Atomic Structure and Periodicity Electromagnetic Radiation and Nature




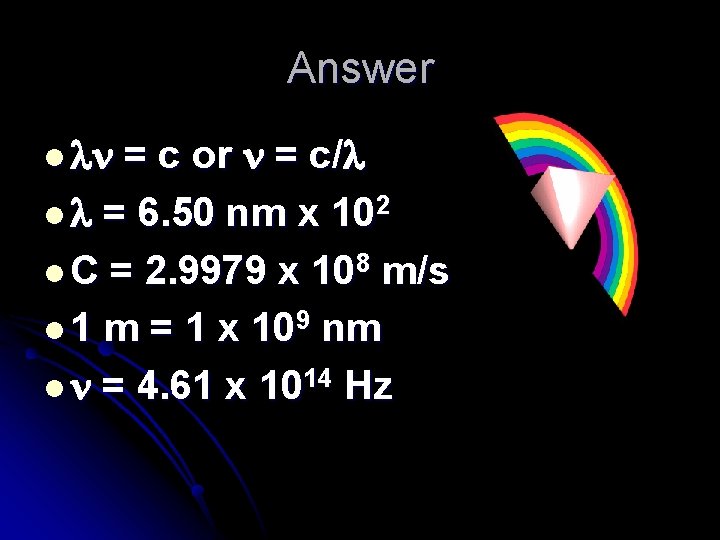






- Slides: 15

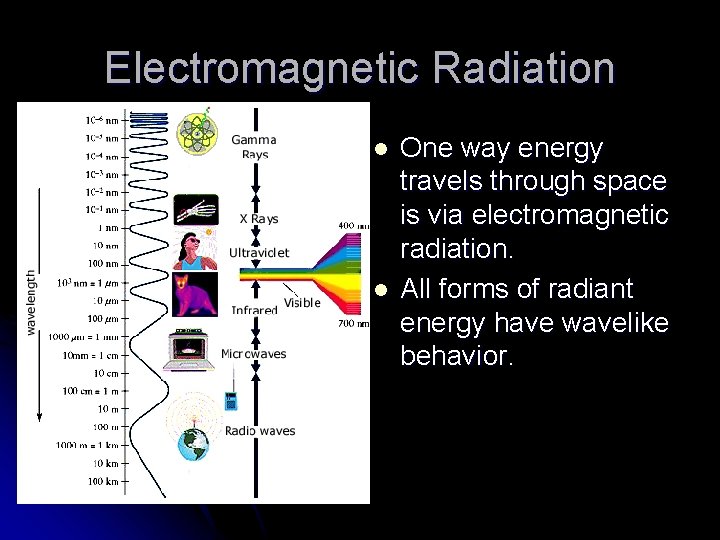
Atomic Structure and Periodicity Electromagnetic Radiation and Nature of Matter

Quantum Theory l l From Democritus to Einstein has come the modern atomic theory. What is the nature of atoms? Remember Dalton’s theory?

Electromagnetic Radiation l l One way energy travels through space is via electromagnetic radiation. All forms of radiant energy have wavelike behavior.

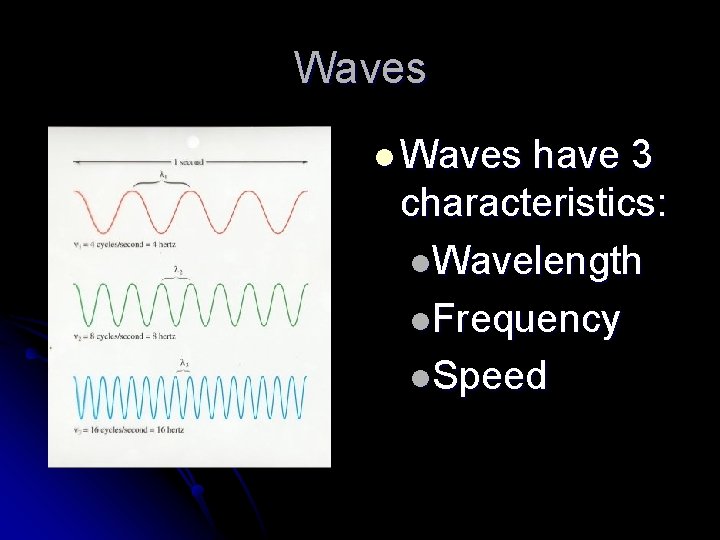
Waves l Waves have 3 characteristics: l. Wavelength l. Frequency l. Speed

Wave characteristics l is the symbol for wavelength, the distance between two consecutive peaks. l is the frequency and is number of waves that pass through a given point in space in 1 second. l The speed of all electromagnetic radiation is the speed of light or c.

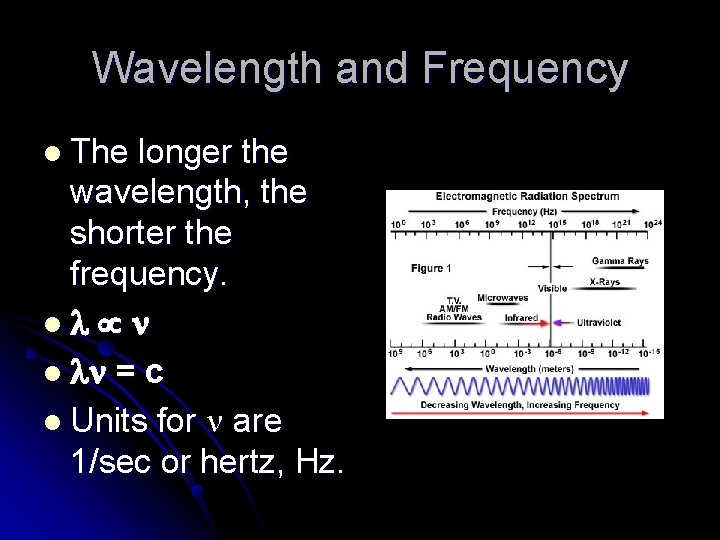
Wavelength and Frequency l The longer the wavelength, the shorter the frequency. l l = c l Units for are 1/sec or hertz, Hz.

Waves have energy! l Microwaves passing through water transfer their energy to the water and eventually heating the water to boiling. l Fire transfers heat by infrared radiation. l Which is higher energy? Red or blue light?

Flame tests! l Example: l Sr(NO 3)2 gives off a red flame of 650 nm. l What is its frequency?
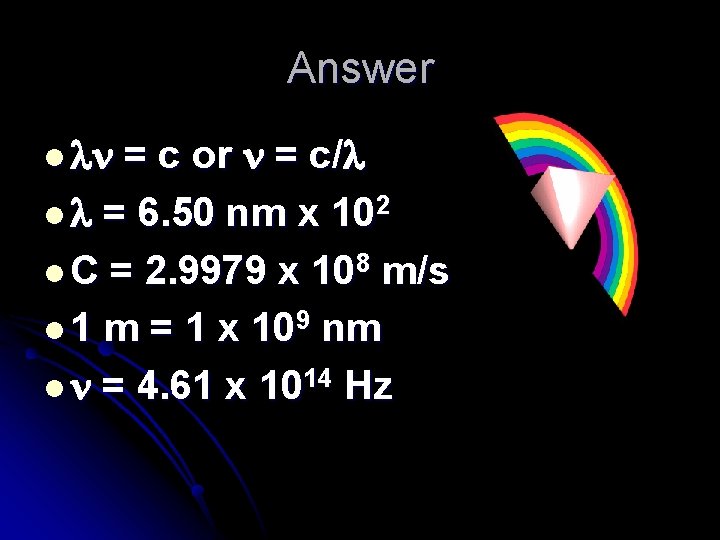
Answer l = c or = c/ l = 6. 50 nm x 102 l C = 2. 9979 x 108 m/s l 1 m = 1 x 109 nm l = 4. 61 x 1014 Hz

Nature of Matter 19 th century thought: Matter and energy are distinct. l Matter was made of particles. l Light was an energy that moved in waves. l With that the study of physics was complete, there would be no new information, or was there? l

Max Planck l Studied radiation of soft bodies heated to incandescence. (light) l Originally thought matter could absorb or emit any quantity of energy. l Max found that the energy could only be gained or lost in whole number multipliers of the equation h

Planck’s Constant lh is Planck’s constant and was found to equal 6. 626 x 10 -34 J s. l The change in energy for a system may be found by ΔE=nh l This equation states that energy is given off in packets or quantized. l Each packet of energy is a quantum.

Example l The blue in fireworks is often from Copper (I) chloride heated to 1200°C. Blue light has a wavelength of 450 nm. What is the quantum of energy that may be emitted by light at 450 nm?

Answer: l ΔE=nh l n=1 = c/ 8 l =2. 9979 x 10 m/s 4. 5 x 10 -7 m =6. 6 x 10 14 s-1

Answer continued: l ΔE=nh x 10 -34 J s) l = (1)(6. 626 x 10 -34 J s)(6. 6 x 1014 s-1) l ΔE=4. 417 x 10 -19 J l ΔE=(1)(6. 626