Atomic Physics Lecture 24 From Wikipedia the free











- Slides: 34

Atomic Physics Lecture 24 From Wikipedia, the free encyclopedia Titus Lucretius Carus ~94 BC to ~49 BC Chapter 29 All nature, then, as self-sustained, consists Of twain of things: of bodies and of void In which they're set, and where they're moved around. …. This ultimate stock we have devised to name Procreant atoms, matter, seeds of things, Or primal bodies, as primal to the world. …. For just as all things of creation are, In their whole nature, each to each unlike, So must their atoms be in shape unlike. Not since few only are fashioned of like form, But since they all, as general rule, are not The same as all. Project Gutenberg Etext of Of The Nature of Things by Lucretius, Translated by William Ellery Leonard

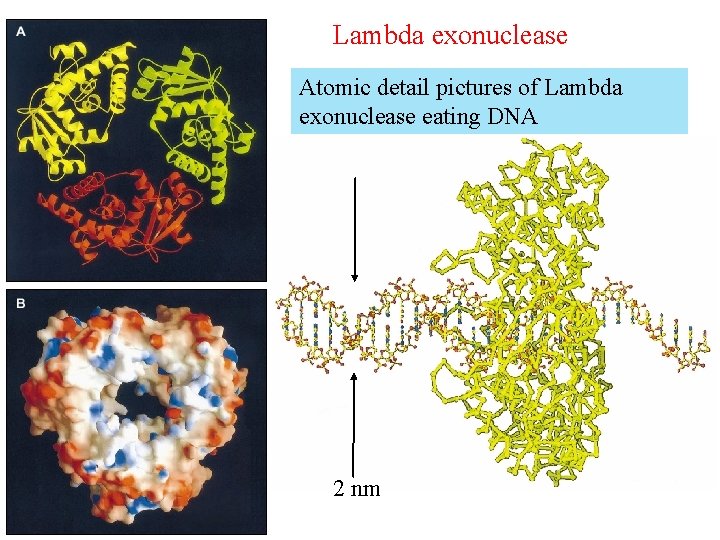
Lambda exonuclease Atomic detail pictures of Lambda exonuclease eating DNA 2 nm

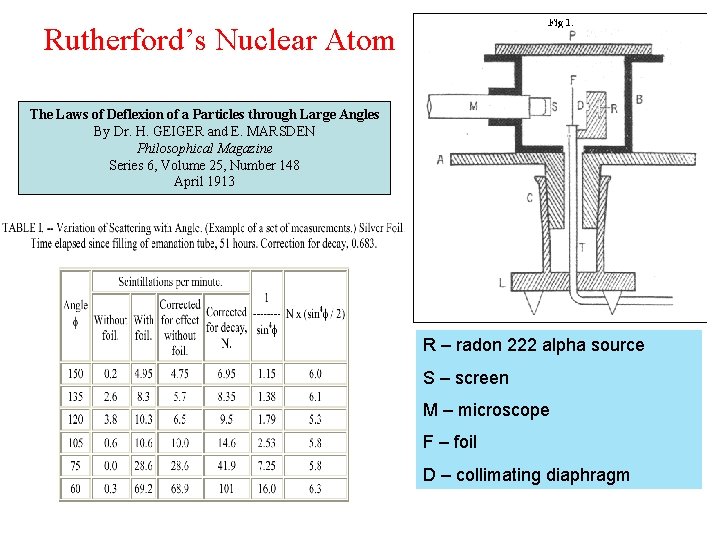
Rutherford’s Nuclear Atom The Laws of Deflexion of a Particles through Large Angles By Dr. H. GEIGER and E. MARSDEN Philosophical Magazine Series 6, Volume 25, Number 148 April 1913 R – radon 222 alpha source S – screen M – microscope F – foil D – collimating diaphragm

An electron is a 1. Wave of probability 2. Particle density 3. Probability amplitude wave 4. Set of quantum numbers 5. Swarm of little point charges Q 1

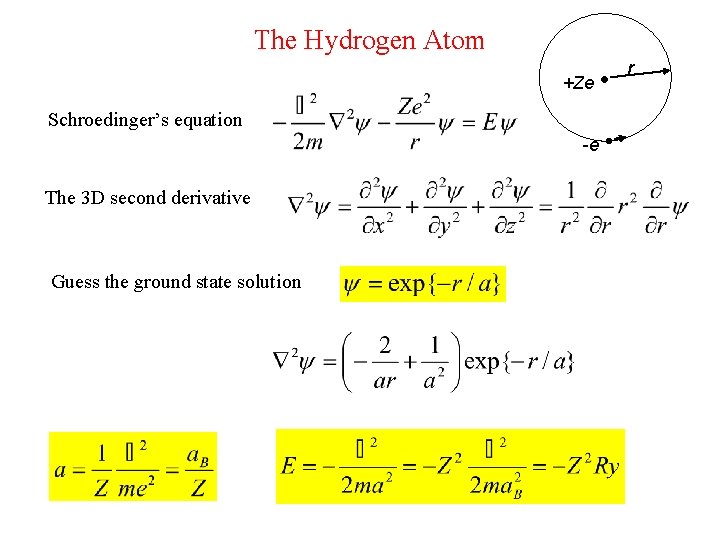
The Hydrogen Atom +Ze -e Schroedinger’s equation The 3 D second derivative Guess the ground state solution r

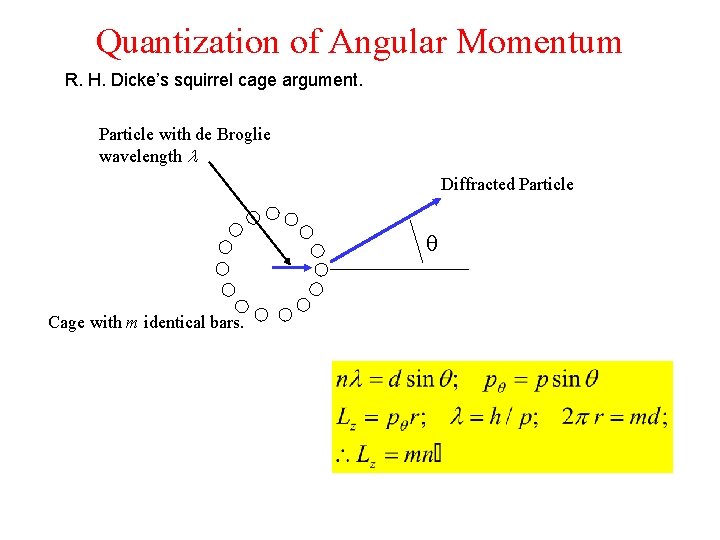
Quantization of Angular Momentum R. H. Dicke’s squirrel cage argument. Particle with de Broglie wavelength l Diffracted Particle q Cage with m identical bars.

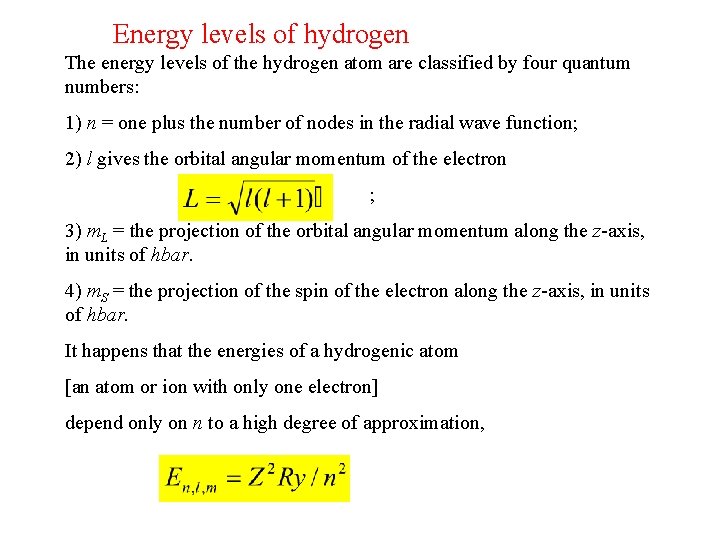
Energy levels of hydrogen The energy levels of the hydrogen atom are classified by four quantum numbers: 1) n = one plus the number of nodes in the radial wave function; 2) l gives the orbital angular momentum of the electron ; 3) m. L = the projection of the orbital angular momentum along the z-axis, in units of hbar. 4) m. S = the projection of the spin of the electron along the z-axis, in units of hbar. It happens that the energies of a hydrogenic atom [an atom or ion with only one electron] depend only on n to a high degree of approximation,

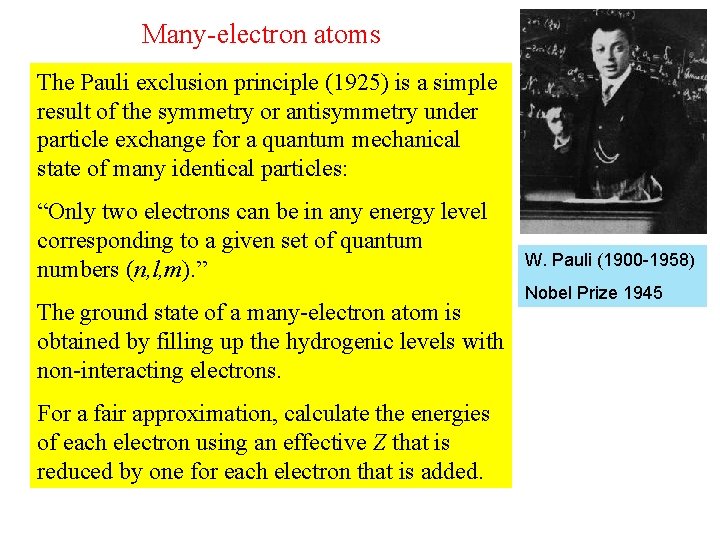
Many-electron atoms The Pauli exclusion principle (1925) is a simple result of the symmetry or antisymmetry under particle exchange for a quantum mechanical state of many identical particles: “Only two electrons can be in any energy level corresponding to a given set of quantum numbers (n, l, m). ” The ground state of a many-electron atom is obtained by filling up the hydrogenic levels with non-interacting electrons. For a fair approximation, calculate the energies of each electron using an effective Z that is reduced by one for each electron that is added. W. Pauli (1900 -1958) Nobel Prize 1945

Many-electron atoms The fact that two electrons could be in any energy level corresponding to a given set of quantum numbers (n, l, m) led eventually to the discovery of the spin of the electron. The energy levels corresponding to n = 1, 2, 3, … are called shells and each can hold 2 n 2 electrons. The shells are labeled K, L, M, … for n = 1, 2, 3, ….

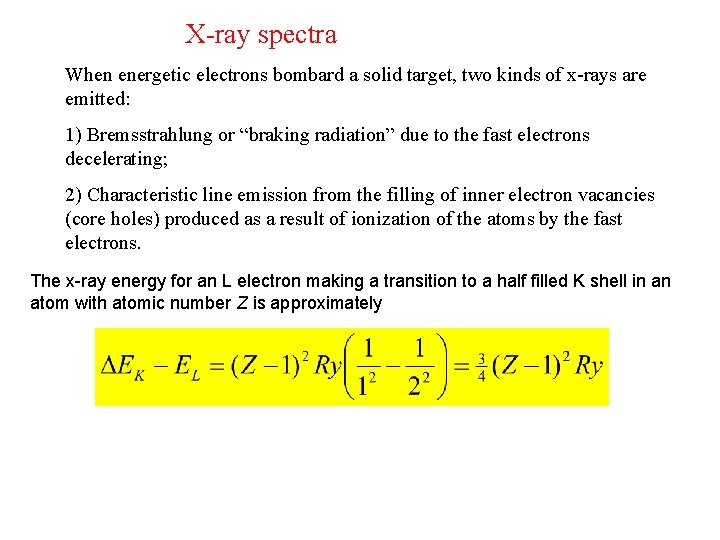
X-ray spectra When energetic electrons bombard a solid target, two kinds of x-rays are emitted: 1) Bremsstrahlung or “braking radiation” due to the fast electrons decelerating; 2) Characteristic line emission from the filling of inner electron vacancies (core holes) produced as a result of ionization of the atoms by the fast electrons. The x-ray energy for an L electron making a transition to a half filled K shell in an atom with atomic number Z is approximately

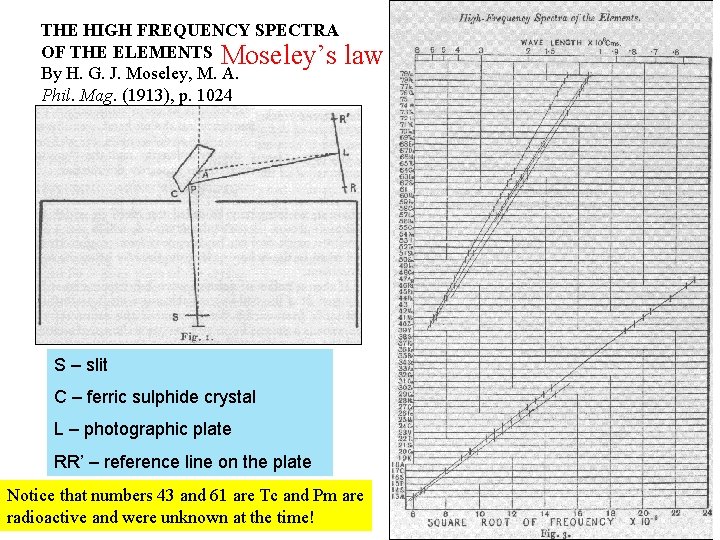
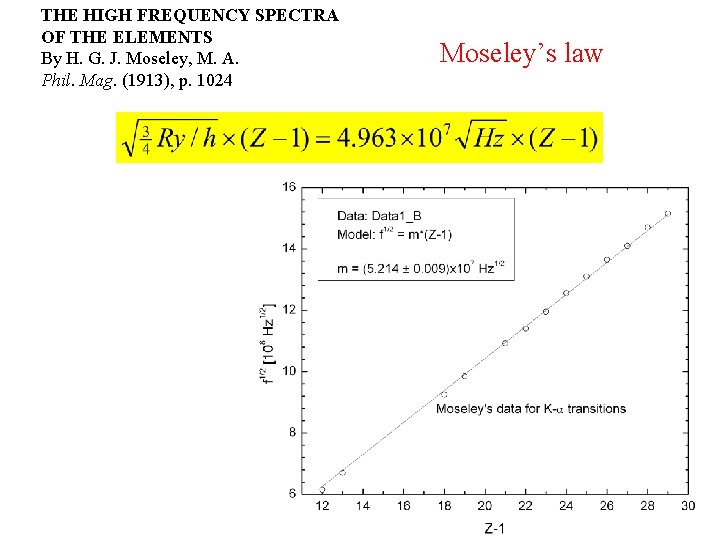
THE HIGH FREQUENCY SPECTRA OF THE ELEMENTS Moseley’s law By H. G. J. Moseley, M. A. Phil. Mag. (1913), p. 1024 S – slit C – ferric sulphide crystal L – photographic plate RR’ – reference line on the plate Notice that numbers 43 and 61 are Tc and Pm are radioactive and were unknown at the time!

THE HIGH FREQUENCY SPECTRA OF THE ELEMENTS By H. G. J. Moseley, M. A. Phil. Mag. (1913), p. 1024 Moseley’s law

Significance of Moseley’s work Moseley found a systematic shift towards shorter wavelengths as one passed from one element to others of higher atomic weight, but there were some irregularities. To get over the difficulty posed by the irregularities, he assigned a number to each element, specifying its position in the periodic table. Then he could assign a relation between the frequency of X-ray lines and the atomic number - a relation known as Moseley's law. When the elements were arranged according to the atomic numbers assigned by Moseley, some inconsistencies apparent in the Mendeleev table were removed. Thus Moseley was the first to arrange the elements in order of atomic number, rather than atomic weight, so he can be considered to be responsible for the present-day arrangement of the elements. from A and B Scott Science History Moseley’s measurements also proved that the nucleus held an integral number of elemental charges, thus placing the nuclear model of the atom on a firm foundation.

Moseley, the man THE HIGH FREQUENCY SPECTRA OF THE ELEMENTS By H. G. J. Moseley, M. A. Phil. Mag. (1913), p. 1024 Henry Moseley (1887 -1915): A British chemist who studied under Rutherford and brilliantly developed the application of X-ray spectra to study atomic structure; his discoveries resulted in a more accurate positioning of elements in the Periodic Table by closer determination of atomic numbers. Tragically for the development of science, Moseley was killed in action at Gallipoli (the Dardanelles campaign) in 1915.

Moseley and WWI http: //en. wikipedia. org/wiki/Battle_of_Gallipoli

Moseley fought and died where lay “the topless towers of Ilium”, and lurk the shades of Ajax, Cassandra, Aeneas, and Anchises.

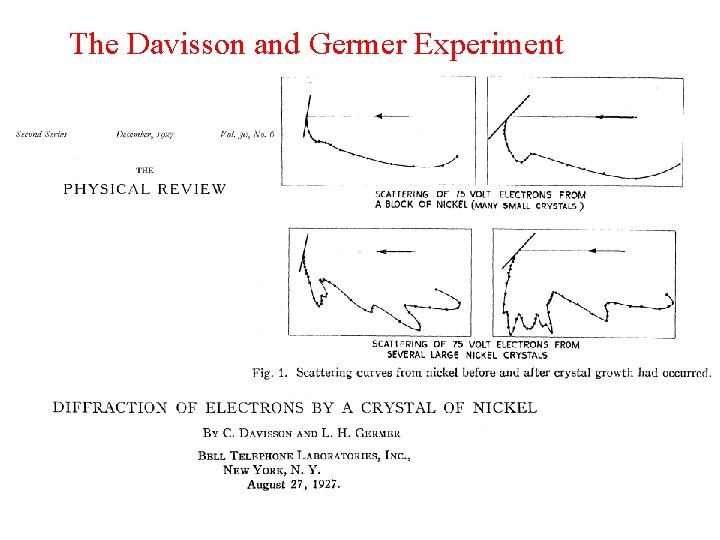
The wave nature of electrons was demonstrated in 1927 by 1. Lewis and Clark 2. Einstein and Planck 3. Davisson and Germer 4. Maxwell and Hertz 5. Michelson and Morley Q 2

The Davisson and Germer Experiment

Disturbing wave-particle duality De Broglie’s hypothesis implied that the double slit experiment should work for electrons and other massive particles. A modern double slit experiment shows that a single photon interferes with itself and goes through both slits or rather that you can’t say which one it went through. We don’t feel so bad about this because we always thought light was waves. However, this experiment is just as disturbing as any other quantum experiment because we detect the light in discrete lumps.

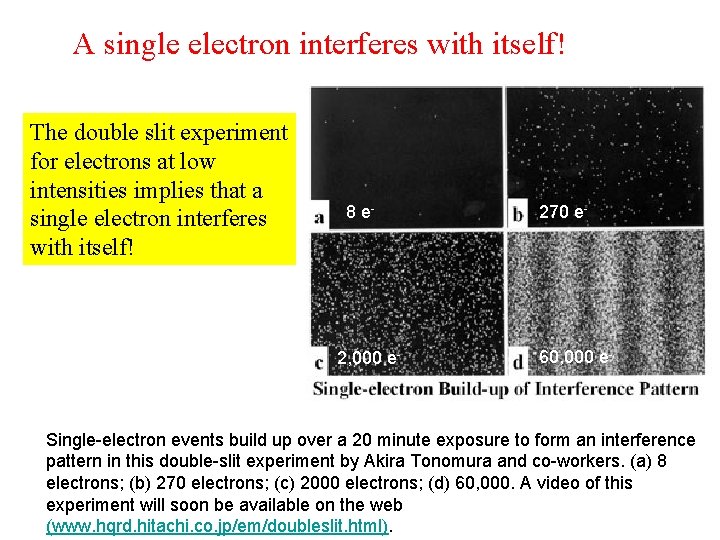
A single electron interferes with itself! The double slit experiment for electrons at low intensities implies that a single electron interferes with itself! 8 e- 2, 000 e- 270 e- 60, 000 e- Single-electron events build up over a 20 minute exposure to form an interference pattern in this double-slit experiment by Akira Tonomura and co-workers. (a) 8 electrons; (b) 270 electrons; (c) 2000 electrons; (d) 60, 000. A video of this experiment will soon be available on the web (www. hqrd. hitachi. co. jp/em/doubleslit. html).

The facts of life are not reasonable! Does one electron go through both slits? Apparently it does in some sense because: If you block one of the slits you get no interference. But if you try to look at which slit the particle went through, there is no interference pattern. If you look gently so as not to disturb the particle too much, you can’t tell which slit it went through and there is interference. No one has been able to make up a model based on some “reasonable” hypotheses that explains the observations.

WHAT DOES IT MEAN? The actual world is much more weird than anything ever cooked up by witches or dreamt of by philosophers! The world is in fact non-local a-causal inscrutable and yielding highly accurate predictions all at the same time!

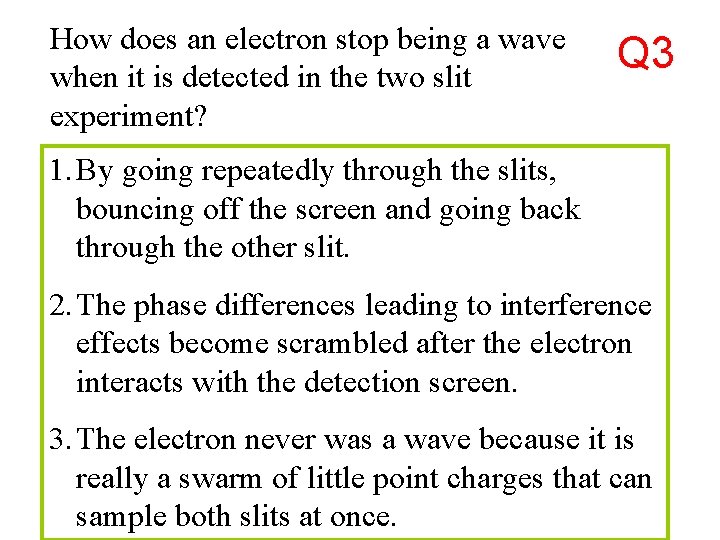
How does an electron stop being a wave when it is detected in the two slit experiment? Q 3 1. By going repeatedly through the slits, bouncing off the screen and going back through the other slit. 2. The phase differences leading to interference effects become scrambled after the electron interacts with the detection screen. 3. The electron never was a wave because it is really a swarm of little point charges that can sample both slits at once.

OUTLINE 1. Complex atoms and molecules. 2. Precision atomic physics. 3. The many-body problem. 4. Emergent properties and empirical law.

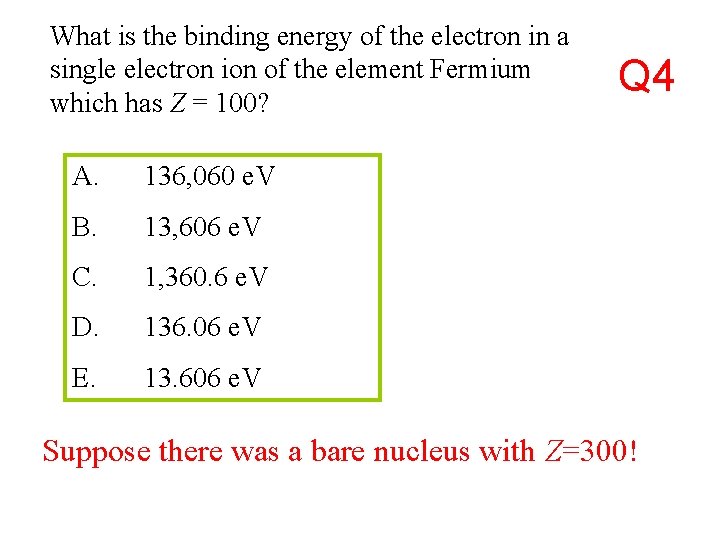
What is the binding energy of the electron in a single electron ion of the element Fermium which has Z = 100? A. 136, 060 e. V B. 13, 606 e. V C. 1, 360. 6 e. V D. 136. 06 e. V E. 13. 606 e. V Q 4 Suppose there was a bare nucleus with Z=300!

Complex atoms and molecules Finding the ground state of a complex atomic system: 1. Variational principle: the ground state has the lowest possible energy. 2. Approximate the non-local exchange-correlation energy by a local function of the density and its gradients.

Protein molecules Fully automated ab initio protein structure prediction using I-SITES, HMMSTR and ROSETTA. Bystroff C, Shao Y. Department of Biology, Rensselaer Polytechnic Institute, Troy, NY 12180, USA. bystrc@rpi. edu MOTIVATION: The Monte Carlo fragment insertion method for protein tertiary structure prediction (ROSETTA) of Baker and others, has been merged with the I-SITES library of sequence structure motifs and the HMMSTR model for local structure in proteins, to form a new public server for the ab initio prediction of protein structure. The server performs several tasks in addition to tertiary structure prediction, including a database search, amino acid profile generation, fragment structure prediction, and backbone angle and secondary structure prediction. Meeting reasonable service goals required improvements in the efficiency, in particular for the ROSETTA algorithm. RESULTS: The new server was used for blind predictions of 40 protein sequences as part of the CASP 4 blind structure prediction experiment.

Precision atomic physics 1. The hydrogen atom, atomic clocks, GPS 2. Cold atoms and Bose-Einstein condensation

Atomic fountain clock

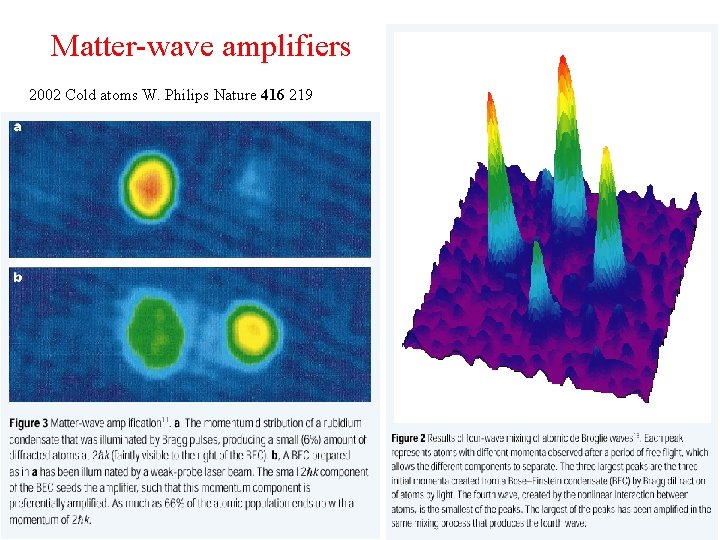
Matter-wave amplifiers 2002 Cold atoms W. Philips Nature 416 219

The many-body problem 1. Solid state physics 2. Phase transitions

Emergent properties 1. The whole is greater than the sum of its parts. 2. The properties of a large system cannot be predicted on the basis of the properties of the components and are largely independent of these properties. 3. Empirical laws are found for larger systems that cannot be related directly to the fundamental postulates of quantum mechanics. 4. The behavior of large systems can only be calculated with precision using the systems themselves.

So why do we need to know quantum mechanics and atomic physics? 1. It is a stupid academic exercise 2. To appreciate what is the playing field Q 5

So why do we need to know quantum mechanics and atomic physics? Diffraction, radiation, knowledge of atomic scale structure, etc. are indispensable in many other fields. Fear holds dominion over mortality Only because, seeing in land sky So much the cause whereof no wise they know, Men think Divinities are working woe.