Atomic Structure and Periodicity Mr Guerrero AP Chemistry











- Slides: 41

Atomic Structure and Periodicity Mr. Guerrero, AP Chemistry

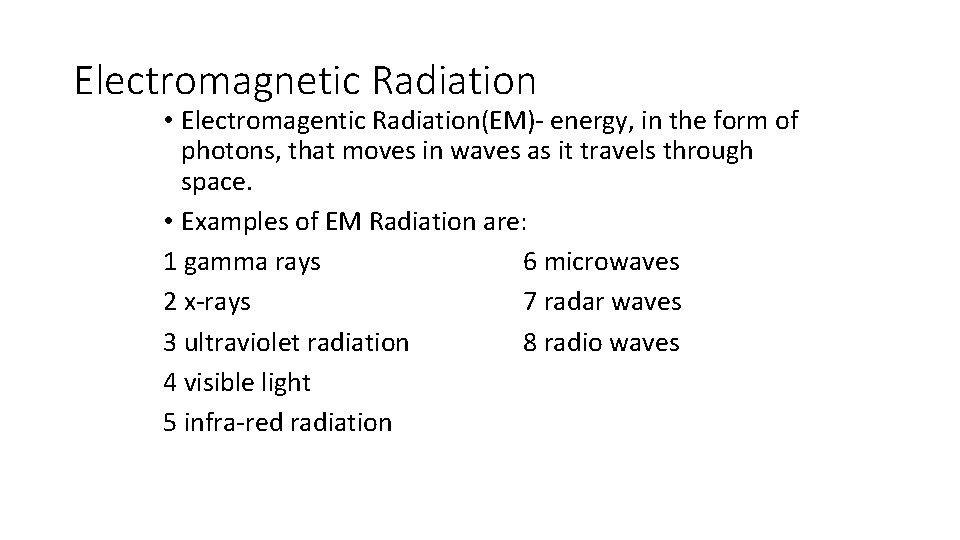
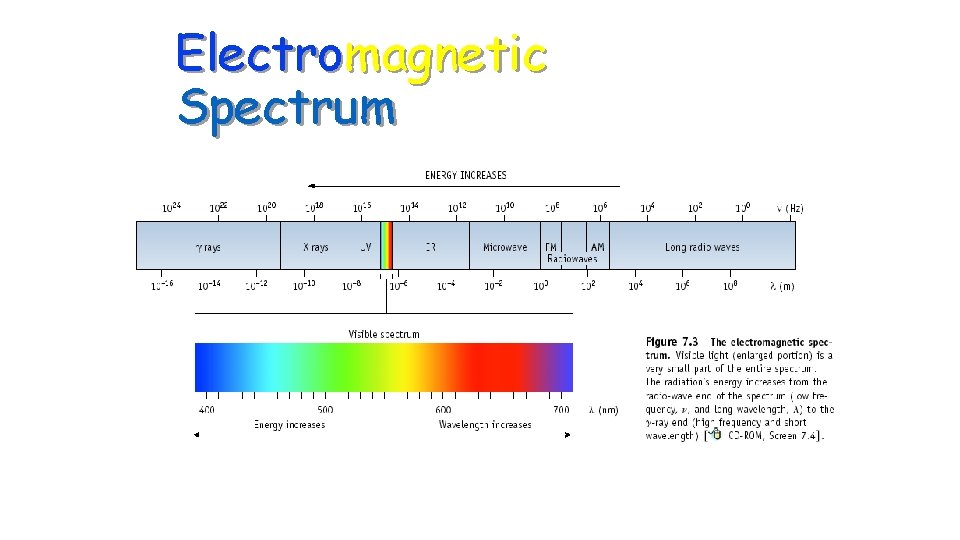
Electromagnetic Radiation • Electromagentic Radiation(EM)- energy, in the form of photons, that moves in waves as it travels through space. • Examples of EM Radiation are: 1 gamma rays 6 microwaves 2 x-rays 7 radar waves 3 ultraviolet radiation 8 radio waves 4 visible light 5 infra-red radiation

Electromagnetic Spectrum

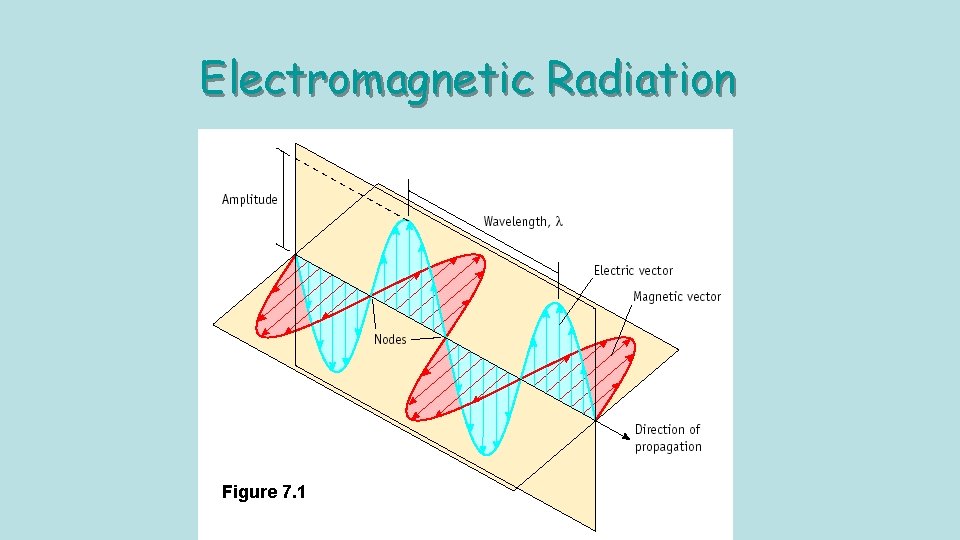
Electromagnetic Radiation Figure 7. 1

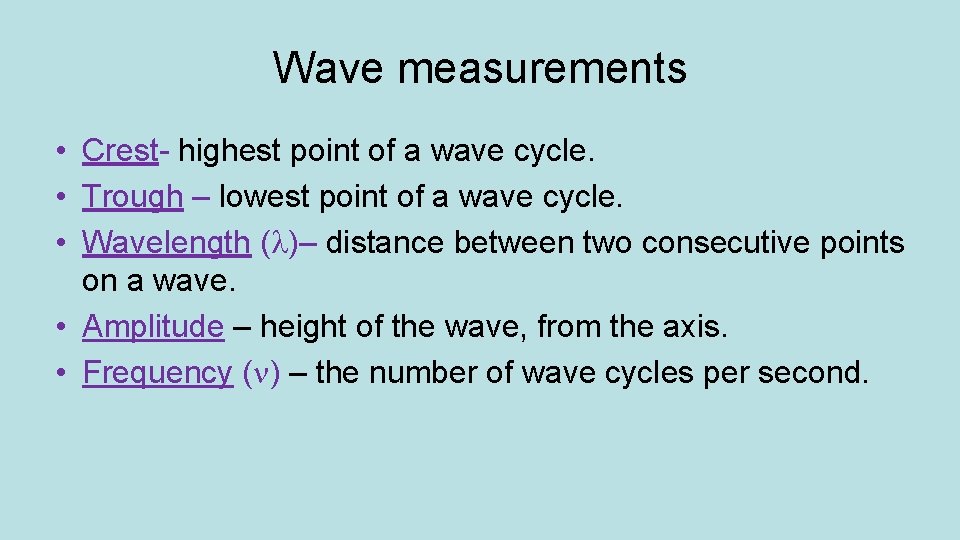
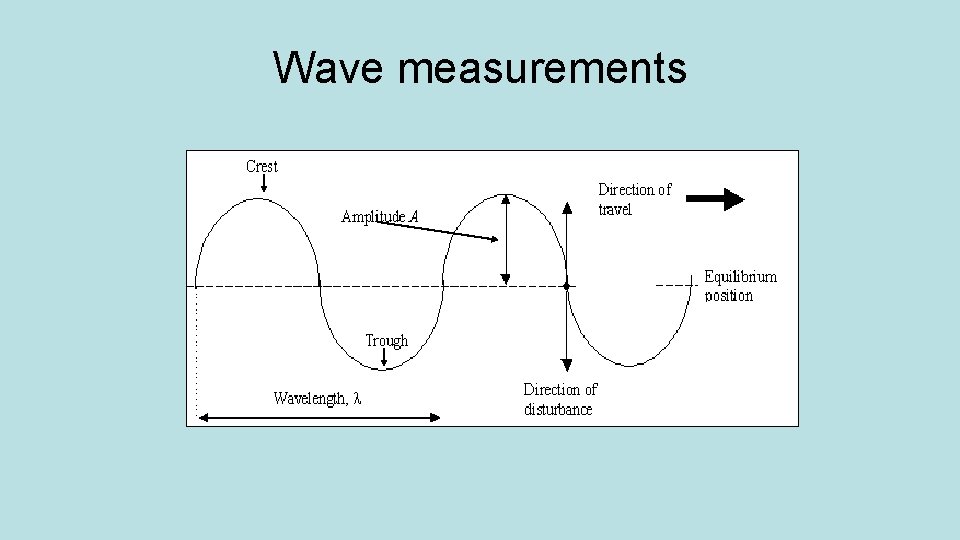
Wave measurements • Crest- highest point of a wave cycle. • Trough – lowest point of a wave cycle. • Wavelength ( )– distance between two consecutive points on a wave. • Amplitude – height of the wave, from the axis. • Frequency ( ) – the number of wave cycles per second.

Wave measurements

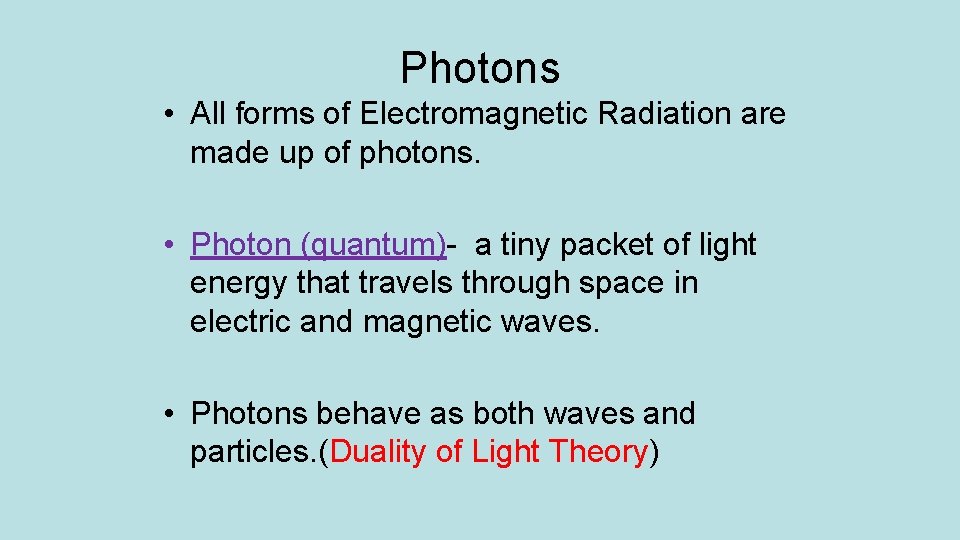
Photons • All forms of Electromagnetic Radiation are made up of photons. • Photon (quantum)- a tiny packet of light energy that travels through space in electric and magnetic waves. • Photons behave as both waves and particles. (Duality of Light Theory)

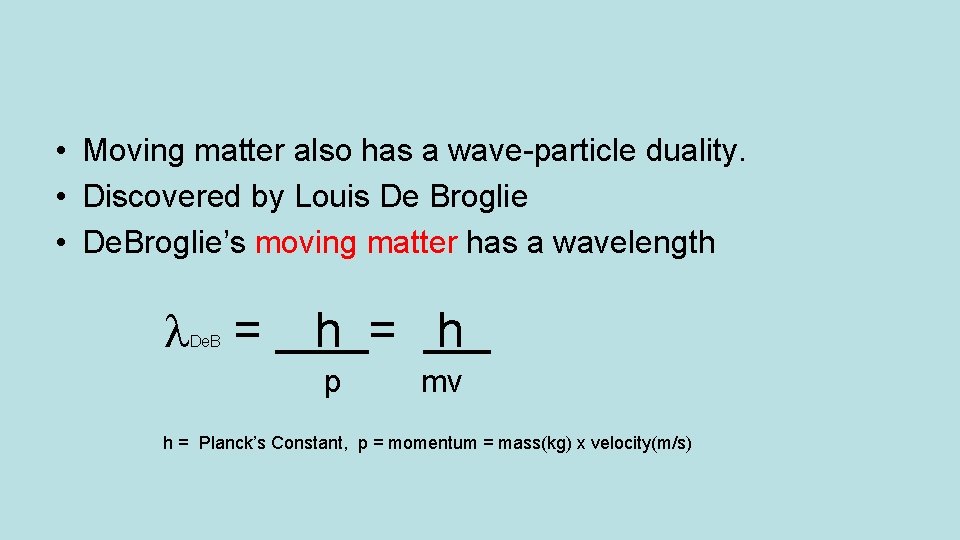
• Moving matter also has a wave-particle duality. • Discovered by Louis De Broglie • De. Broglie’s moving matter has a wavelength = De. B h = h p mv h = Planck’s Constant, p = momentum = mass(kg) x velocity(m/s)

Find the De. Broglie wavelength of: • A 2. 35 kg bowing ball moving at 7. 00 m/s. • An electron travelling at ½ the speed of light. • A 75 Kg football player running at 8. 55 m/s.

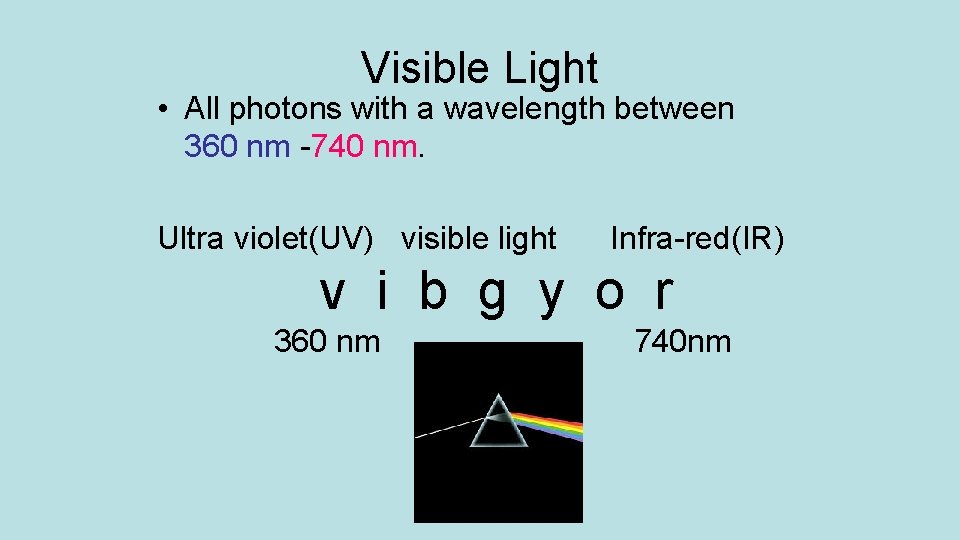
Visible Light • All photons with a wavelength between 360 nm -740 nm. Ultra violet(UV) visible light Infra-red(IR) v i b g y o r 360 nm 740 nm

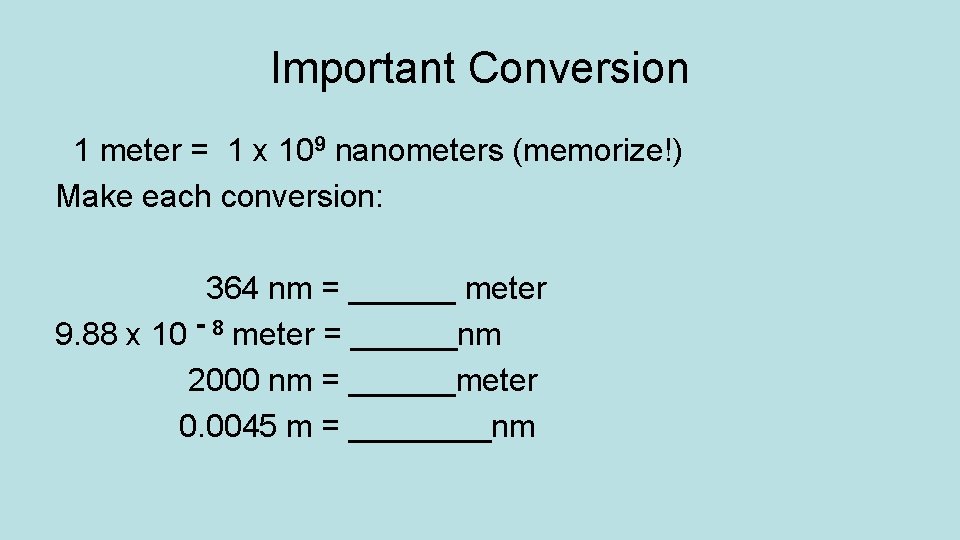
Important Conversion 1 meter = 1 x 109 nanometers (memorize!) Make each conversion: 364 nm = ______ meter 9. 88 x 10 - 8 meter = ______nm 2000 nm = ______meter 0. 0045 m = ____nm

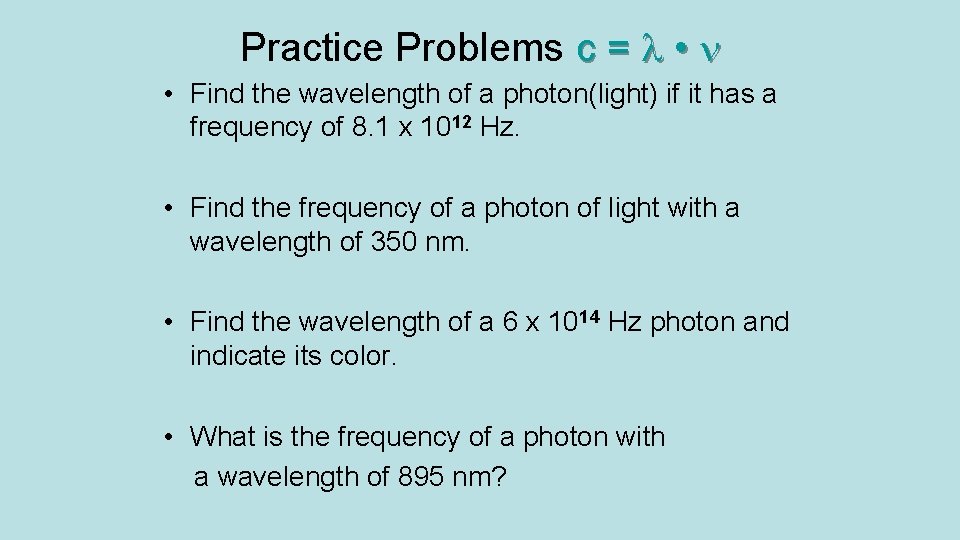
Practice Problems c = • • Find the wavelength of a photon(light) if it has a frequency of 8. 1 x 1012 Hz. • Find the frequency of a photon of light with a wavelength of 350 nm. • Find the wavelength of a 6 x 1014 Hz photon and indicate its color. • What is the frequency of a photon with a wavelength of 895 nm?

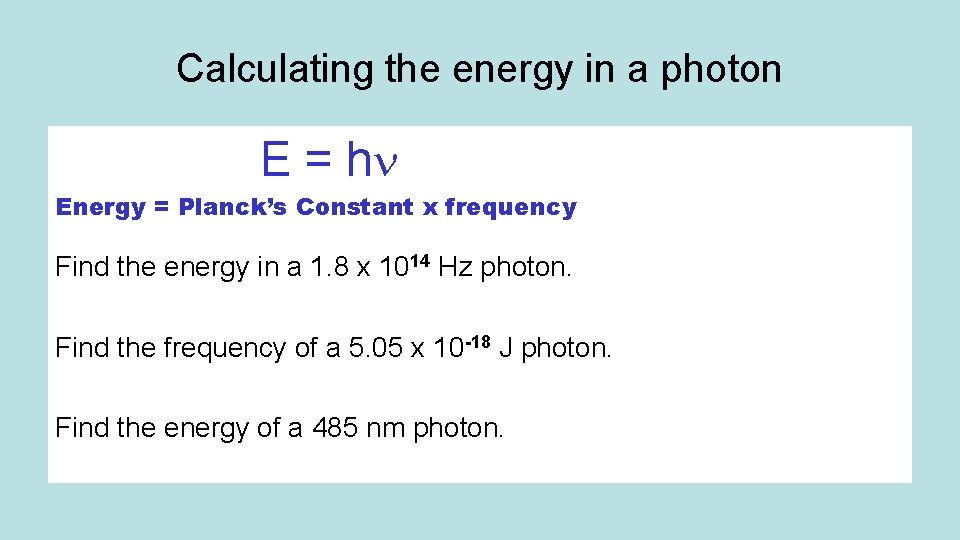
Calculating the energy in a photon E = h Energy = Planck’s Constant x frequency Find the energy in a 1. 8 x 1014 Hz photon. Find the frequency of a 5. 05 x 10 -18 J photon. Find the energy of a 485 nm photon.

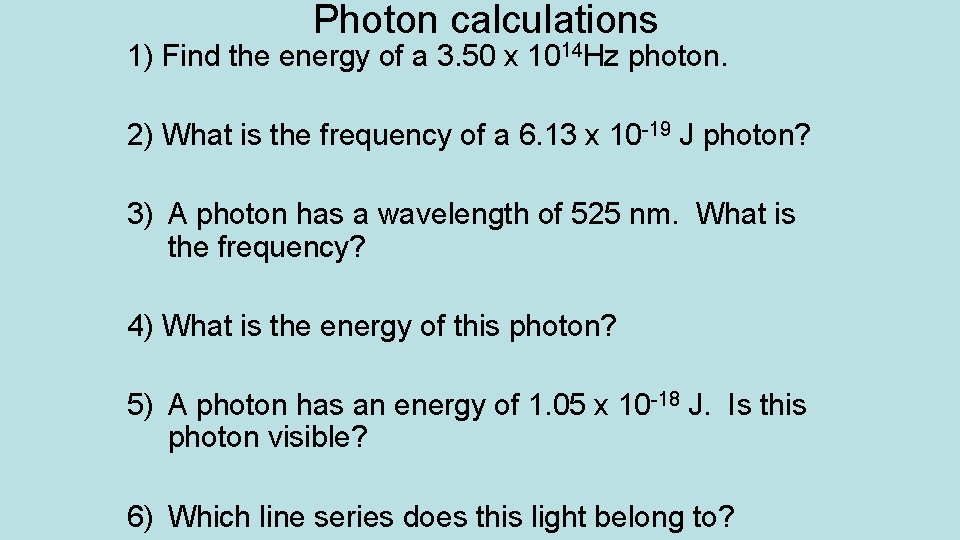
Photon calculations 1) Find the energy of a 3. 50 x 1014 Hz photon. 2) What is the frequency of a 6. 13 x 10 -19 J photon? 3) A photon has a wavelength of 525 nm. What is the frequency? 4) What is the energy of this photon? 5) A photon has an energy of 1. 05 x 10 -18 J. Is this photon visible? 6) Which line series does this light belong to?

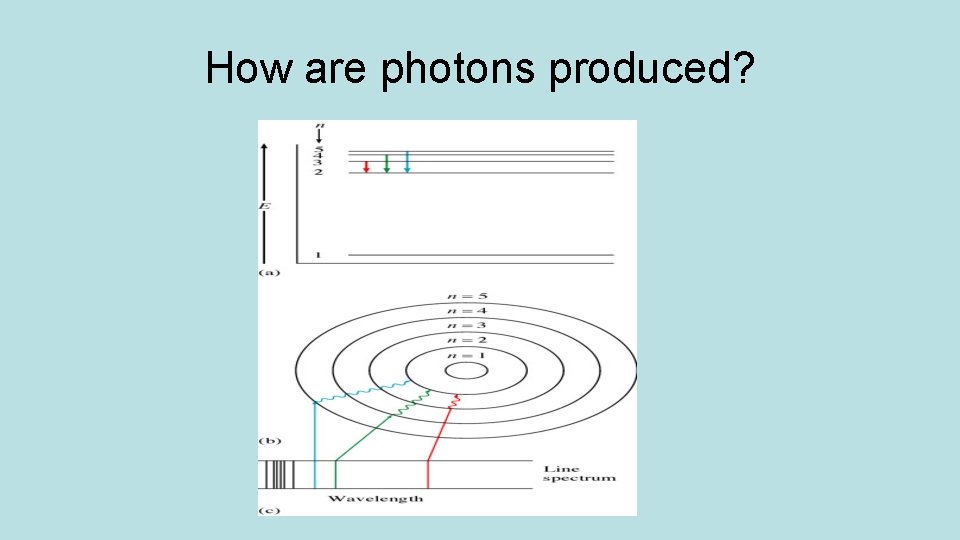
How are photons produced?

How are photons produced?

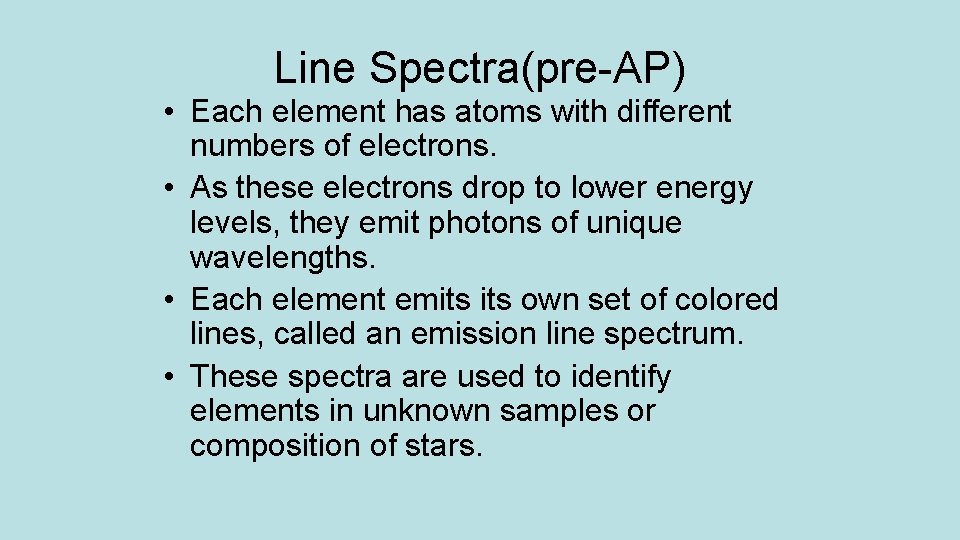
Line Spectra(pre-AP) • Each element has atoms with different numbers of electrons. • As these electrons drop to lower energy levels, they emit photons of unique wavelengths. • Each element emits own set of colored lines, called an emission line spectrum. • These spectra are used to identify elements in unknown samples or composition of stars.

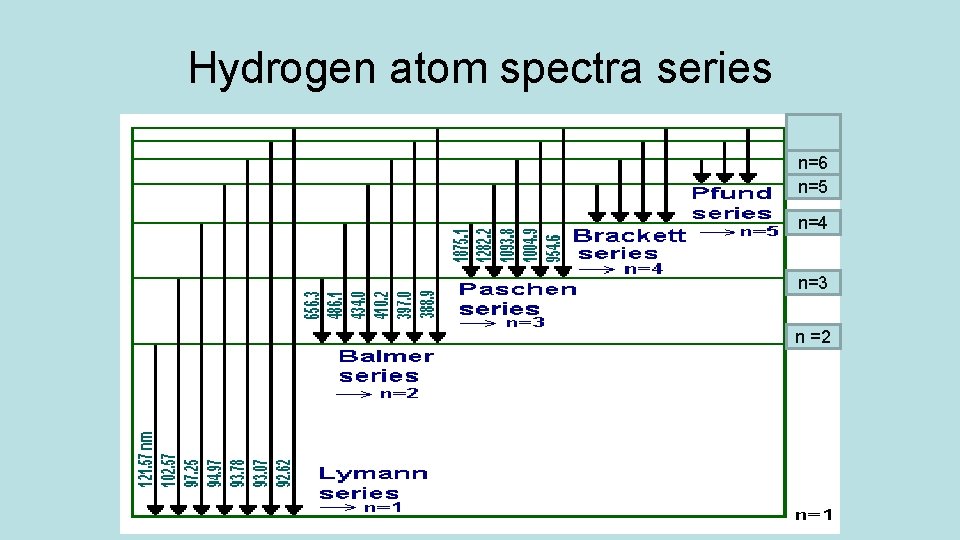
Hydrogen atom spectra series n=6 n=5 n=4 n=3 nn=2 =2

Line spectra series Lyman Series- e- drops from n = X to n=1 lights are in Ultraviolet Region Balmer Series- e- drops from n = X to n =2 lights are in Visible Region Paschen Series- e- drops from n = X to n = 3 lights are in Infrared Region

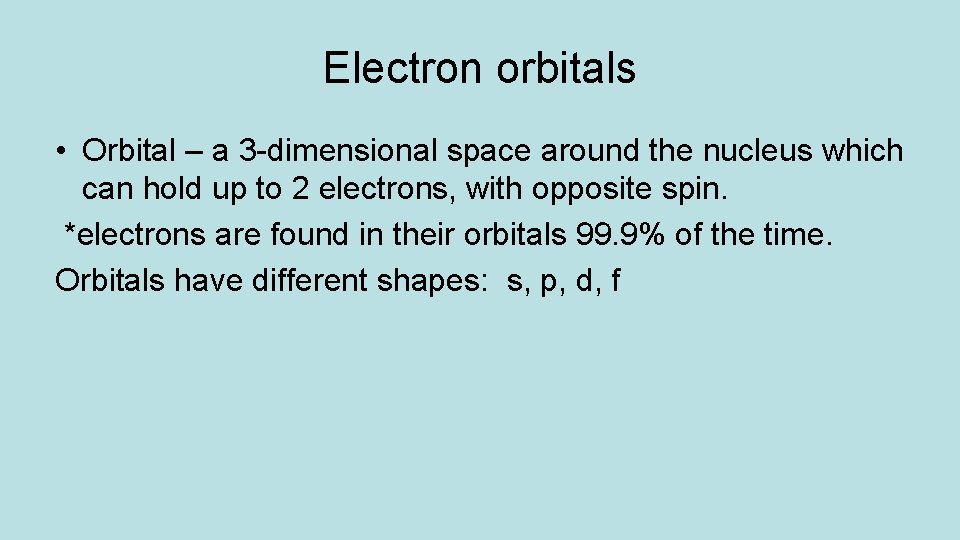
Electron orbitals • Orbital – a 3 -dimensional space around the nucleus which can hold up to 2 electrons, with opposite spin. *electrons are found in their orbitals 99. 9% of the time. Orbitals have different shapes: s, p, d, f

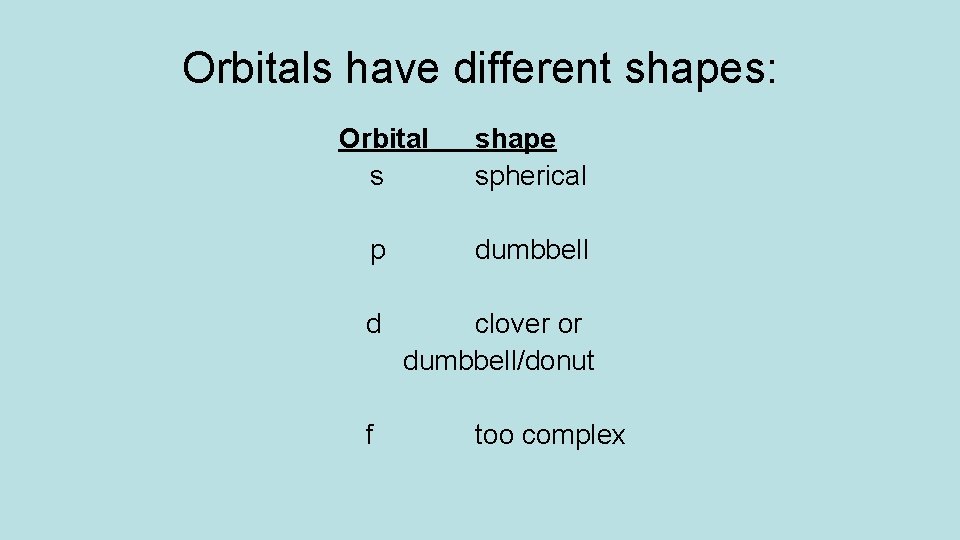
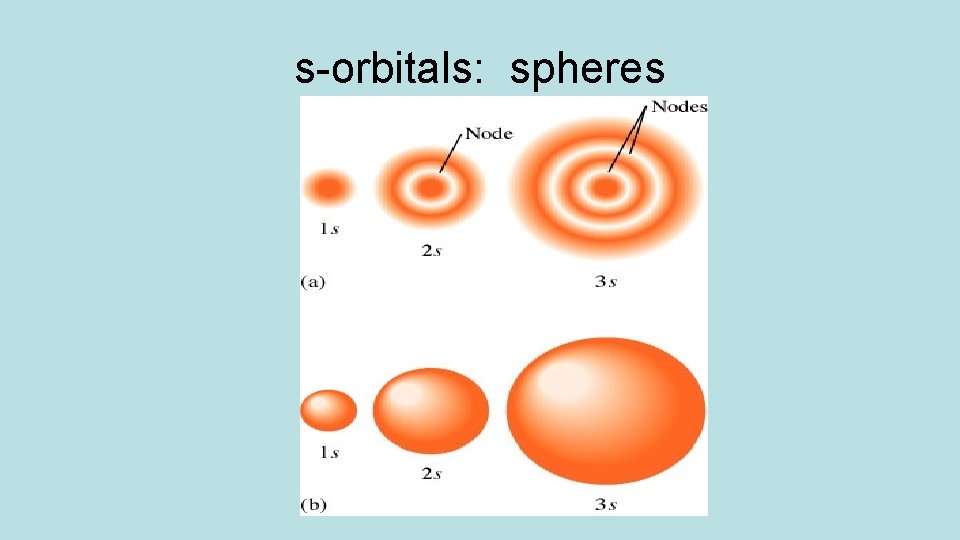
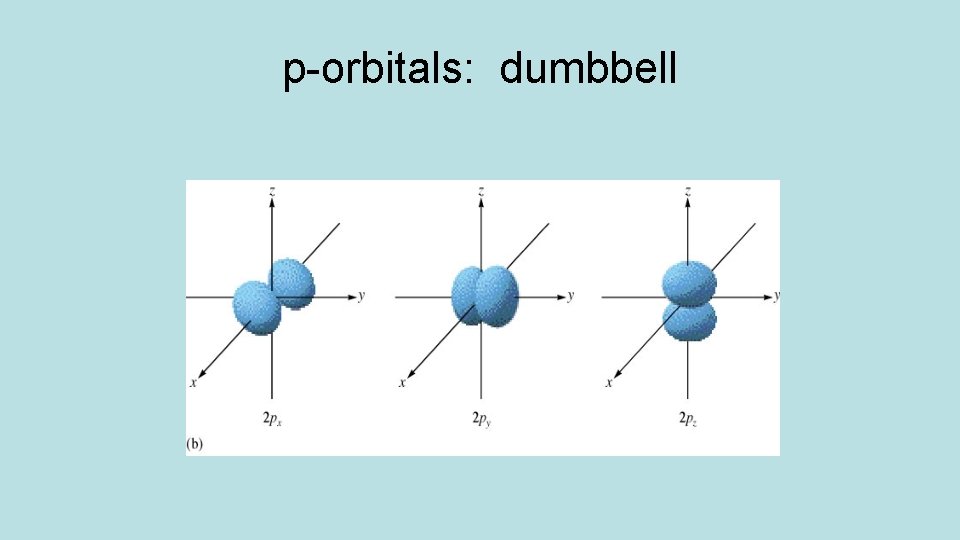
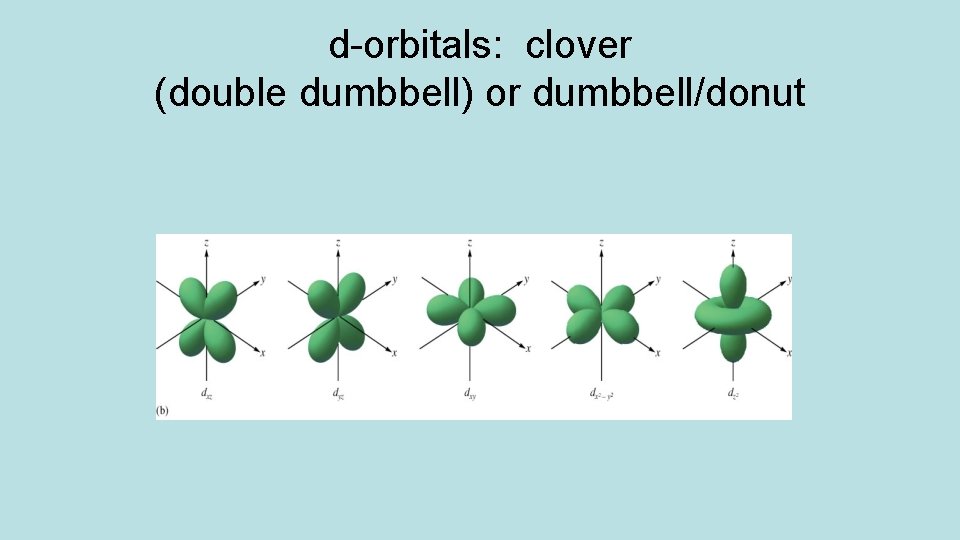
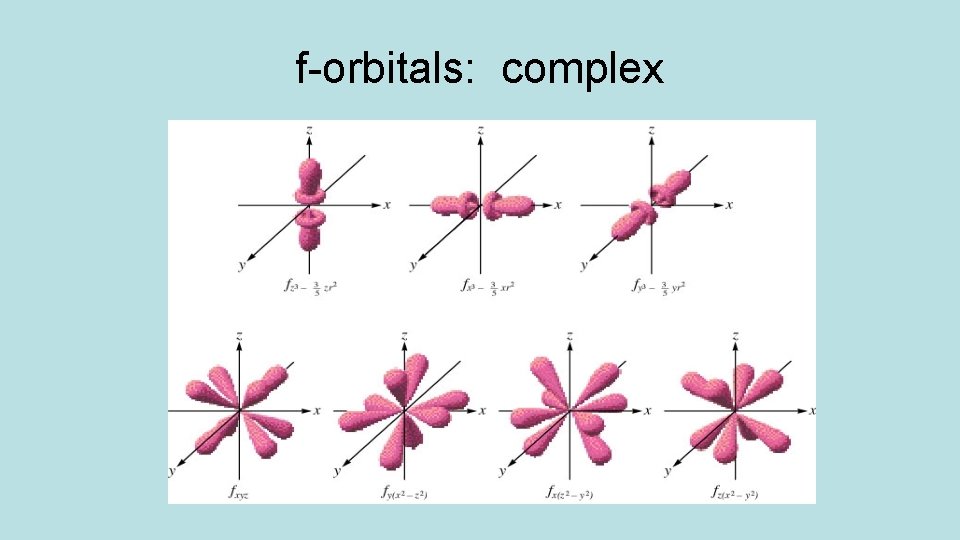
Orbitals have different shapes: Orbital s shape spherical p dumbbell d clover or dumbbell/donut f too complex

s-orbitals: spheres

p-orbitals: dumbbell

d-orbitals: clover (double dumbbell) or dumbbell/donut

f-orbitals: complex

Quantum Numbers • The location of each electron in an atom can be determined by assigning each electron a four-number code called quantum numbers.

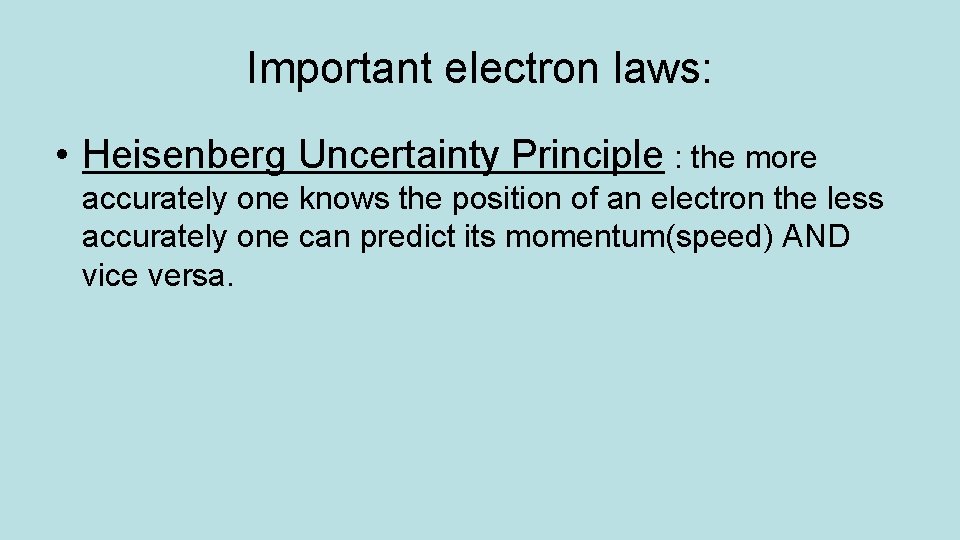
Important electron laws: • Heisenberg Uncertainty Principle : the more accurately one knows the position of an electron the less accurately one can predict its momentum(speed) AND vice versa.

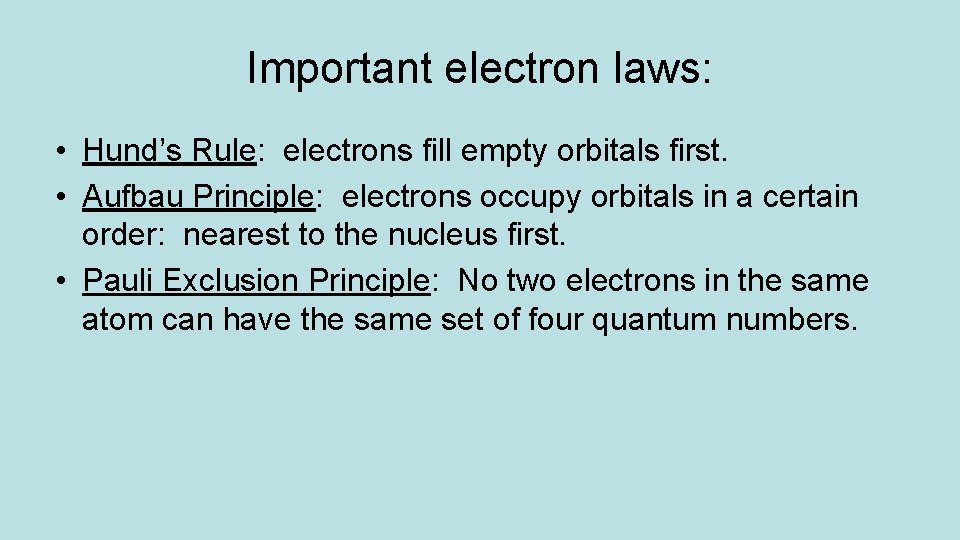
Important electron laws: • Hund’s Rule: electrons fill empty orbitals first. • Aufbau Principle: electrons occupy orbitals in a certain order: nearest to the nucleus first. • Pauli Exclusion Principle: No two electrons in the same atom can have the same set of four quantum numbers.

4 Quantum Numbers: • 1 Principal Quantum Number (n), gives the energy level (aka shell) of the electron. • 2 Orbital Quantum Number (l) = gives the shape of orbital of the electron. • 3 Magnetic Quantum Number(ml) = gives the orientation(direction) of the orbital. • 4 Spin Quantum Number(ms) = gives the direction of spin of the electron.

Allowable ranges for quantum numbers • • n (energy level) = 1 infinity l (type of orbital = 0 n-1 ml (direction) = -l +l ms (spin) = -1/2 or +1/2

Determine whether each set of quantum numbers is valid or invalid: • • • 1) 3, 2, -1, +1/2 2) 1, 2, 0, -1/2 3) 4, 3, -3, +1/4 4) 88, 67, -55, -1/2 5) -2, 1, -1, +1/2 6) 1, 0, 1, -1/2 7) 2, 0, 0, +1/2 8) 4, 2, -3, +1/2 9) 3, 1, 2, -1/2 10) 4, 2, -2, +1/2 • • • Valid Invalid Valid Invalid Valid

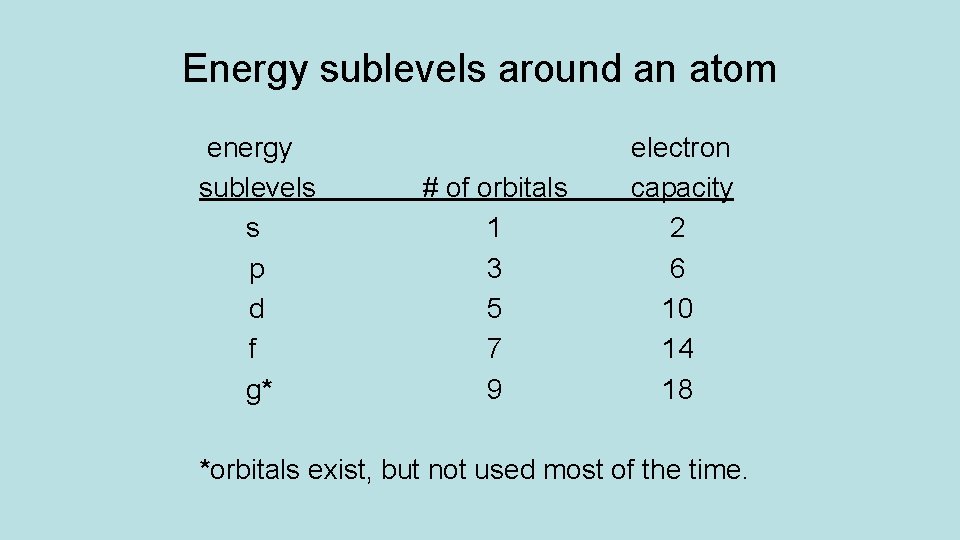
Energy sublevels around an atom energy sublevels s p d f g* # of orbitals 1 3 5 7 9 electron capacity 2 6 10 14 18 *orbitals exist, but not used most of the time.

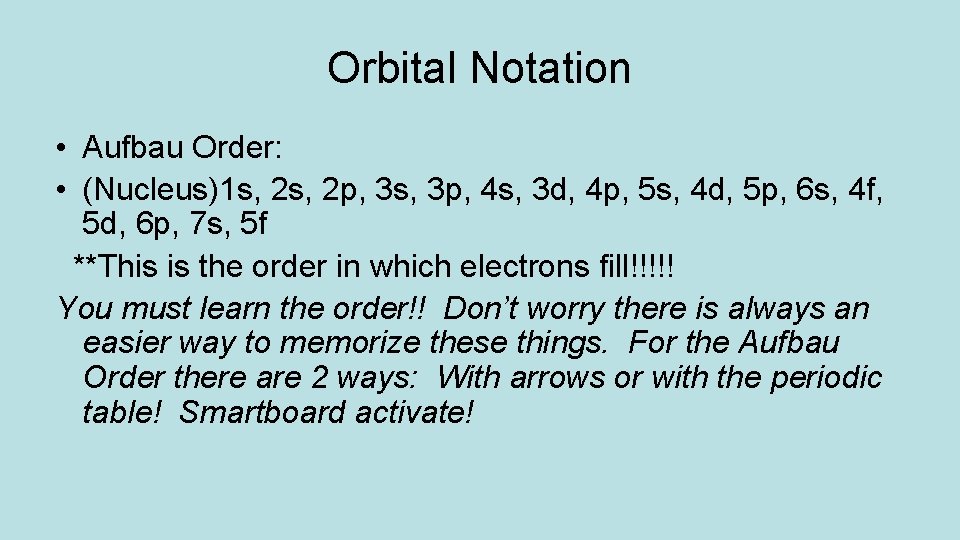
Orbital Notation • Aufbau Order: • (Nucleus)1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f **This is the order in which electrons fill!!!!! You must learn the order!! Don’t worry there is always an easier way to memorize these things. For the Aufbau Order there are 2 ways: With arrows or with the periodic table! Smartboard activate!

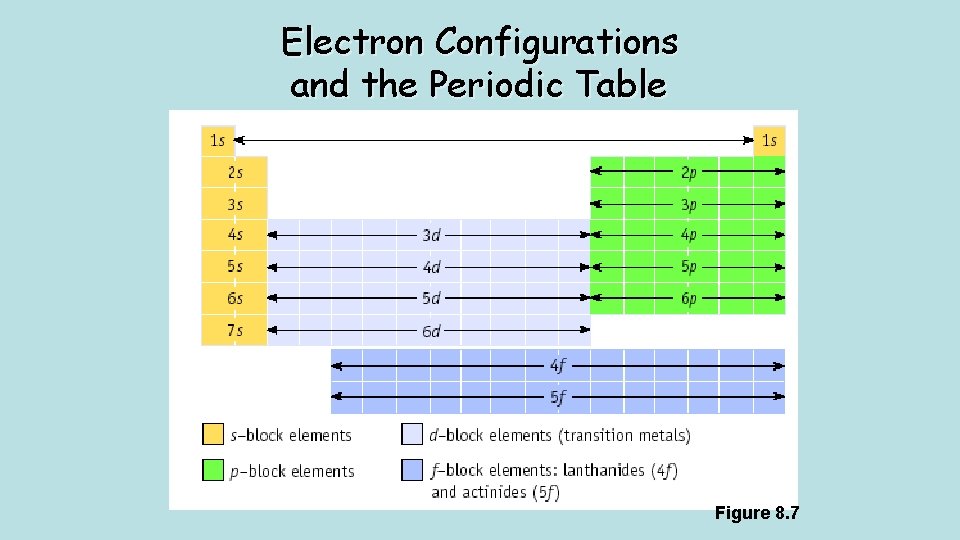
Electron Configurations and the Periodic Table Figure 8. 7

Electron Spin Quantum Number Diamagnetic: NOT attracted to a magnetic field all electrons are spin paired. Paramagnetic: substance is attracted to a magnetic field. Substance has unpaired electrons.

Orbital Notation • Write the orbital notation for each atom: Nitrogen, N(7 electrons) Sodium, Na(11 e-) Iron, Fe(__ e-) Antimony, Sb(__ e-) Gold, Au(__ e-)

• Write the orbital notation for each atom: • Silver, Ag(__ e-) • Silver cations • Aluminum cations • Copper (I) & Copper (II) cations • Zinc atoms & Zinc ions • Iron(II) & Iron(III) ions

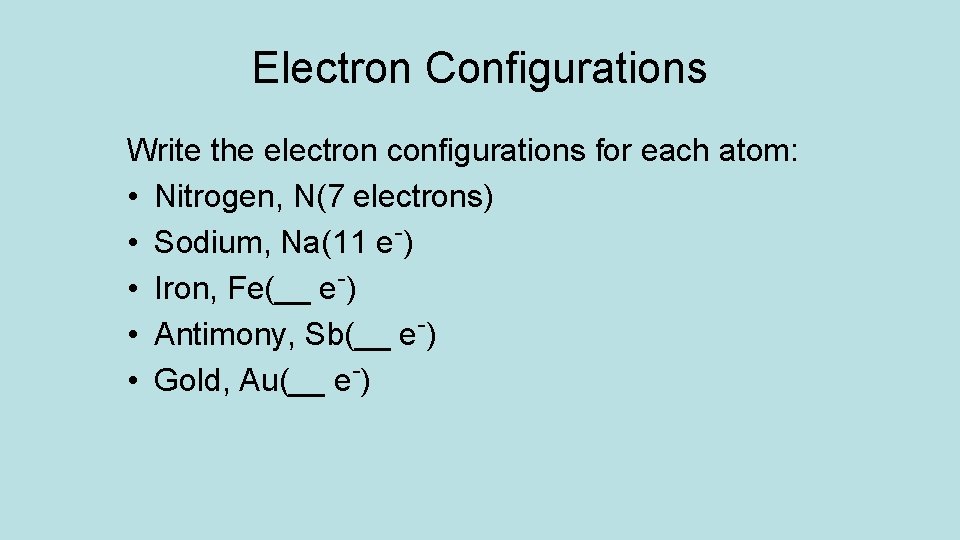
Electron Configurations Write the electron configurations for each atom: • Nitrogen, N(7 electrons) • Sodium, Na(11 e-) • Iron, Fe(__ e-) • Antimony, Sb(__ e-) • Gold, Au(__ e-)

Quantum Numbers • Write the four quantum numbers for the last electron to fill each atom: • Nitrogen, N(7 electrons) • Sodium, Na(11 e-) • Iron, Fe(__ e-) • Antimony, Sb(__ e-) • Gold, Au(__ e-)

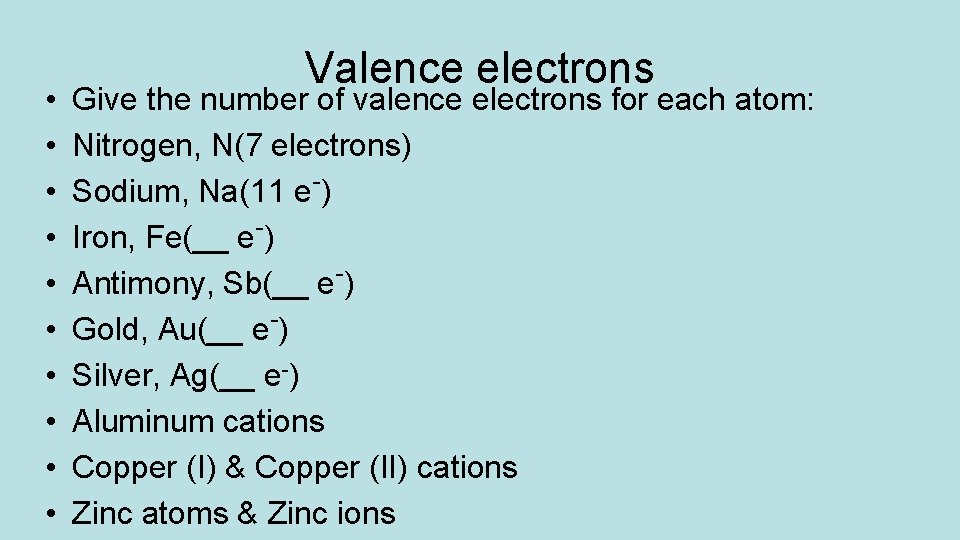
• • • Valence electrons Give the number of valence electrons for each atom: Nitrogen, N(7 electrons) Sodium, Na(11 e-) Iron, Fe(__ e-) Antimony, Sb(__ e-) Gold, Au(__ e-) Silver, Ag(__ e-) Aluminum cations Copper (I) & Copper (II) cations Zinc atoms & Zinc ions

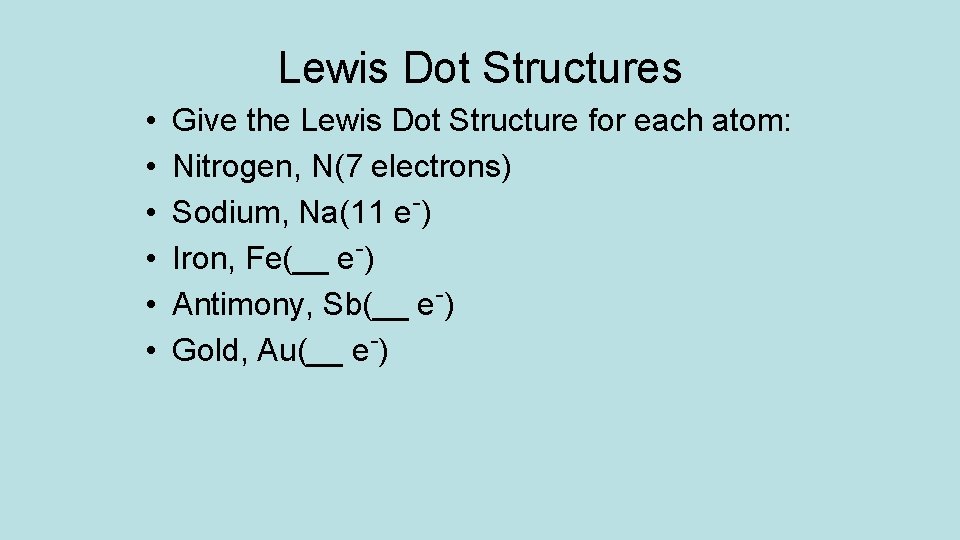
Lewis Dot Structures • • • Give the Lewis Dot Structure for each atom: Nitrogen, N(7 electrons) Sodium, Na(11 e-) Iron, Fe(__ e-) Antimony, Sb(__ e-) Gold, Au(__ e-)
 Ap chemistry chapter 7
Ap chemistry chapter 7 Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Chapter 8
Chapter 8 Orbital diagram for k
Orbital diagram for k Ib chemistry atomic structure
Ib chemistry atomic structure First ionization energy definition ib
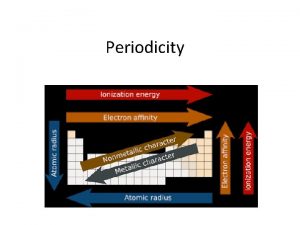
First ionization energy definition ib What is periodicity?
What is periodicity? Texas health steps check up forms
Texas health steps check up forms Feeding periodicity adalah
Feeding periodicity adalah Chemsheets periodicity
Chemsheets periodicity Aap bright futures periodicity schedule
Aap bright futures periodicity schedule Periodic table trends
Periodic table trends Wuchereria bancrofti periodicity
Wuchereria bancrofti periodicity Relative formula mass of hcl
Relative formula mass of hcl O
O Difference between atomic mass and atomic number
Difference between atomic mass and atomic number What are the trends in periodic table
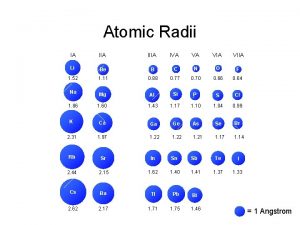
What are the trends in periodic table Fr atomic radius
Fr atomic radius Atomic number vs atomic radius
Atomic number vs atomic radius Departamentos de presidencia municipal
Departamentos de presidencia municipal Constelacion guerrero
Constelacion guerrero Mario guerrero corzo
Mario guerrero corzo Jessica guerrero jarama
Jessica guerrero jarama Claudia patricia guerrero chaparro
Claudia patricia guerrero chaparro Jesus alberto guerrero rojas
Jesus alberto guerrero rojas Tst rlr
Tst rlr Curso exel
Curso exel Jose elias ganem guerrero
Jose elias ganem guerrero Quien no haya sido soldado de infanteria
Quien no haya sido soldado de infanteria He is the author of icnofalangometrica
He is the author of icnofalangometrica Jessica renee johnson photo
Jessica renee johnson photo Jose guerrero torre
Jose guerrero torre Cuentan que un dios hace maravillas
Cuentan que un dios hace maravillas Tlamacazapa guerrero
Tlamacazapa guerrero Liz guerrero switch
Liz guerrero switch Guerrero nato
Guerrero nato Eduardo guerrero villegas
Eduardo guerrero villegas Luis felipe vázquez guerrero
Luis felipe vázquez guerrero Un arbol y sus partes
Un arbol y sus partes Mayra guerrero
Mayra guerrero