ADVANCED PLACEMENT CHEMISTRY ATOMIC STRUCTURE AND PERIODICITY Electromagnetic







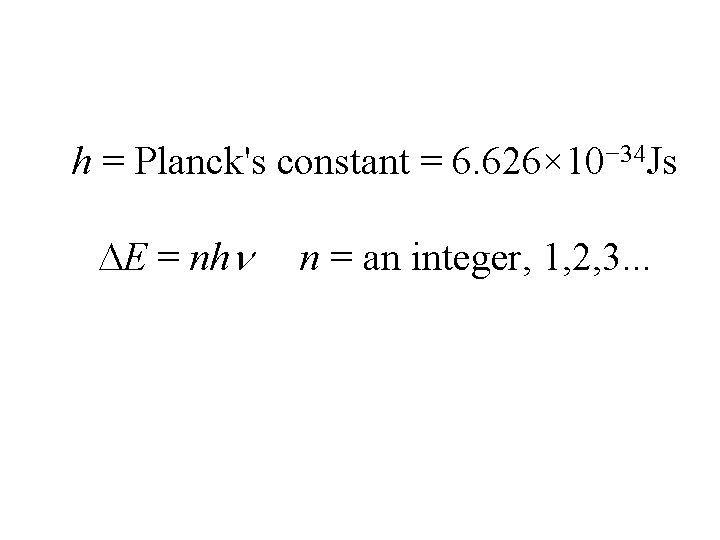
































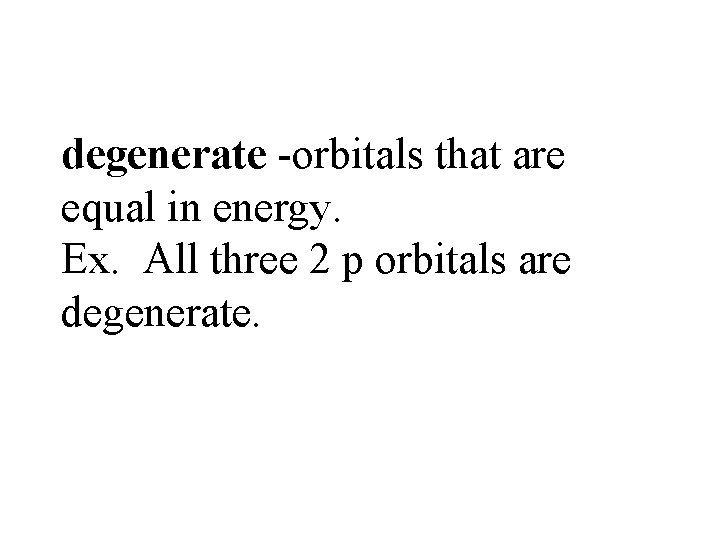





































- Slides: 105

ADVANCED PLACEMENT CHEMISTRY ATOMIC STRUCTURE AND PERIODICITY

Electromagnetic radiationradiant energy that exhibits wavelike behavior and travels through space at the speed of light in a vacuum.


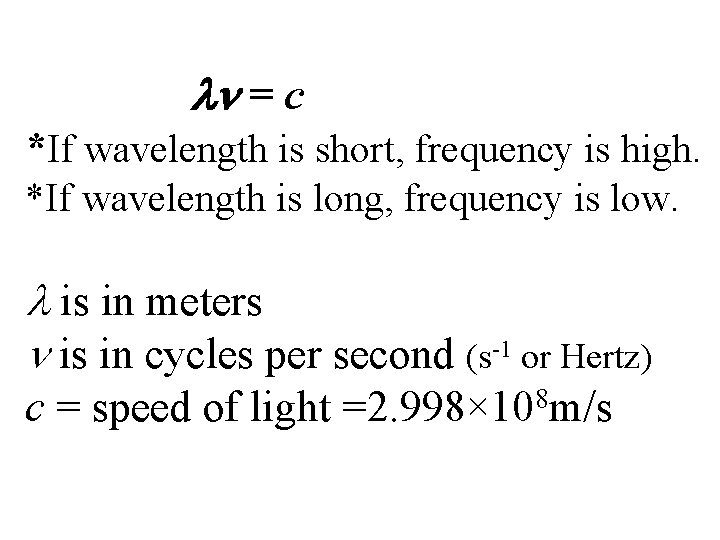
Wavelength ( ) -distance between two consecutive peaks or troughs in a wave. Frequency ( ) -number of waves per second that pass a given point in space


= c *If wavelength is short, frequency is high. *If wavelength is long, frequency is low. is in meters is in cycles per second (s-1 or Hertz) c = speed of light 8 =2. 998× 10 m/s

Ex. The yellow light given off by a sodium lamp has a wavelength of 589 nm. What is the frequency of this radiation? 589 nm 1 m = 5. 89× 10− 7 m 109 nm = c/ = 2. 998× 108 m/s = 5. 09× 1014 s− 1 5. 89× 10− 7 m

Until the early 1900's, it was believed that matter and energy were very different. Matter was composed of particles and energy was composed of waves.

In 1901, Max Planck found that when solids were heated strongly, they absorbed and emitted energy. He determined that energy can be gained or lost only in integer multiples of h.
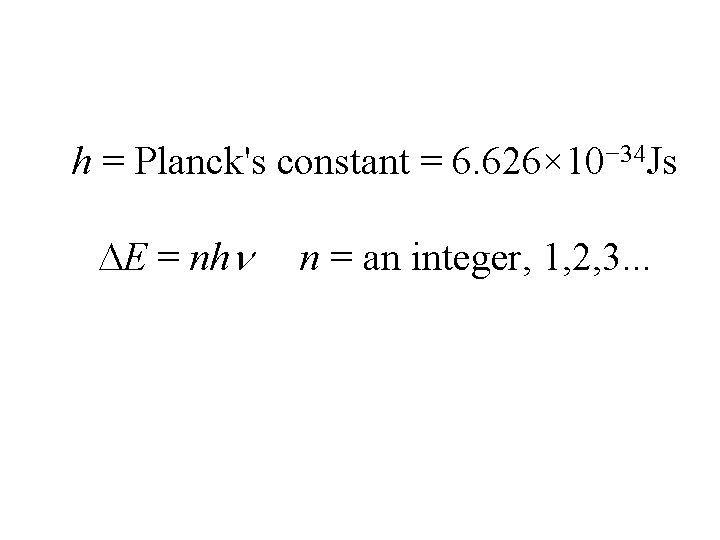
h = Planck's constant = 6. 626× 10− 34 Js E = nh n = an integer, 1, 2, 3. . .

This shows that energy is quantized. The "packets" of energy are quanta(plural) quantum (singular).

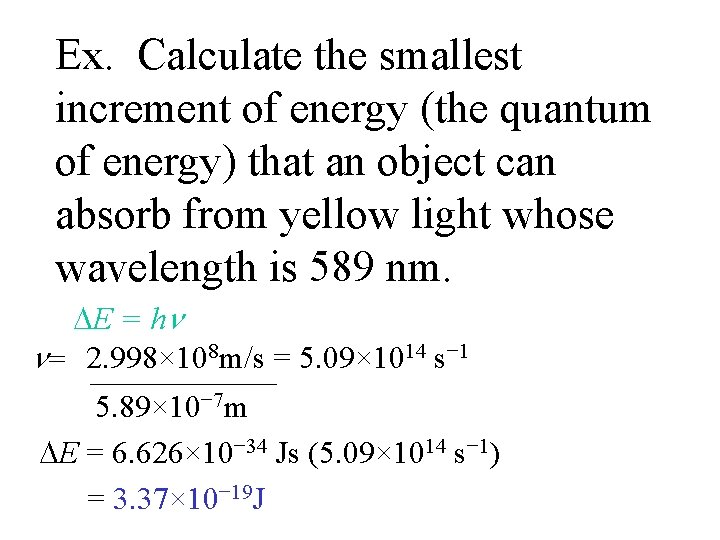
Ex. Calculate the smallest increment of energy (the quantum of energy) that an object can absorb from yellow light whose wavelength is 589 nm. E = h = 2. 998× 108 m/s = 5. 09× 1014 s− 1 5. 89× 10− 7 m E = 6. 626× 10− 34 Js (5. 09× 1014 s− 1) = 3. 37× 10− 19 J

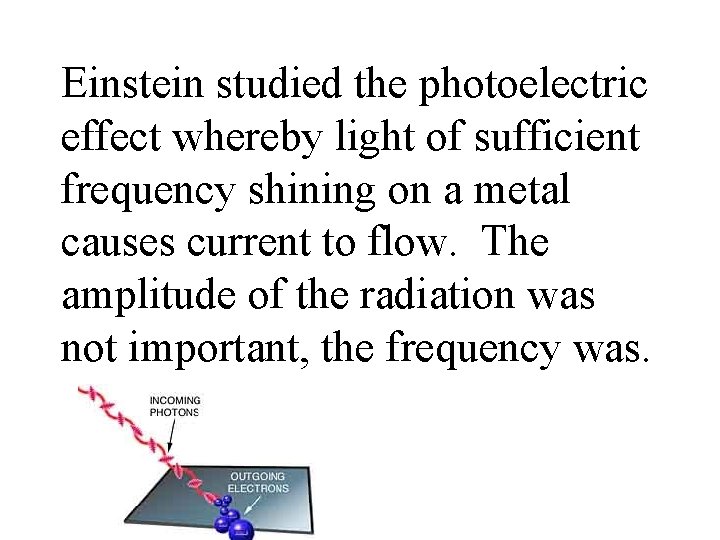
Einstein studied the photoelectric effect whereby light of sufficient frequency shining on a metal causes current to flow. The amplitude of the radiation was not important, the frequency was.

This told him that the light must be in particles, each having a given energy. Einstein proposed that electromagnetic radiation can be viewed as a stream of particles called photons. Energy of a photon: E = hc

Notice that the red light does not have sufficient energy to knock electrons off of potassium. The green and blue light both can achieve the photoelectric effect. Since the blue light has more energy than green light, the electrons emitted using blue light have a higher velocity than those emitted when green light is used.

Einstein's special theory of 2 relativity: E = mc Matter and energy are different forms of the same entity.

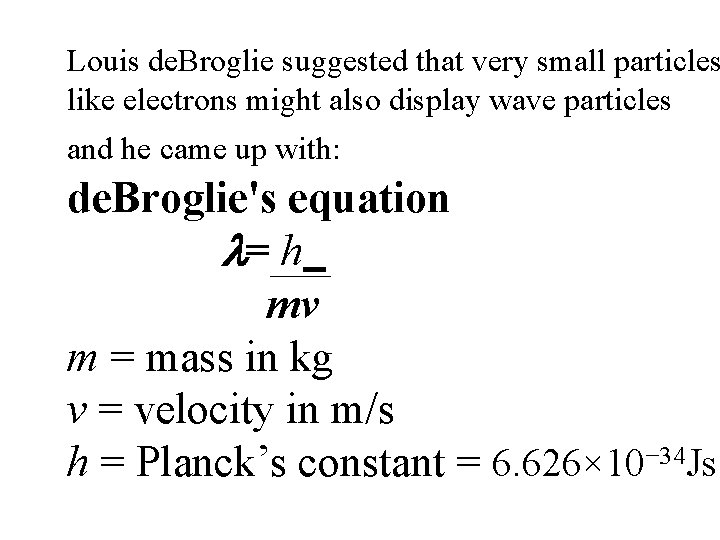
Louis de. Broglie suggested that very small particles like electrons might also display wave particles and he came up with: de. Broglie's equation = h mv m = mass in kg v = velocity in m/s h = Planck’s constant = 6. 626× 10− 34 Js

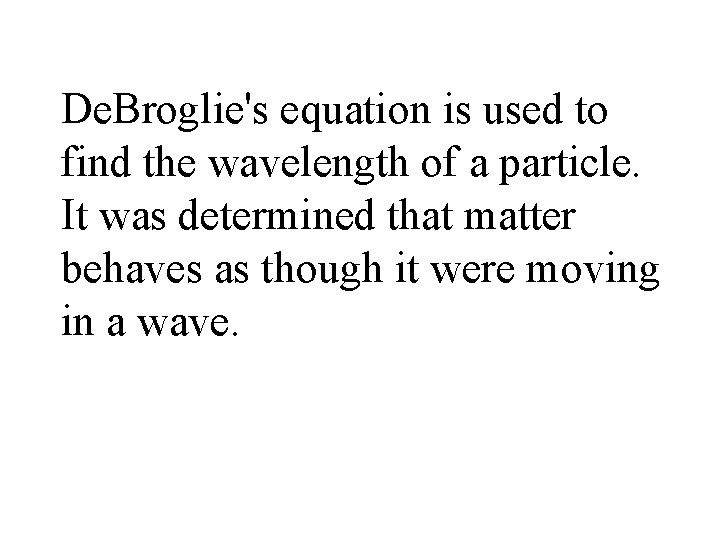
De. Broglie's equation is used to find the wavelength of a particle. It was determined that matter behaves as though it were moving in a wave.

This is important in small objects such as electrons but is negligible in larger objects such as baseballs. Heavy objects have very short wavelengths.

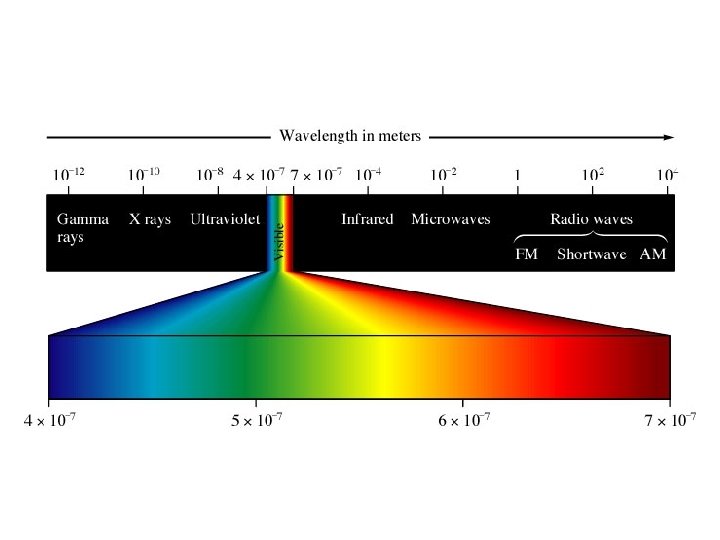
When radiation is separated into its single wavelength components, a spectrum is produced. A prism is used to separate the components. White light produces a continuous spectrum. Ex. rainbow

Some emitters of light radiate only certain colors and wavelengths. This produces a bright line spectrum. When various gases at low pressures are put in tubes and a high voltage is applied, they glow in various colors.

If this light is passed through a prism, a series of lines of color is produced. This series identifies the element.



Niels Bohr found that the absorptions and emissions of light by hydrogen atoms correspond to energy changes of electrons within the atom. Bohr proposed that the electron in hydrogen travels only in certain allowed orbits.

Bohr calculated the energy differences between these orbits and predicted the wavelengths at which lines would be found. His research worked great with the hydrogen atom but did not work correctly for polyelectronic atoms.

ground state- lowest energy level of an electron


wave mechanical model. Schrodinger and de Broglie worked on a wave mechanical model of the atom. The electron is visualized as a standing wave (opposite of a traveling wave- like a vibrating guitar string instead of an ocean wave).

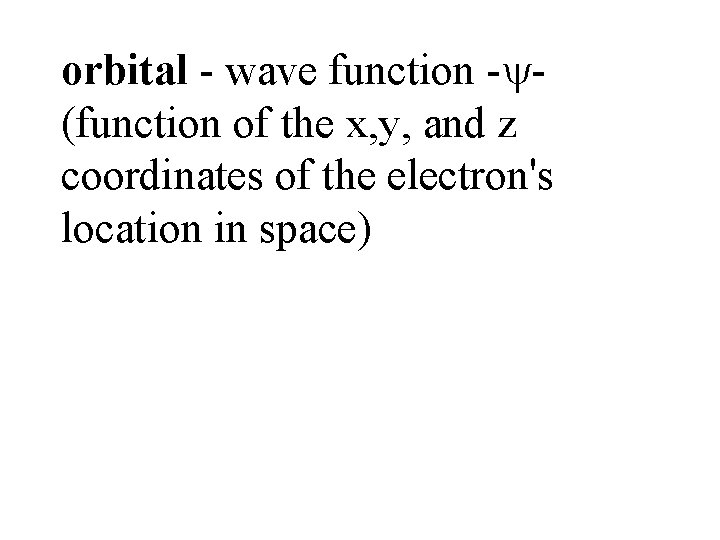
orbital - wave function - (function of the x, y, and z coordinates of the electron's location in space)

Heisenberg's Uncertainty Principle-There is a fundamental limitation to just how precisely we can know both the position and momentum of a particle at a given time. This is negligible with macroscopic objects such as a baseball but very important with an electron.

The accepted definition of the size of the hydrogen 1 s orbital is the radius of the sphere that encloses 90% of the total electron probability.


Electrons are arranged in the atom in a series of levels and sublevels (shells and subshells).

Energy Levels (n)= 1, 2, 3. . . (energy level or shells) as n increases higher energy larger orbital farther from the nucleus can hold increasing numbers of electrons

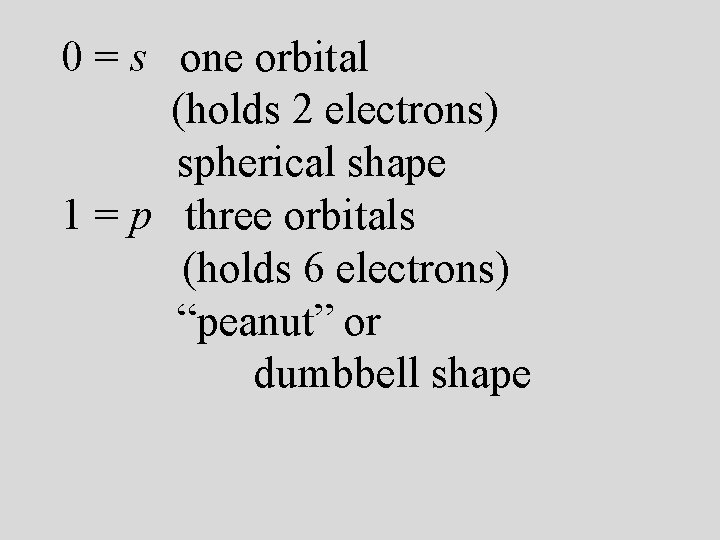
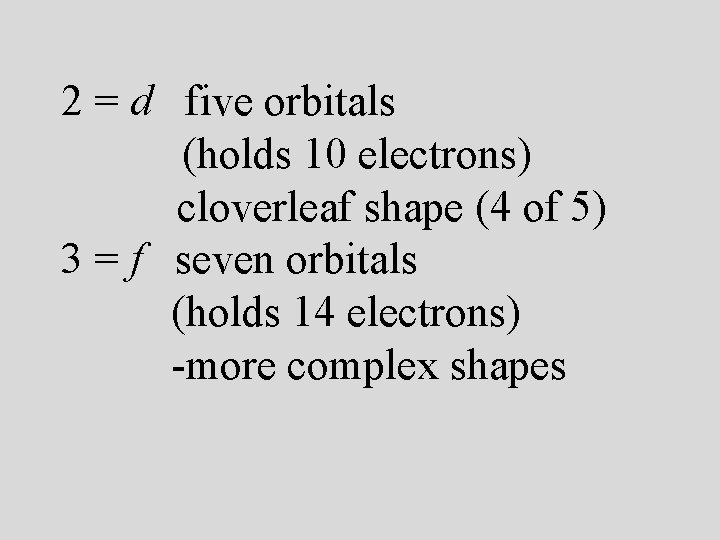
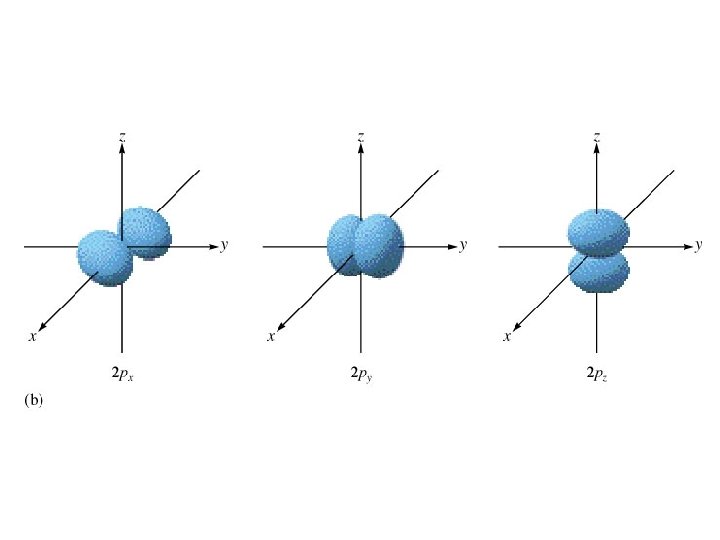
Energy sublevels 4 types of sublevels apply to electrons in their ground state. s = 1 orbital, holds 2 electrons (spherical shape p = 3 orbitals, holds 6 electrons (peanut or dumbbell shape) d = 5 orbitals, holds 10 electrons (daisy shape) f = 7 orbitals, holds 14 electrons (fancy shape)

Quantum numbers - the "address" of an electron Principle quantum number (n)= 1, 2, 3. . . (energy level or shells) as n increases higher energy larger orbital farther from the nucleus

Azimuthal or angular momentum quantum number (l) *values of 0 to n-1 *shape of atomic orbitals *sublevels

0 = s one orbital (holds 2 electrons) spherical shape 1 = p three orbitals (holds 6 electrons) “peanut” or dumbbell shape

2 = d five orbitals (holds 10 electrons) cloverleaf shape (4 of 5) 3 = f seven orbitals (holds 14 electrons) -more complex shapes




Magnetic quantum number (ml) *-l to +l , including 0 *orientation of the orbital in space

Spin quantum number (ms) +1/2, -1/2 *direction of electron spin


Practice determining possible sets of four quantum numbers!

One of the outer most electrons in a strontium atom in the ground state can be described by which of the following sets of four quantum numbers? (A) 5, 2, 0, 1/2 (B) 5, 1, 1, 1/2 (C) 5, 1, 0, 1/2 (D) 5, 0, 1, 1/2 (E) 5, 0, 0, 1/2

Which of the following sets of quantum numbers (n, l, ms) best describes the valence electron of highest energy in a ground-state gallium atom (atomic number 31)? (A) 4, 0, 0, 1/2 (B) 4, 0, 1, 1/2 (C) 4, 1, 1, 1/2 (D) 4, 1, 2, 1/2 (E) 4, 2, 0, 1/2

Orbital shapes and energies Orbitals have regions of high electron probability and regions of zero electron probability. Areas of zero electron probability are called nodes.

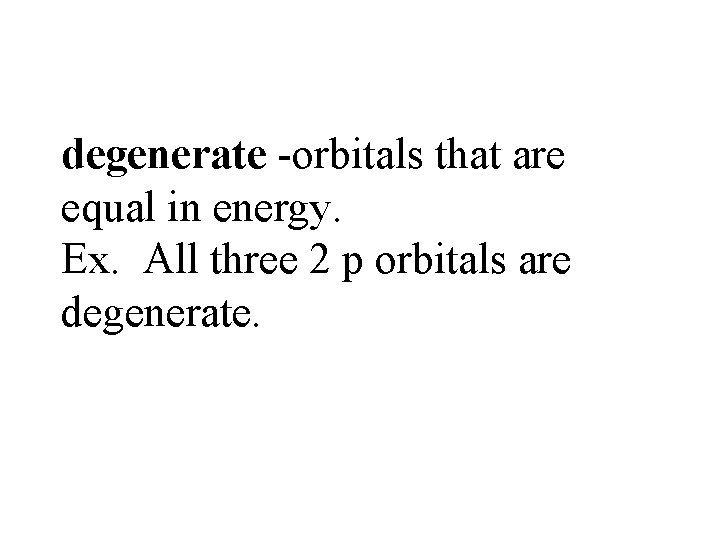
degenerate -orbitals that are equal in energy. Ex. All three 2 p orbitals are degenerate.

Pauli's Exclusion Principle- In a given atom, no two electrons can have the same set of four quantum numbers. An orbital can only hold two electrons, and they must have opposite spins.

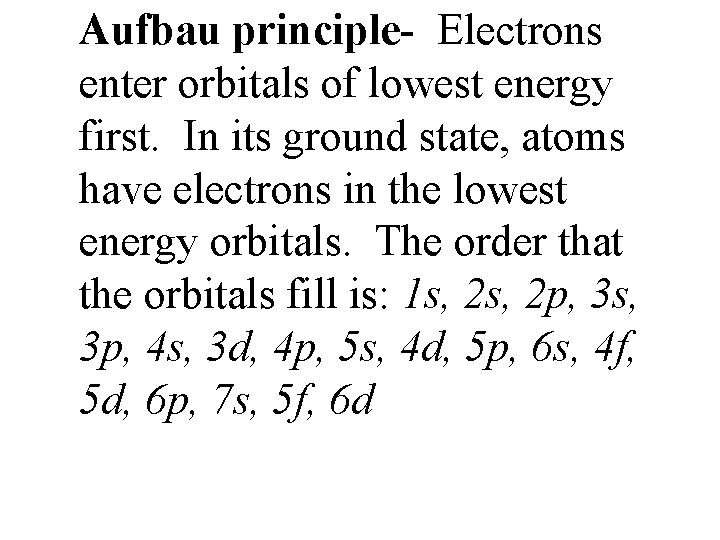
Aufbau principle- Electrons enter orbitals of lowest energy first. In its ground state, atoms have electrons in the lowest energy orbitals. The order that the orbitals fill is: 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f, 6 d

Hund's rule- All orbitals in a sublevel must be half-filled with electrons having parallel spins before any may be completely filled.

Review relationships between location on the periodic table and electron configuration.


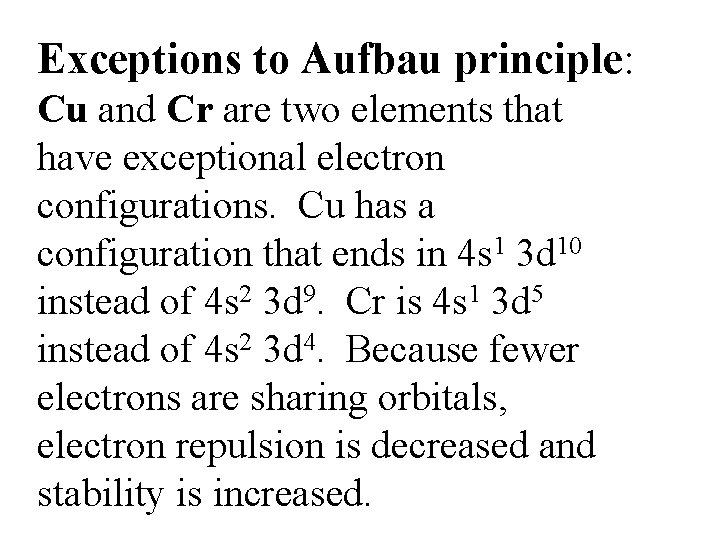
Exceptions to Aufbau principle: Cu and Cr are two elements that have exceptional electron configurations. Cu has a configuration that ends in 4 s 1 3 d 10 instead of 4 s 2 3 d 9. Cr is 4 s 1 3 d 5 instead of 4 s 2 3 d 4. Because fewer electrons are sharing orbitals, electron repulsion is decreased and stability is increased.

These exceptions do not need to be memorized for the AP test, but if given the exceptions, you should be able to explain why.

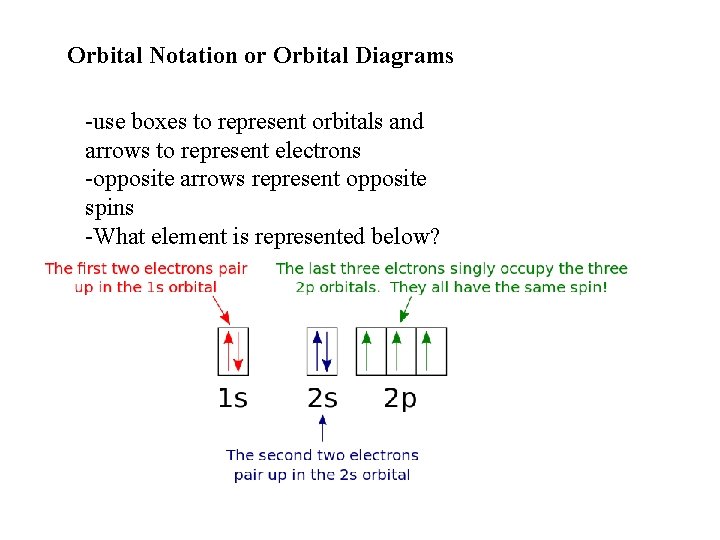
Orbital Notation or Orbital Diagrams -use boxes to represent orbitals and arrows to represent electrons -opposite arrows represent opposite spins -What element is represented below?

2 2 6 2 3 1 s 2 s 2 p 3 s 3 p Atoms of an element, X, have the electronic configuration shown above. The compound most likely formed with magnesium. (A) Mg 2 X (B) Mg. X 2 (C) Mg 2 X 3 (D) Mg 3 X 2

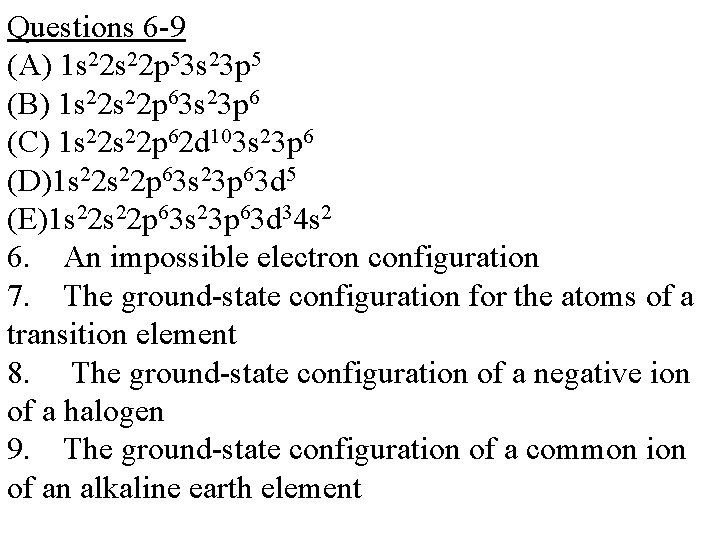
Questions 6 -9 (A) 1 s 22 p 53 s 23 p 5 (B) 1 s 22 p 63 s 23 p 6 (C) 1 s 22 p 62 d 103 s 23 p 6 (D)1 s 22 p 63 s 23 p 63 d 5 (E)1 s 22 p 63 s 23 p 63 d 34 s 2 6. An impossible electron configuration 7. The ground-state configuration for the atoms of a transition element 8. The ground-state configuration of a negative ion of a halogen 9. The ground-state configuration of a common ion of an alkaline earth element

Polyelectronic atoms- atoms with more than one electron (anything beyond hydrogen)

When an atom has more than one electron, these electrons tend to repel each other. The effect of the electron repulsions is to reduce the nuclear charge (pull of the nucleus on the electron). The pull of the nucleus on an electron is sometimes referred to as Zeff (effective nuclear charge).

Shielding- the effect by which the other electrons screen or shield a given electron from some of the nuclear charge.

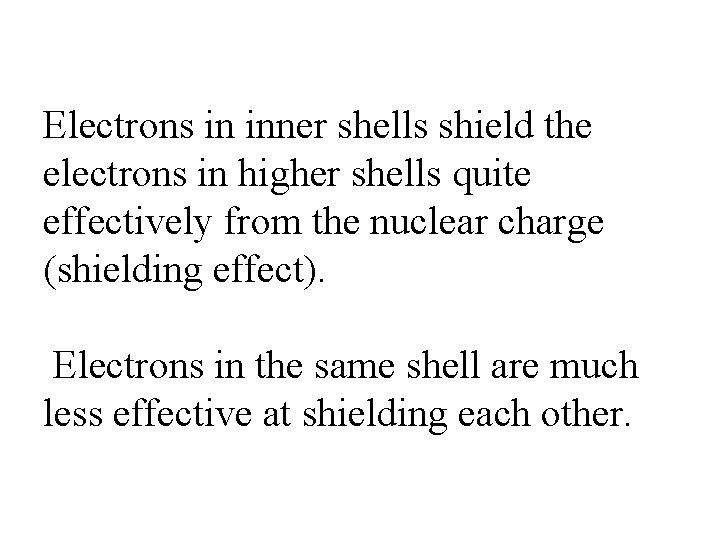
Electrons in inner shells shield the electrons in higher shells quite effectively from the nuclear charge (shielding effect). Electrons in the same shell are much less effective at shielding each other.

Penetration effect- the effect whereby a valence electron penetrates the core electrons, thus reducing the shielding effect and increasing the pull from the nucleus. (The electron is temporarily closer to the nucleus than normal. )

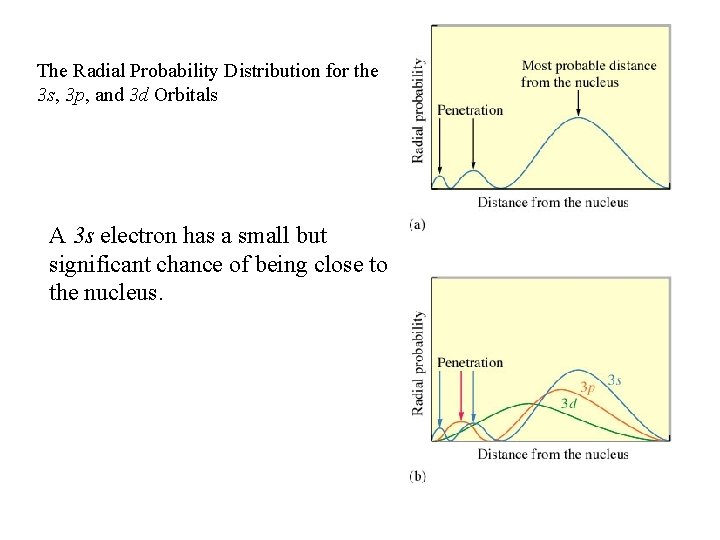
The Radial Probability Distribution for the 3 s, 3 p, and 3 d Orbitals A 3 s electron has a small but significant chance of being close to the nucleus.

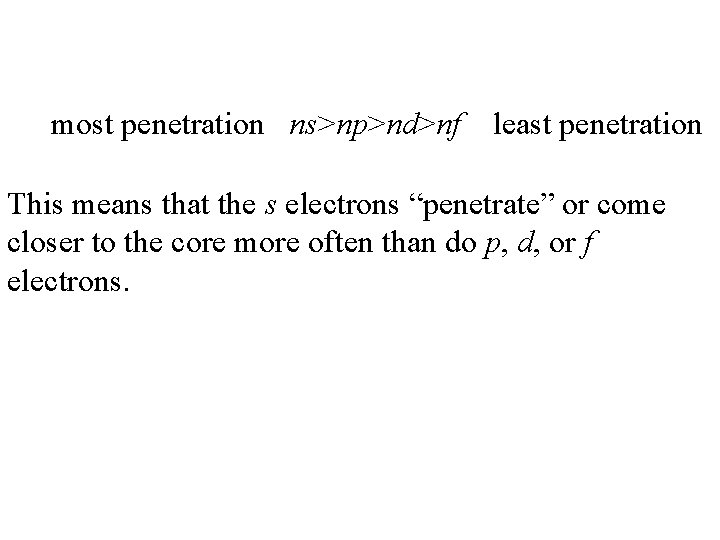
most penetration ns>np>nd>nf least penetration This means that the s electrons “penetrate” or come closer to the core more often than do p, d, or f electrons.

Electrons fill orbitals in order of increasing energy. Because of the penetration effect, electrons fill (n+1)s before nd. ((n+1)s has lower energy).

Electrons sharing an orbital do not shield each other as well as core electrons shield outer electrons.

Valence electrons- electrons in the outermost principle quantum number Core electrons- inner electrons

Ionization energy (IE)- energy required to remove an electron from a gaseous atom or ion. + − X(g) X (g) + e

- a measure of how much work it is to remove an electron or how tightly the electron is held by the nucleus. -units are usually k. J/mol

-IE depends mainly on two factors: 1. the nuclear charge 2. the average distance of the electron from the nucleus

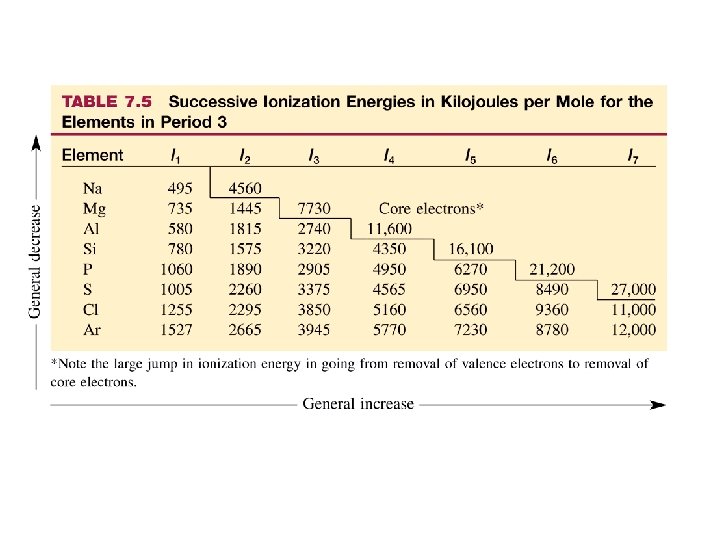
The lowest energy valence electrons are the first to be removed. Successive electrons take much more energy to remove because the atom now has a positive charge (more protons than electrons). IE takes a large jump going from a valence electron to a core electron. The 2 nd IE of sodium is much higher than the 1 st. The 3 rd IE of magnesium is much higher than that of the first and second electron. Core electrons are much more tightly held than valence electrons.


The first IE generally increases going from left to right across a period because of the increasing nuclear charge. IE decreases going down a group because electrons being removed are farther from the nucleus and are well shielded from the nuclear charge.

Exceptions to these general trends occur at group 3 (shielding of p electron by s electrons) and group 6 (paired electron repulsion).

Atoms with a low IE 1 tend to form cations during reactions, whereas those with a high IE 1(except noble gases) tend to form anions.



AP Test Hint #1 When asked to explain a periodic trend on the AP test, simply stating that the trend increases or decreasing across a period from left to right or up/down a group will result in no credit. The reasons for the change must be clearly stated!

AP Test Hint #2 If two elements/ions are given and you are asked to compare a property of theirs, you must discuss both elements/ions to get full credit.

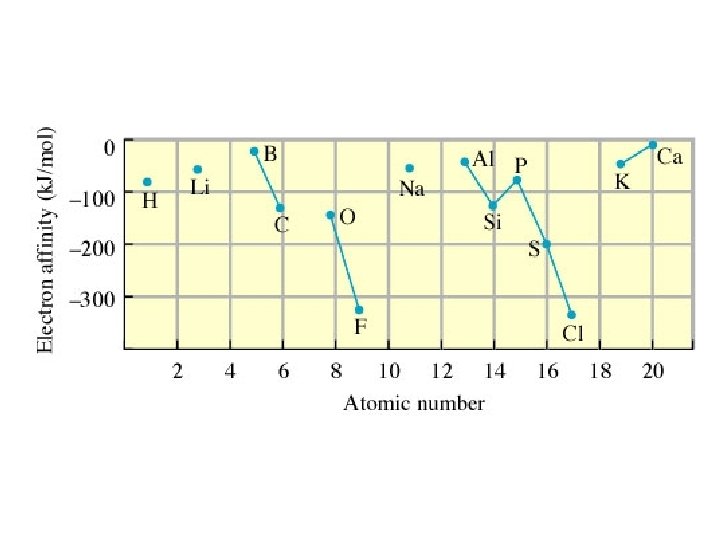
Electron Affinity (EA) - the energy associated with the addition of an electron to a gaseous atom X(g) + e− X−(g) -If the addition is exothermic, EA is negative. The more negative, the greater the amount of energy released.

In going down a group, EA generally becomes more positive (less energy released) because the electron is farther from the nucleus. There are many exceptions. EA generally becomes more negative going across a period (again, many exceptions)


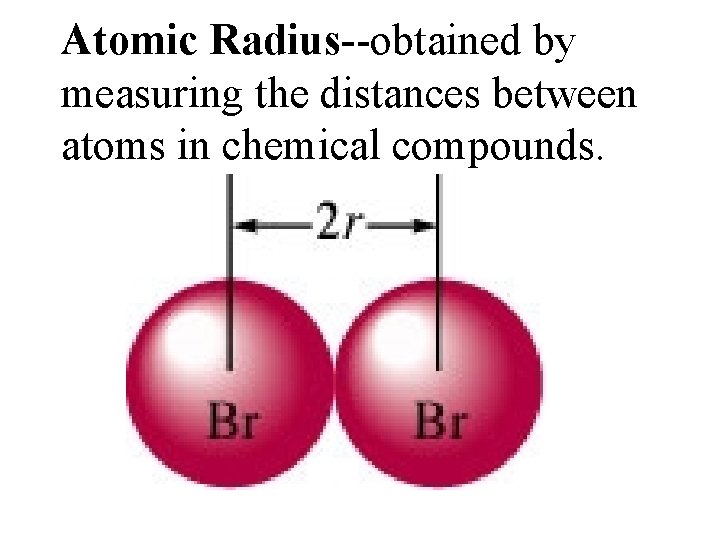
Atomic Radius--obtained by measuring the distances between atoms in chemical compounds.

-decreases going from left to right across a period because the nuclear charge increases from left to right. -increases going down a group because of larger orbitals. The nuclear charge stays about the same because of shielding.


Metals have low IE. Nonmetals have high IE and very negative EA. Density generally increases going down the periodic table because the atomic number increases faster than the atomic size.

Transition Metal Size and Lanthanide Contractions The variation in size as we go across a row of transition metals is much less than among the representative elements. This is because electrons are being added to an inner shell as the nuclear charge gets larger.

The inner shell electrons are almost completely effective at shielding the outer shell from the nuclear charge, so the outer electrons experience only a small, gradual increase in the nuclear charge. Therefore, only small size decreases occur.

A similar phenomenon occurs among the inner transition metals (Ex. lanthanides) The lanthanides fall between La and 1 Hf, La last fills the 5 d electron, Ce ends in 5 d 1 4 f 1. In this 6 th period we have a much larger decrease in size occurring between La and Hf because of the intervening lanthanide elements.

This additional decrease in size is known as the lanthanide contraction. It causes Hf to be the same size as Zr, even though Hf is below Zr.

All of the rest of the transition elements in the 6 th period are nearly the same size as the elements above them in the fifth period. This causes the 6 th period transition metals to be extremely dense. This even influences lead and bismuth beyond the transition metals.


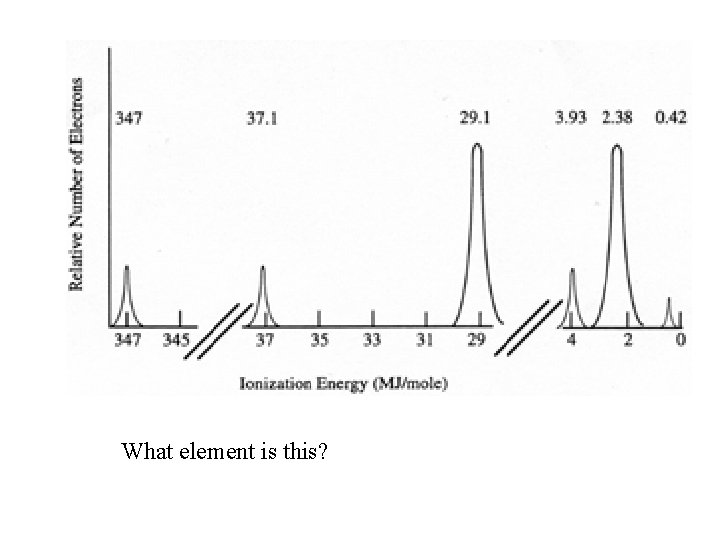
Photoelectron Spectroscopy -uses the energy from electrons emitted via the photoelectric effect to gain information about the electronic structure of a substance. Very high energy photons of light of a given wavelength shine on a gas phase sample of the element. If the photons are higher in energy than the ionization of the electrons, one electron per atom is ejected. Since there a limited number of electrons (and energy levels) in an atom, there a limited number of energies of electrons emitted. These energies can be measured. When the kinetic energy of the emitted electron is subtracted from the energy of the light photons, the resulting value is the ionization energy of that particular electron.

The photons used for photoelectron spectroscopy range from ultraviolet light to X-rays. Analysis of photoelectron spectra for a single element can give us information about the shells and subshells of electrons and the number of electrons in each.

The x-axis has units of ionization energy of the electron. Electrons closer to the nucleus have greater Coulombic attraction and thus have a higher ionization energy. Some scales show the energies going from highest to lowest and some go from lowest to highest, so attention needs to be paid to these numbers. Don’t worry about the units, because they may vary. The height of the peak indicates the number of electrons in the atom having that energy (the number of electrons in a sublevel).

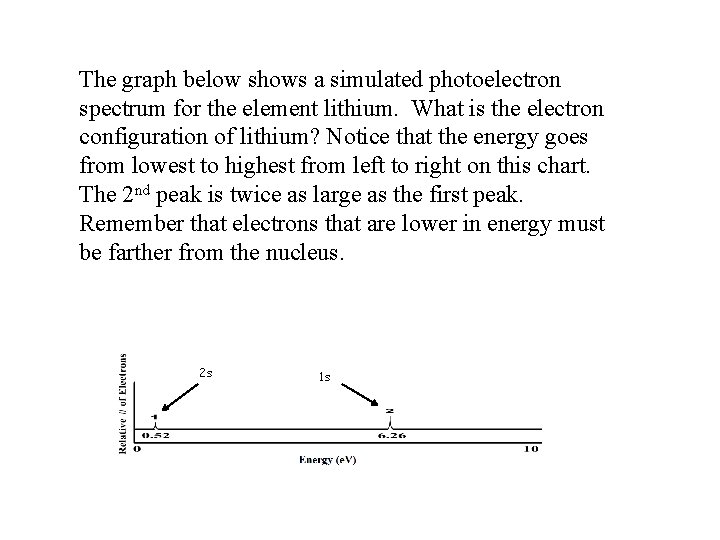
The graph below shows a simulated photoelectron spectrum for the element lithium. What is the electron configuration of lithium? Notice that the energy goes from lowest to highest from left to right on this chart. The 2 nd peak is twice as large as the first peak. Remember that electrons that are lower in energy must be farther from the nucleus. 2 s 1 s

This website allows us to investigate the spectra of several different elements. PES examples

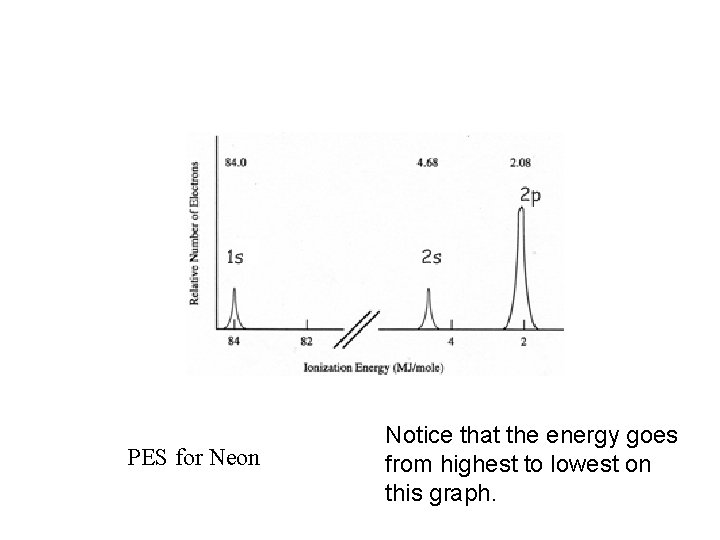
PES for Neon Notice that the energy goes from highest to lowest on this graph.

What element is this?