr. O 4(aq) + 2 Ag. NO 3(aq) 2 Na. NO 3(aq) + Ag 2 Cr. O 4(s) + 2. NIE: Cr. O 42 -(aq) + 2 Ag (aq) Ag 2 Cr. O 4(s) 3. # moles of titrant (Cr. O 42 -) added to reach the equivalence point: # mol Cr. O 42 - = (0. 03750 L) x (0. 152 mol/L) = 0. 00570 mol Cr. O 42 - (Na 2 Cr. O 4) 4. # moles of Ag+ (Ag. NO 3) reacting with Cr. O 42 - (Na 2 Cr. O 4): # mol Ag+ = 2 x (# mol Cr. O 42 - ) = 2 x 0. 00570 = 0. 0114 mol Ag+ (Ag. NO 3) 5. Molar Concentrationolume of the Ag. NO 3 solution: MAg. NO 3 = (0. 0114 mol Ag. NO 3) / (0. 02500 Liter) = 0. 456 M