Air Pollution Thank God men cannot fly and





























































- Slides: 103

Air Pollution Thank God men cannot fly, and lay waste the sky as well as the earth. - Henry David Thoreau 1

Early Atmosphere • • The earliest atmosphere consisted primarily of lighter gases (H, He) -> like the gas giants. The Earth's gravitation was not sufficient to retain these gases and they dissipated into space. Secondary atmosphere formed from degassing from volcanoes and hot springs and from space: N 2, H 2 S, CO 2, HCl, H 2 O Oxygen was released by photosynthesis (~2 billion years ago).

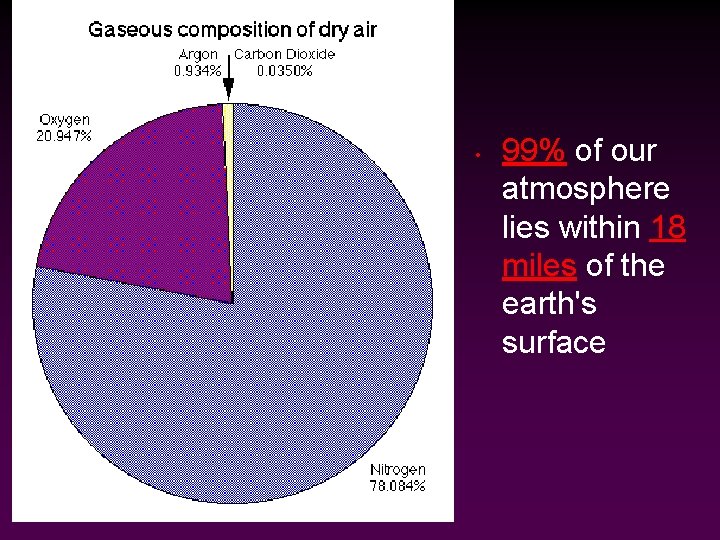
• 99% of our atmosphere lies within 18 miles of the earth's surface

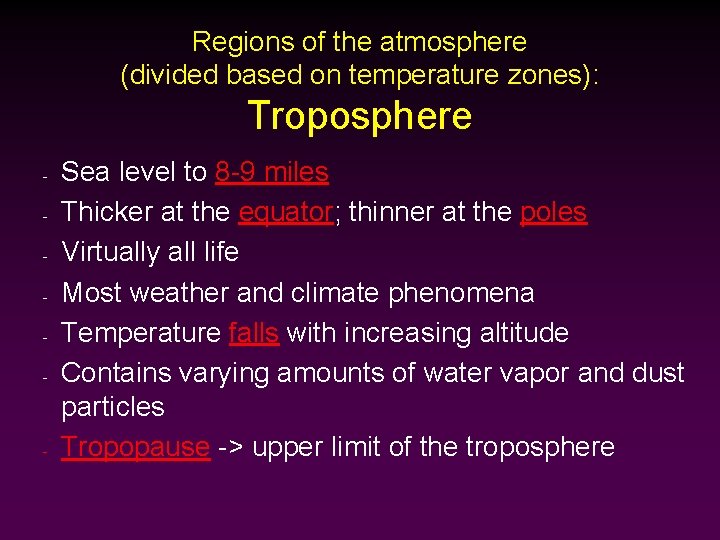
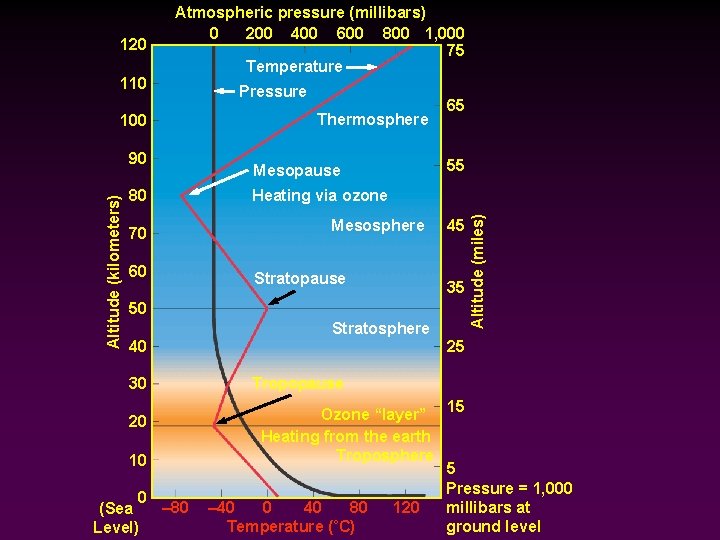
Regions of the atmosphere (divided based on temperature zones): Troposphere - - Sea level to 8 -9 miles Thicker at the equator; thinner at the poles Virtually all life Most weather and climate phenomena Temperature falls with increasing altitude Contains varying amounts of water vapor and dust particles Tropopause -> upper limit of the troposphere

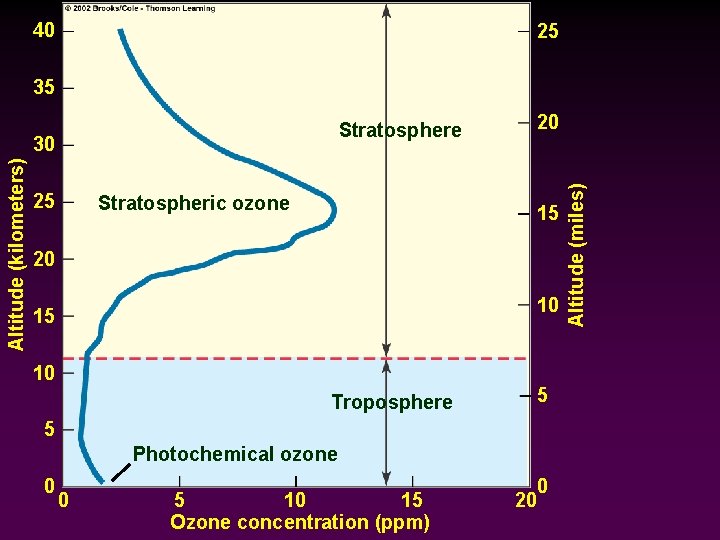
Regions of the atmosphere (divided based on temperature zones): Stratosphere - - - Marked by a temperature gradient reversal -> Temperature rises with increasing altitude until it reaches 0 C at ~ 30 miles (stratopause) Ozone layer -> higher than usual concentrations of the rare gas ozone at ~10 -30 miles above sea level Ozone concentrations increase in the winter; and decrease in the spring/summer

110 100 Altitude (kilometers) 90 80 70 60 Pressure Thermosphere Mesopause 65 55 Heating via ozone Mesosphere Stratopause 45 35 50 Stratosphere 40 30 20 10 0 – 80 (Sea Level) Altitude (miles) 120 Atmospheric pressure (millibars) 0 200 400 600 800 1, 000 75 Temperature 25 Tropopause Ozone “layer” 15 Heating from the earth Troposphere 5 Pressure = 1, 000 – 40 0 40 80 120 millibars at Temperature (˚C) ground level

40 25 35 Altitude (kilometers) 30 25 Stratospheric ozone 20 15 20 10 15 10 Troposphere 5 5 Photochemical ozone 0 0 5 10 15 Ozone concentration (ppm) 0 20 Altitude (miles) Stratosphere

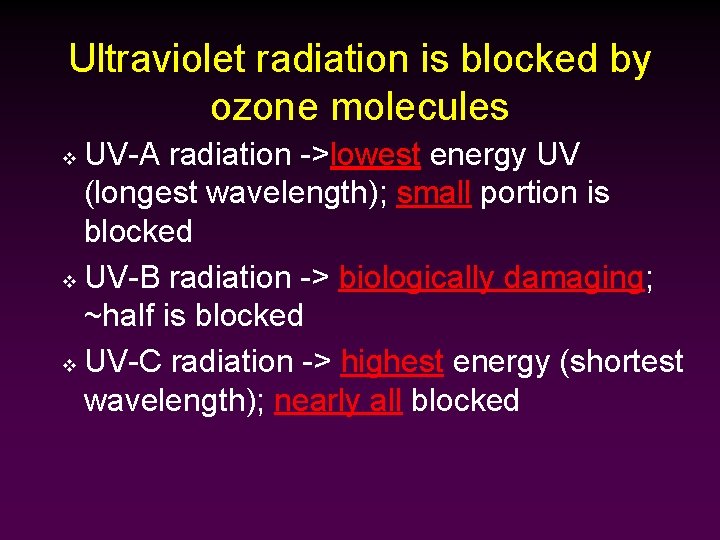
Ultraviolet radiation is blocked by ozone molecules UV-A radiation ->lowest energy UV (longest wavelength); small portion is blocked v UV-B radiation -> biologically damaging; ~half is blocked v UV-C radiation -> highest energy (shortest wavelength); nearly all blocked v

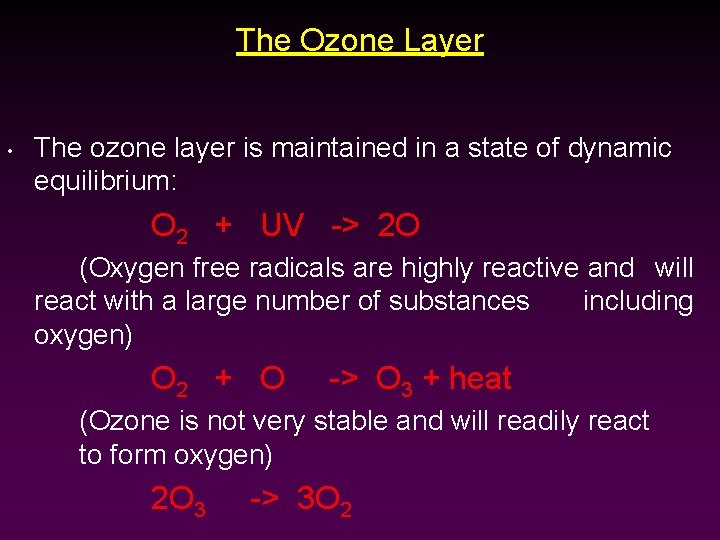
The Ozone Layer • The ozone layer is maintained in a state of dynamic equilibrium: O 2 + UV -> 2 O (Oxygen free radicals are highly reactive and will react with a large number of substances including oxygen) O 2 + O -> O 3 + heat (Ozone is not very stable and will readily react to form oxygen) 2 O 3 -> 3 O 2

• Ozone (like oxygen) can absorb UV light and produce oxygen atoms: O 3 + UV -> O 2 + O

Chlorofluorocarbons • • CFCs are nonpoisonous, nonflammable, and noncorrosive CFCs uses: v as coolants in refrigerators and air conditioners v styrofoam v propellants in spray cans v solvents

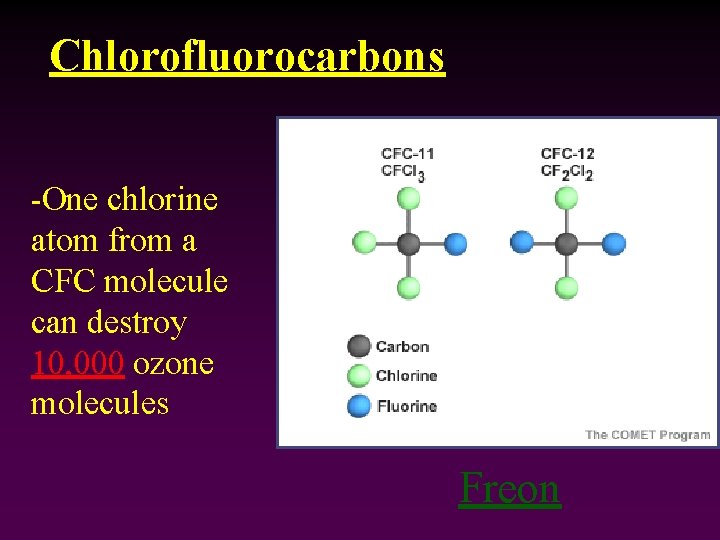
Chlorofluorocarbons -One chlorine atom from a CFC molecule can destroy 10, 000 ozone molecules Freon

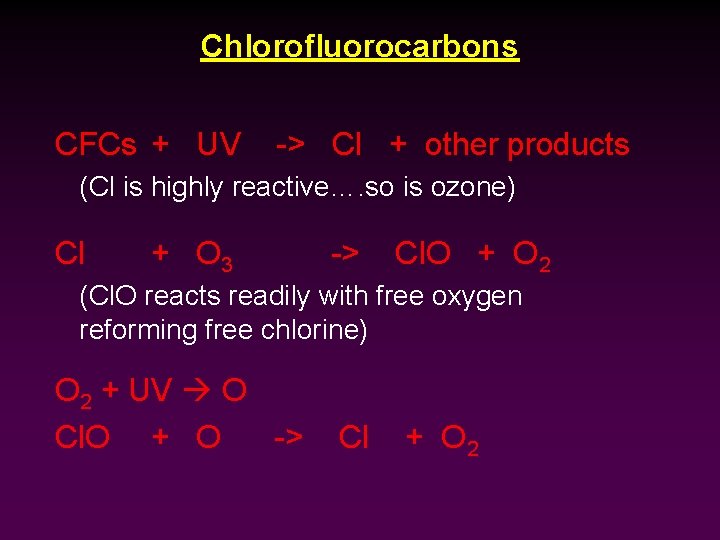
Chlorofluorocarbons CFCs + UV -> Cl + other products (Cl is highly reactive…. so is ozone) Cl + O 3 -> Cl. O + O 2 (Cl. O reacts readily with free oxygen reforming free chlorine) O 2 + UV O Cl. O + O -> Cl + O 2

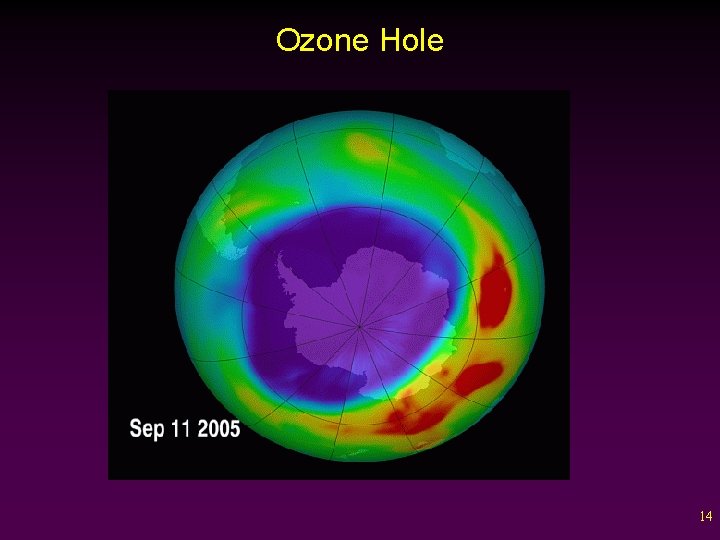
Ozone Hole 14

Atmospheric Ozone • Scientists discovered that atmospheric ozone levels were dropping rapidly every year, during September and October. v Occurring since at least 1960. v A 1% decrease in ozone results in a 2% increase in UV rays reaching the earth. v The ozone was being depleted by pollutants containing chlorine. 15

Stratospheric Ozone Cont’d • A concentration of pollution at the poles and other factors caused chlorine pollution to be concentrated in Antarctica. - When the sun returns in the spring, the energy liberates the chlorine from ice. - Chlorine causes ozone (O 3) to be broken down into oxygen (O 2). 16

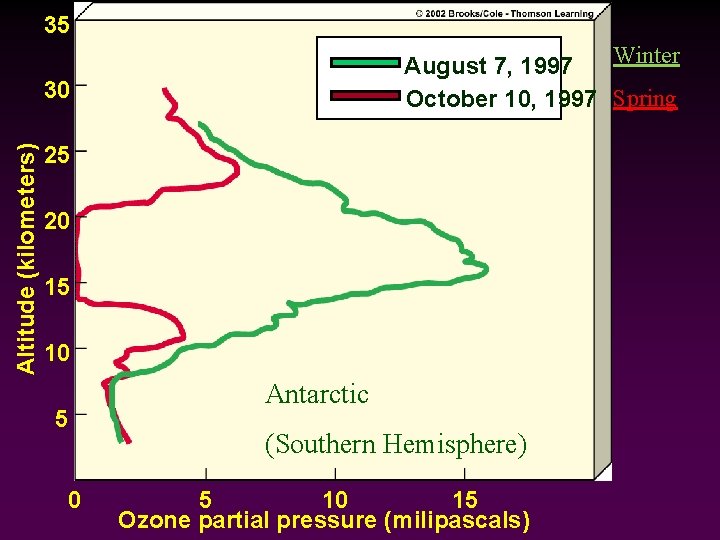
35 Winter August 7, 1997 October 10, 1997 Spring Altitude (kilometers) 30 25 20 15 10 5 0 Antarctic (Southern Hemisphere) 5 10 15 Ozone partial pressure (milipascals)

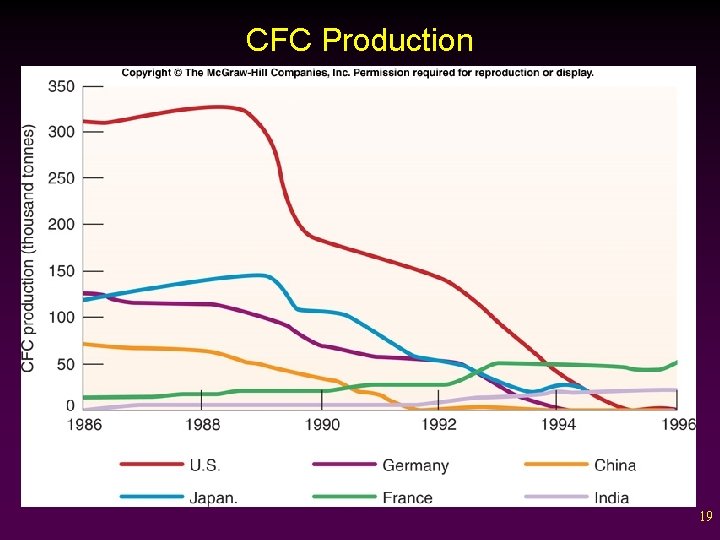
Montreal Protocol • The Montreal Protocol was passed in 1989. v Countries agreed to phase out CFC use by the year 2000. v CFC levels in the atmosphere decreased and the ozone layer is beginning to recover. 18

CFC Production 19

Other chemicals that deplete the ozone layer include: • • • halons (3 -10 times more destructive than CFCs; now responsible for ~20% of ozone layer destruction) carbon tetrachloride methyl chloroform hydrochlorofluorocarbons methyl bromide

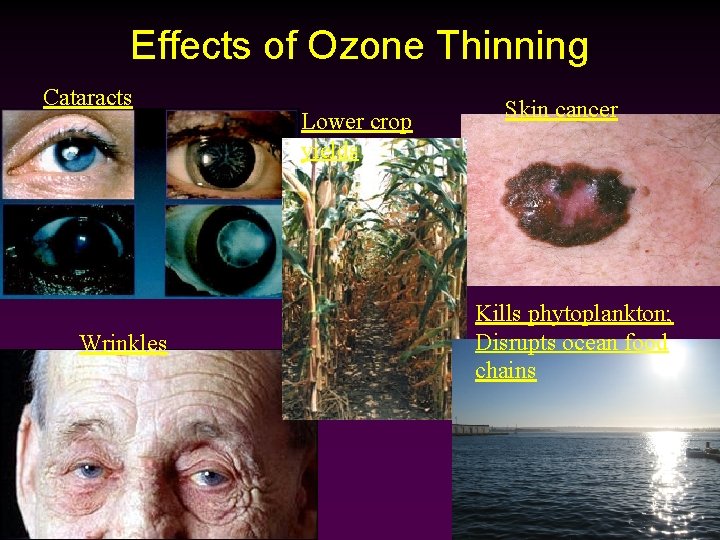
Depletion of ozone • • increase incidence of sunburn and skin cancers increase incidence of cataracts suppression of the immune system lower crop yields decline in forest productivity increased breakdown of materials (paints, plastics, outdoor materials) reduction in phytoplankton productivity

Effects of Ozone Thinning Cataracts Wrinkles Lower crop yields Skin cancer Kills phytoplankton; Disrupts ocean food chains


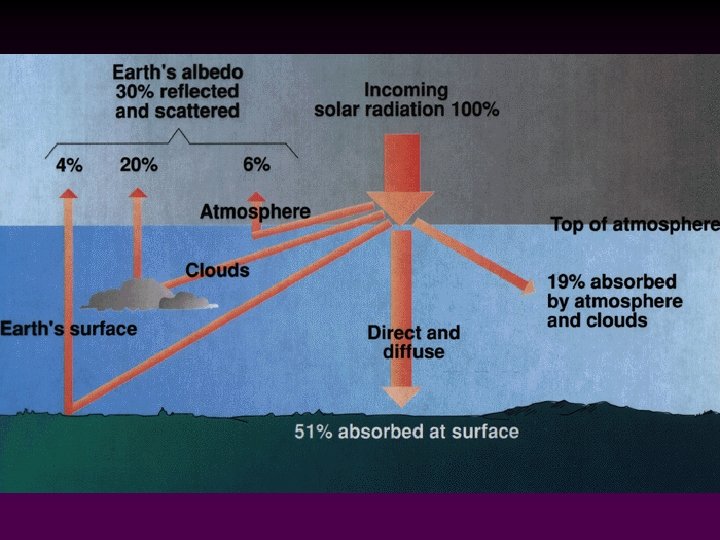
Incoming solar radiation may be: Scattered and reflected due to contact with dust particles and clouds -> some is reradiated to outer space; some to the Earth's surface v Absorbed by dust, carbon dioxide, water vapor in the atmosphere v Reflected from the ground v Absorbed by the ground v


• • Ground radiation (long-wave radiation heat) is continuously radiated back into the atmosphere. Some is absorbed by water vapor and carbon dioxide (and other greenhouse gases) and is re-radiated back to the Earth's surface, thereby keeping the Earth's warmer than it would otherwise be. Imbalances in incoming and outgoing radiation drives our weather patterns.

Human Impact on the Earth. Atmosphere System • • Drastic climate changes have occurred in Earth's history. The extent to which humans are currently causing climate change is under heated debate. The major causes of human-induced atmospheric change are: v Introduction of pollutant gases and particles v Changes in concentration of natural atmospheric gases

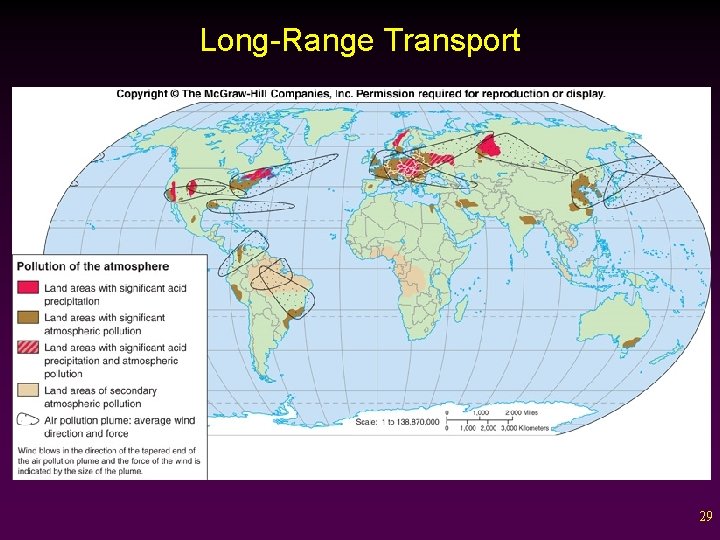
CLIMATE PROCESSES AND AIR POLLUTION • • Air pollution is defined as any contaminant added to the air that is harmful to the health of living organisms. Due to the nature of air and wind, this pollution can be carried great distances. - Industrial contaminants can be found in places that have virtually no population. - Contaminants especially concentrate at the poles. 28

Long-Range Transport 29

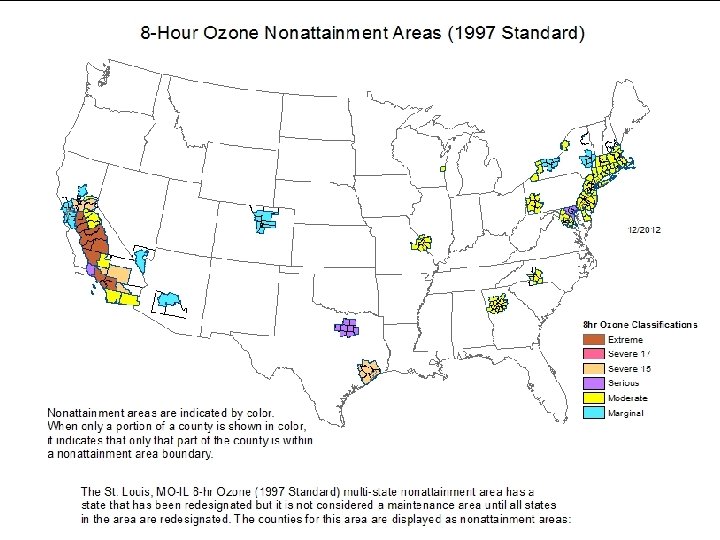
AIR POLLUTION CLASSIFICATION Primary Pollutants - Released directly into the air v Secondary Pollutants - Formed as a result of a chemical reaction in the air. - Smog – Reaction of sunlight with nitrogen oxide. - Ozone – Reaction of nitrogen oxides with volatile organic compounds - Acid Rain – Reaction of sulfur dioxide with water to form sulfuric acid. -. v 30

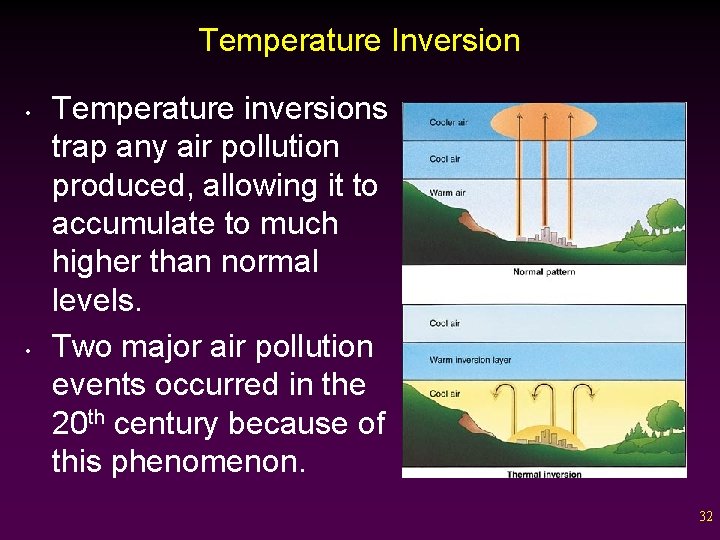
Air Pollution and Topography • • The effects of air pollution are also influenced by the shape of the land. Temperature inversions occur when a layer of dense, cool air is trapped below a layer of lighter, warmer air. v Most likely to occur in valleys and canyons. v May also occur in any area where the wind is typically calm. 31

Temperature Inversion • • Temperature inversions trap any air pollution produced, allowing it to accumulate to much higher than normal levels. Two major air pollution events occurred in the 20 th century because of this phenomenon. 32

Air Pollution History • The London Smog of 1952 v London naturally has very calm air, and regularly experiences thick sea fog. v The weather turned unusually cold, causing the residents to burn greater amounts of coal to heat their homes. v This combined with a temperature inversion to create a thick smog of sulfur dioxide over the city. v The number of fatalities is unknown, but estimated to be around 12, 000. 33

The London Smog of 1952 • • As a result of this disaster, London passed its own Clean Air Act. One of the specific changes made was to make chimneys taller to reach above a temperature inversion. 34

Clean Air Act • • • The most significant parts of the U. S. Clean Air Act took effect in 1970. Initially, the law required the EPA to set and enforce limits for 6 different air pollutants. These are called criteria pollutants. v Sulfur Dioxide v Carbon Monoxide v Particulates v Ozone v Nitrogen Oxides v Lead 35

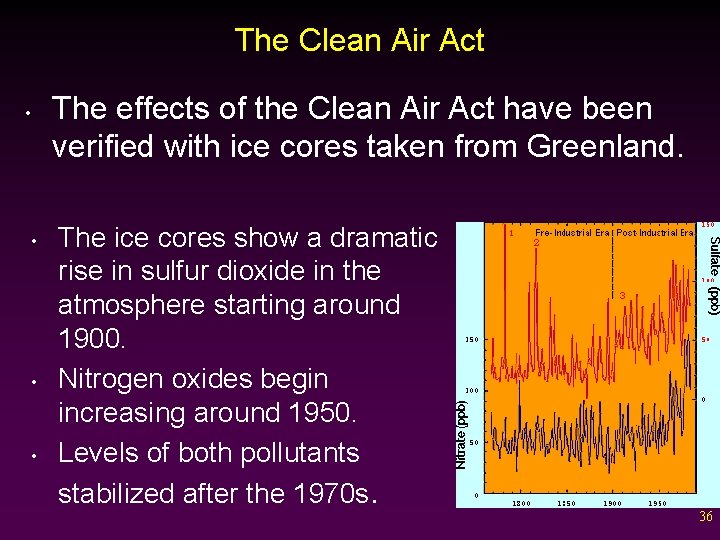
The Clean Air Act • • The effects of the Clean Air Act have been verified with ice cores taken from Greenland. The ice cores show a dramatic rise in sulfur dioxide in the atmosphere starting around 1900. Nitrogen oxides begin increasing around 1950. Levels of both pollutants stabilized after the 1970 s. 36

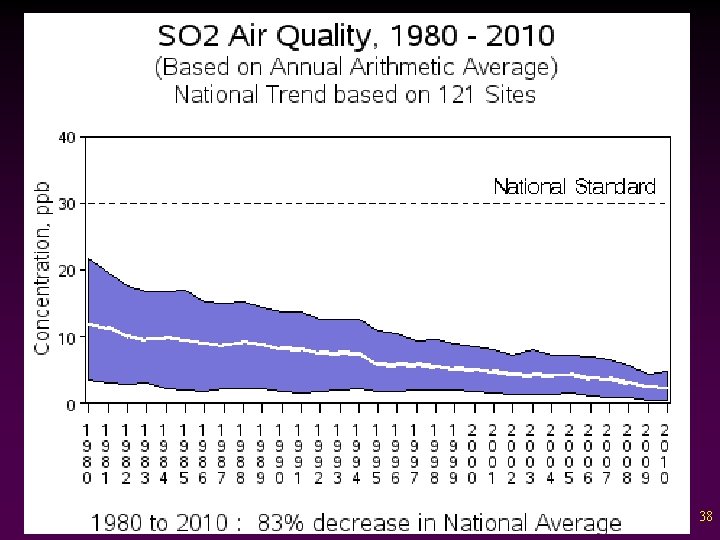
Criteria Pollutants v Sulfur dioxide - Colorless gas often associated with “rotten eggs” smell - Forms sulfuric acid in clouds causing acid rain. - Biggest source: coal burning power plants 37

38

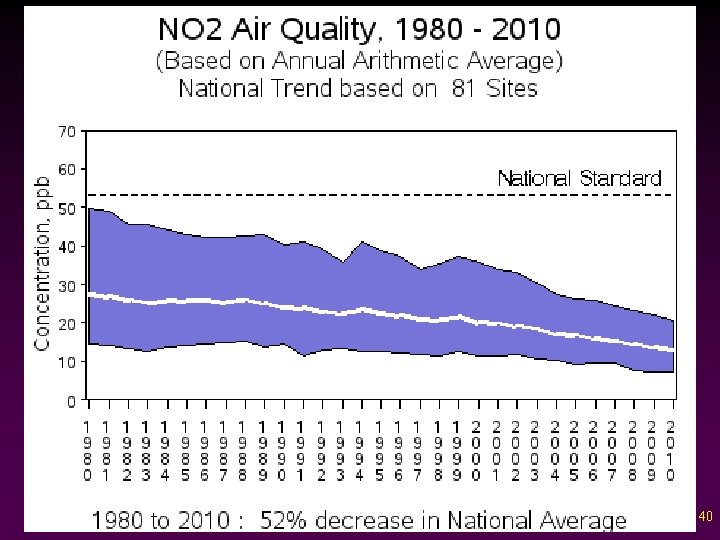
Criteria Pollutants v Nitrogen oxides - Reddish brown gas - Reacts with water vapor to form nitric acid resulting in acid rain. - Reacts with sunlight to form smog. - Biggest source: car exhaust 39

40

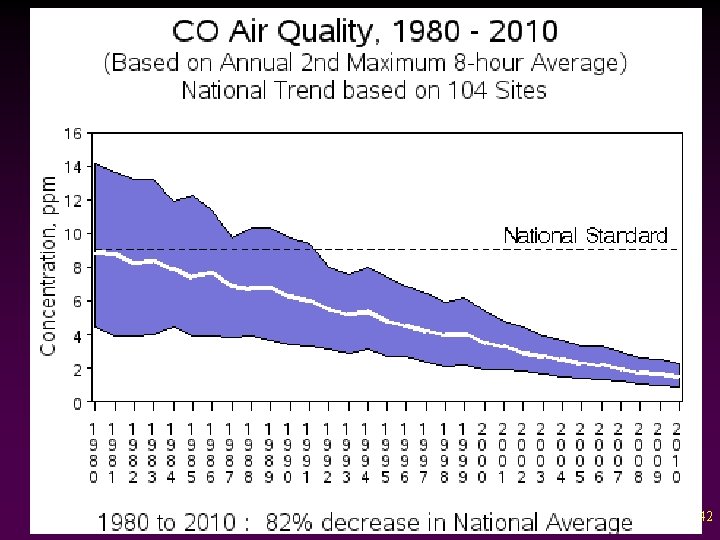
Criteria Pollutants v Carbon Monoxide - Colorless, odorless, highly toxic gas - Binds to hemoglobin in red blood cells, interfering with oxygen transport causing headaches, etc. - Biggest source: car exhaust 41

42

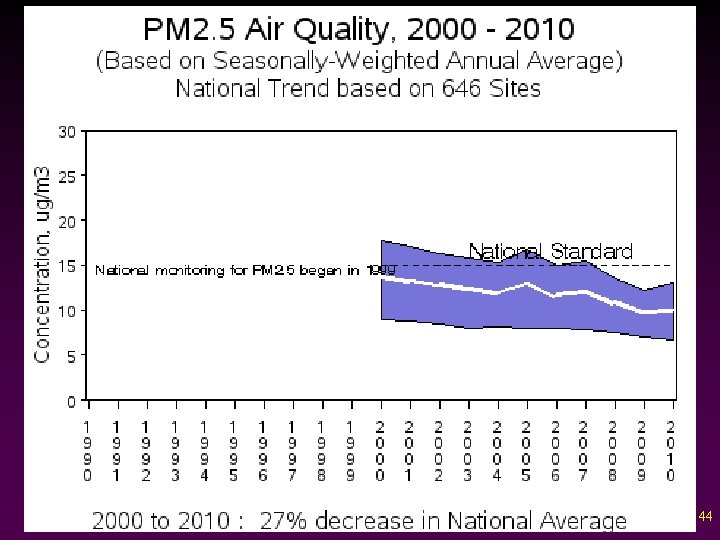
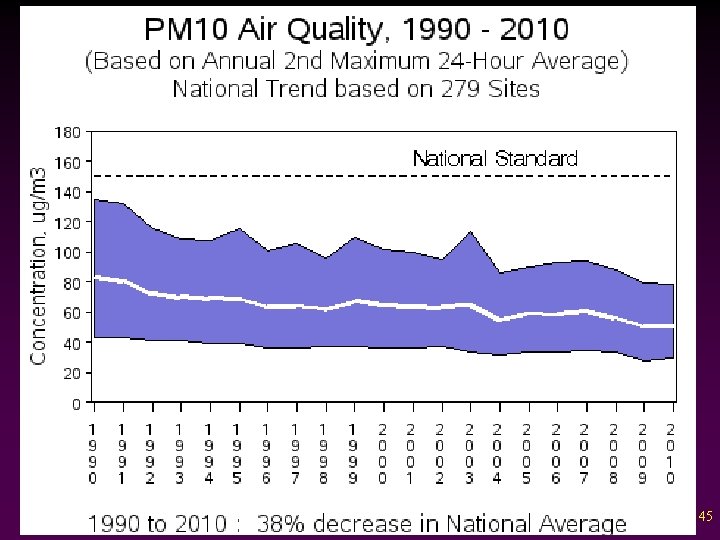
Criteria Pollutants v Particulate Matter - Dust, ash, soot, lint, smoke, pollen, spores, and all other suspended matter. - Cause the most visibility problems - Biggest source: unpaved road dust and construction 43

44

45

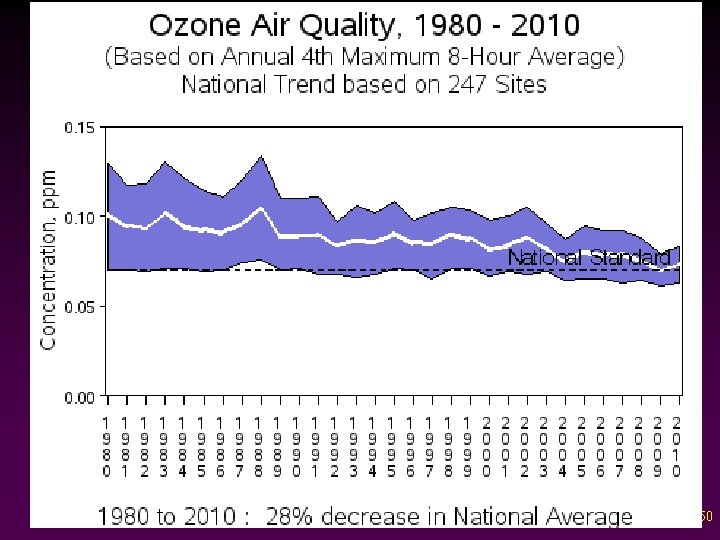
Criteria Pollutants v Ozone - Molecule made of three oxygen atoms - Pale blue gas, odor resembling chlorine bleach - Secondary pollutant; not released directly 46

Photochemical Smog is a secondary air pollutant -> most difficult to control

Criteria Air Pollutants: Ground Level OZONE • • • Required for ozone to accumulate: VOCs + NOx + Sunlight The hotter the day, the higher the levels of ozone and other photochemical oxidants Ozone season: May 1 - Sept 30

Criteria Air Pollutants: Ground Level OZONE (O 3 ) • Ozone, NO 2 , and PANs are the main constituents of photochemical smog: NOx + VOCs + sunlight O 3 + PANs + other hydrocarbons • • VOCs = Volatile Organic Compounds from fuels, paints & glues PANs = Peroxyacetylnitrate

50

Criteria Air Pollutants: Ground Level OZONE • Ozone exposure causes v v v • decreases in lung function inflammation of the airways aggravates lung diseases such as asthma. Ozone also v v reduces agricultural crop and commercial forest yields increases plant susceptibility to disease, pests, and other environmental stresses (e. g. , harsh weather).

52

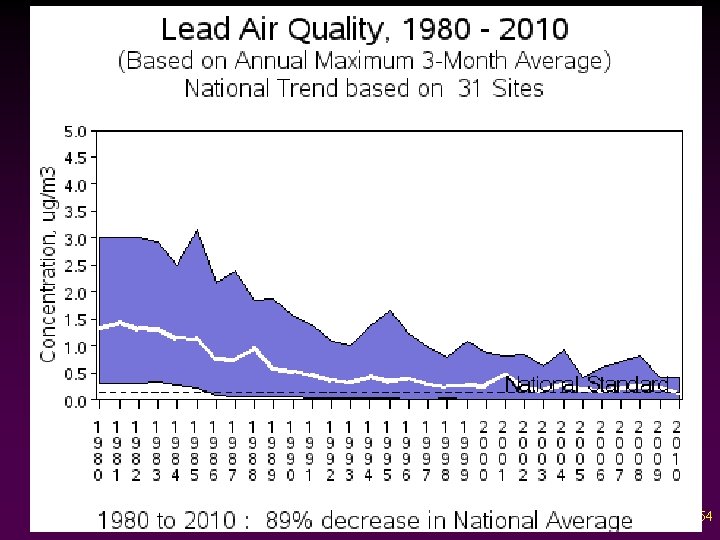
Criteria Pollutants v Lead - Enters the air as particles or part of dust. - The biggest source used to be exhaust from cars using leaded gas; now it is industry and burning fossil fuels. 53

54

QOD 1/25/16 List one major anthropogenic greenhouse gas, and one major ozone depleting chemical. For each selection identify a major source and describe a method that is effective at reducing its emissions. 55

Criteria Air Pollutants: Lead • • Excessive exposure to lead may cause: v seizures v mental retardation v behavioral disorders Even at low doses, lead exposure is associated with damage to the nervous systems of fetuses and young children, resulting in learning deficits and lowered IQ.

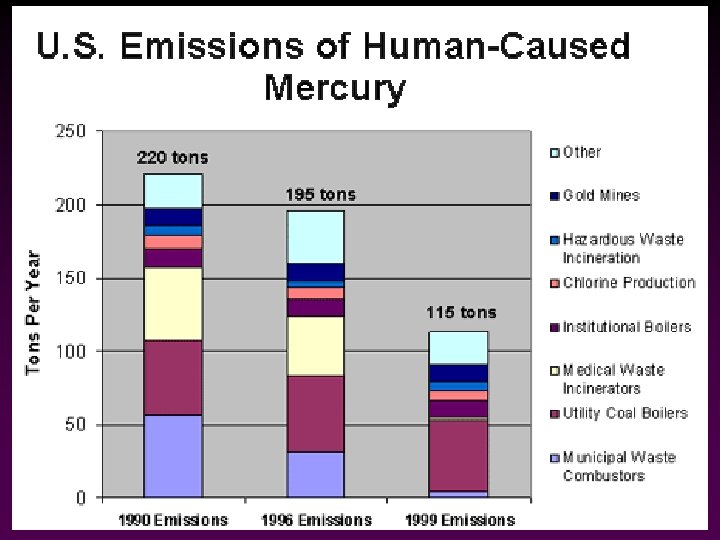
Hazardous Air Pollutants (HAPs) • In 1970 Congress directed the EPA to develop a list of industrial air pollutants that can cause serious health damage even at low concentrations (original HAPs): v v v v • asbestos mercury beryllium benzene vinyl chloride radionuclides coke oven emissions There are now 188 regulated HAPs -> prompted by the tragic chemical accident in Bhopal, India (1984)


Clean Air Act • The Clean Air Act was amended in 1990 and included additional provisions and controls for: v Acid Rain v Urban Smog v Toxic and Hazardous Air Pollutants v Protection of the Ozone Layer v Leakage of volatile organic compounds 59

Other Major Pollutants v Volatile organic compounds - Organic (carbon-based) gases like methane that can decompose or react easily, forming carbon dioxide or carbon monoxide in the air. - Biggest sources: Ø Spilled/leaking gasoline that evaporates Ø Paint and paint cleaners 60

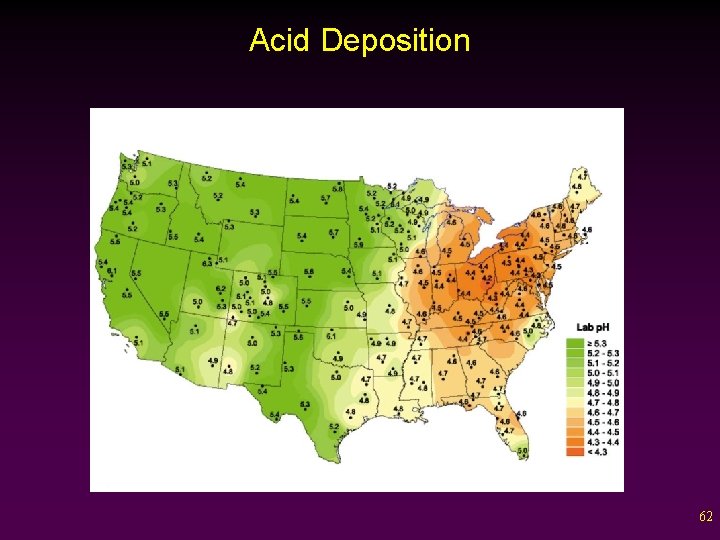
Acid Deposition • Acid Precipitation – Rainfall or snowfall that contains an lower than normal p. H (5. 6). v p. H scale ranges from 0 -14. - 7 = Neutral; <7 = Acidic; >7 = Basic v Unpolluted rain generally has p. H of 5. 6. - Carbonic acid from atmospheric CO 2. v In industrialized areas, the p. H level can reach as low as 4. 3 - Rain of p. H 2. 1 was recorded in the 1970 s and 1980 s 61

Acid Deposition 62

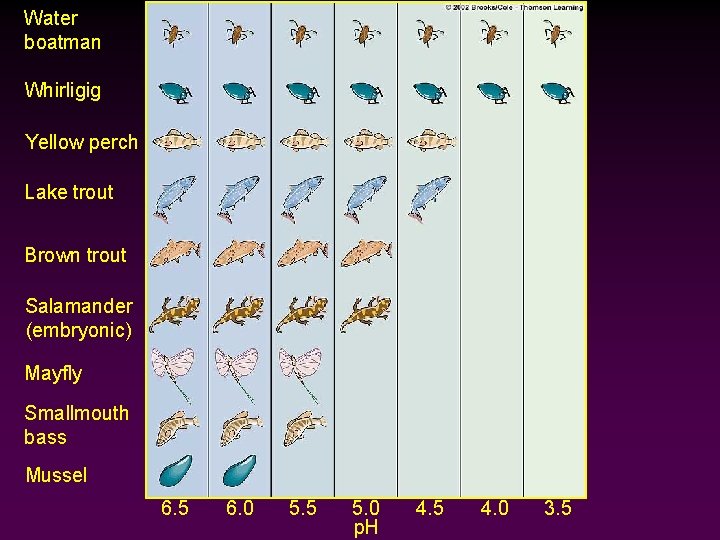
Acid Deposition Cont’d • Aquatic Effects - Fish and other aquatic organisms are extremely sensitive to p. H changes. Ø p. H below 5 = eggs will not hatch Ø p. H below 4 = kills adult fish - Acid shock often follows spring snow melt (also the critical developmental period for many larvae) 63

Water boatman Whirligig Yellow perch Lake trout Brown trout Salamander (embryonic) Mayfly Smallmouth bass Mussel 6. 5 6. 0 5. 5 5. 0 p. H 4. 5 4. 0 3. 5

Regional Vulnerability to Acid Precipitation • An ecosystem’s sensitivity to acid precipitation is determined by the chemical composition of its soil and bedrock (buffering capacity ability to neutralize acids) v v v Low buffering capacity naturally acidic soils or/and granite bedrock High buffering capacity alkaline soils or/and limestone bedrock Over time, a soil’s buffering capacity can be overwhelmed by acidic inputs

Environmental Effects of Acid Deposition: Mobilization of toxic metals • As p. H decreases, toxic metals (Al, Mn, Pb, Zn, Hg, and Cd) dissolve out of bottom sediments or soils and leach into the aquatic environment. These metals can: v v • bioaccumulate in fish tissues, making them dangerous for humans to eat can kill fish by damaging their gills (especially aluminum) Acidification cause the conversion of moderately toxic inorganic mercury Hg to the highly toxic organic methylmercury

Acid Deposition Cont’d • • Forest Damage v Acid rain cause the p. H of soil to decrease. v This interferes with trees’ ability to absorb nutrients properly. Buildings and Monuments v Limestone and marble are slowly dissolved as they are exposed to acid rain. v Acid rain can also corrode steel, weakening structures like bridges. 67

Tombstone in Hamilton, NY Picture by Cassandra Willyard, Smithsonian Magazine 68

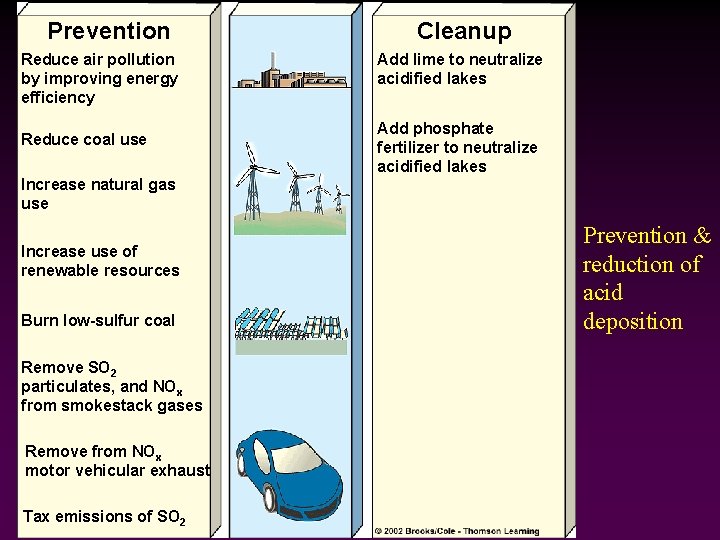
Prevention Reduce air pollution by improving energy efficiency Reduce coal use Increase natural gas use Increase use of renewable resources Burn low-sulfur coal Remove SO 2 particulates, and NOx from smokestack gases Remove from NOx motor vehicular exhaust Tax emissions of SO 2 Cleanup Add lime to neutralize acidified lakes Add phosphate fertilizer to neutralize acidified lakes Prevention & reduction of acid deposition

Clean Air Act • • 1. Spurred by the environmental movement, Congress passed the 1970 CAA. The CAA was strengthened in 1990 under Bush. The CAA has the following provisions: Development of the NAAQS for the 6 criteria air pollutants v penalties were set for nonattainment areas

Clean Air Act 2. Emission limitations for new stationary sources (factories and power plants) v required new stationary sources to obtain operating permits specifying allowable levels of pollutant emissions, as well as required control measures

Clean Air Act 3. Stricter emission standards for automobiles v v Inspection and maintenance programs (some areas) Made catalytic converters standard 1990 CAA mandated reformulated gasoline containing oxygen additives in cities not in compliance with ozone emission standards Service stations in these areas were required to install vapor recovery systems

Clean Air Act 4. Regulation of Hazardous Air Pollutants (HAPs) through technology-based controls

Clean Air Act 5. Acid deposition controls • Mandate to cut sulfur dioxide emissions in half • Allowance trading (Cap & Trade): v v v let utility companies buy and sell allowances for SO 2 emissions encourages companies to reduce emissions below the legal limit 1 allowance = 1 ton of SO 2 annually market forces control the cost of each allowance; in 1993, 1 allowance cost $250; in 1998 -9, 1 allowance cost under $100 the EPA-imposed penalty for exceeding allowance allocations is $2000/ton

QOD 1/28/16 You’ve been promoted to king or Queen of the world. List 3 strategies you will implement to deal with outdoor air pollution. 75

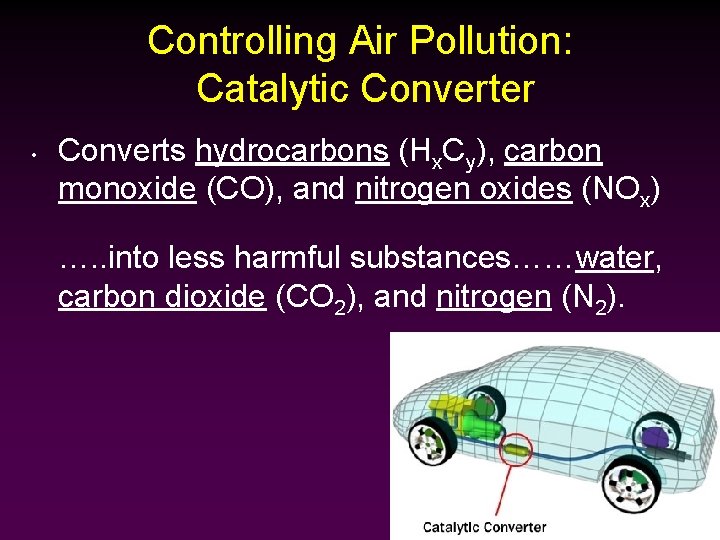
Controlling Air Pollution: Catalytic Converter • Converts hydrocarbons (Hx. Cy), carbon monoxide (CO), and nitrogen oxides (NOx) …. . into less harmful substances……water, carbon dioxide (CO 2), and nitrogen (N 2).

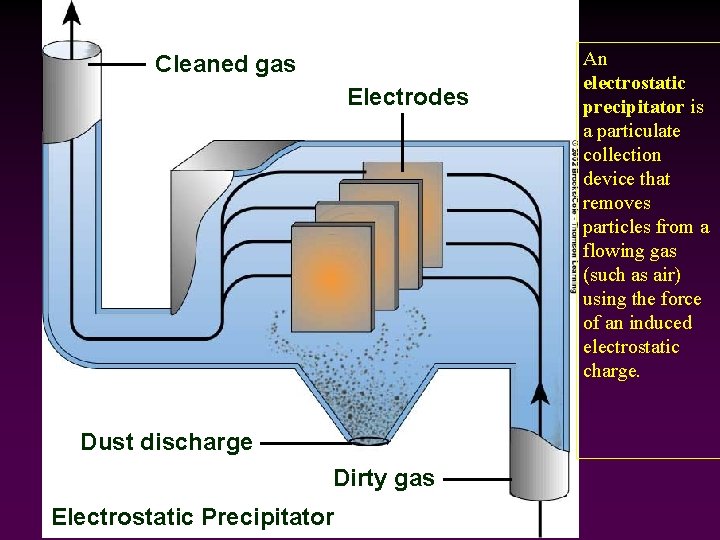
Cleaned gas Electrodes Dust discharge Dirty gas Electrostatic Precipitator An electrostatic precipitator is a particulate collection device that removes particles from a flowing gas (such as air) using the force of an induced electrostatic charge.

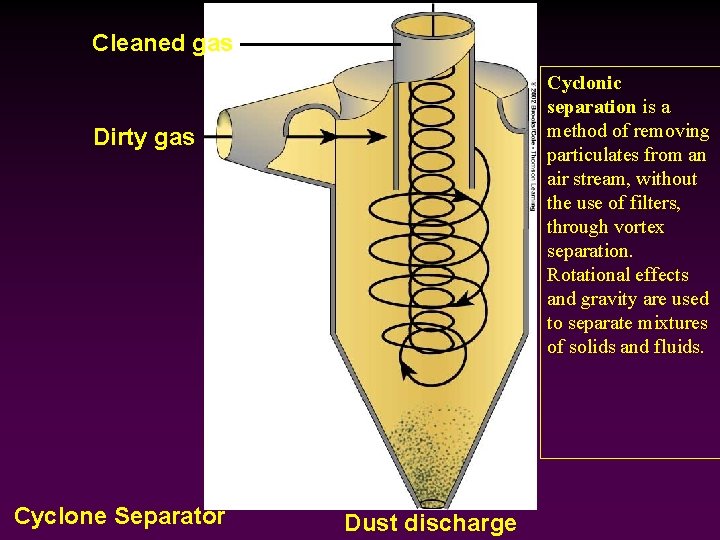
Cleaned gas Cyclonic separation is a method of removing particulates from an air stream, without the use of filters, through vortex separation. Rotational effects and gravity are used to separate mixtures of solids and fluids. Dirty gas Cyclone Separator Dust discharge

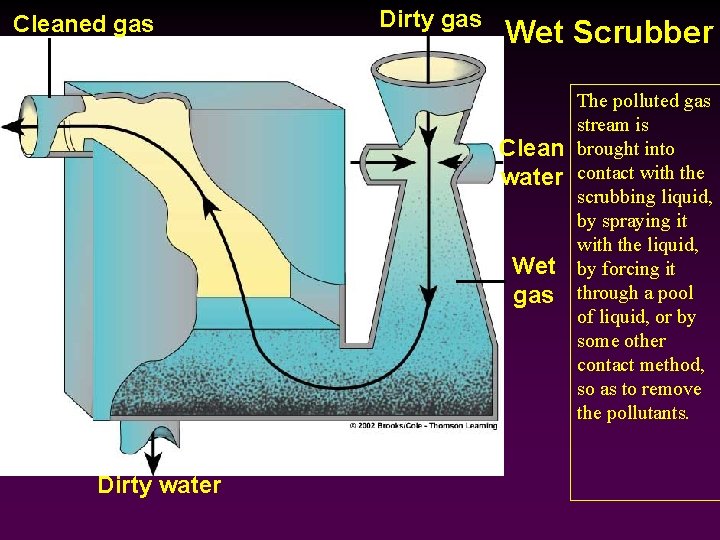
Cleaned gas Dirty gas Wet Scrubber Clean water Wet gas Dirty water The polluted gas stream is brought into contact with the scrubbing liquid, by spraying it with the liquid, by forcing it through a pool of liquid, or by some other contact method, so as to remove the pollutants.

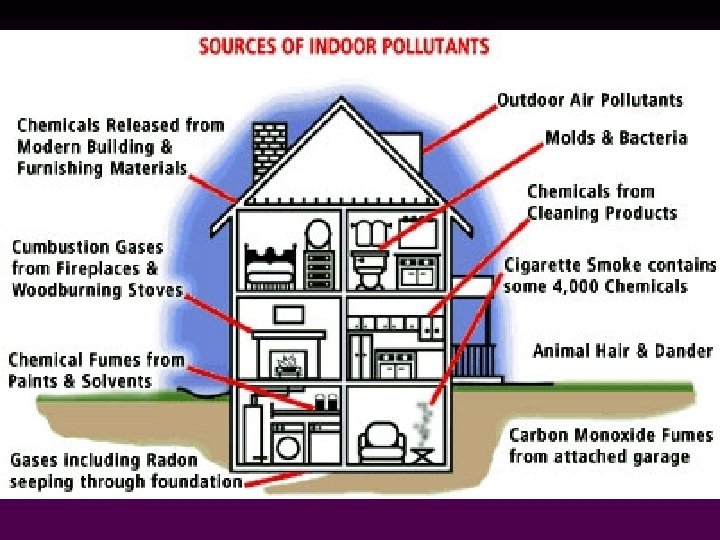
Indoor Air Pollution • Indoor air pollution can have more significant effects on human health than outdoor pollution. v People generally spend more time indoors. v Cigarette smoke is the most common indoor air pollutant in the U. S. - 430, 000 die annually from a disease related to smoking. 80

Indoor Air Pollution Cont’d • Less-developed countries also suffer from indoor air pollution. v Organic fuels (wood, etc) make up majority of household energy. v These fuels are often burned in smoky, poorly ventilated heating and cooking fires. 81

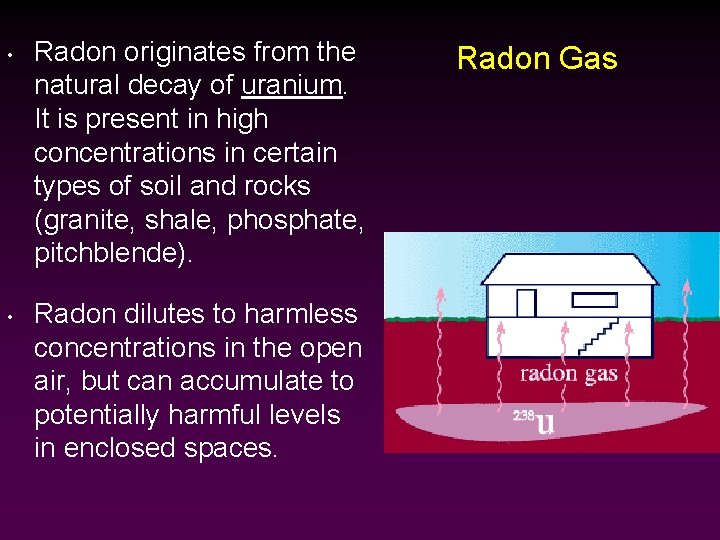
• • Radon originates from the natural decay of uranium. It is present in high concentrations in certain types of soil and rocks (granite, shale, phosphate, pitchblende). Radon dilutes to harmless concentrations in the open air, but can accumulate to potentially harmful levels in enclosed spaces. Radon Gas

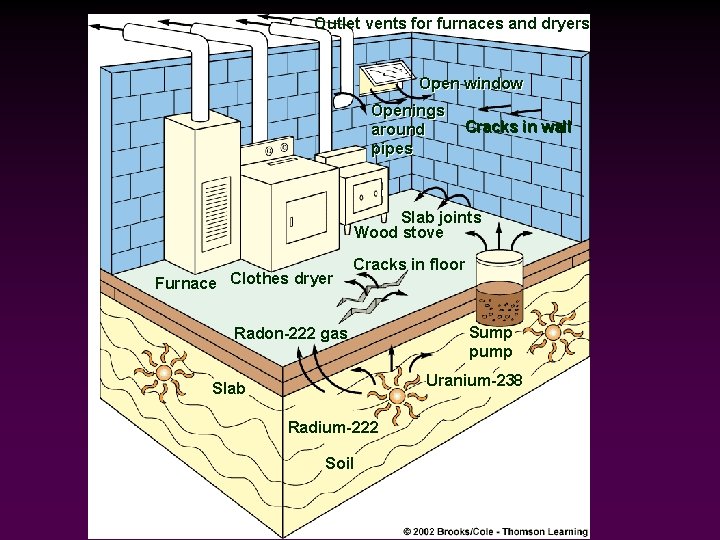
Outlet vents for furnaces and dryers Open window Openings around pipes Cracks in wall Slab joints Wood stove Furnace Clothes dryer Cracks in floor Radon-222 gas Sump pump Uranium-238 Slab Radium-222 Soil

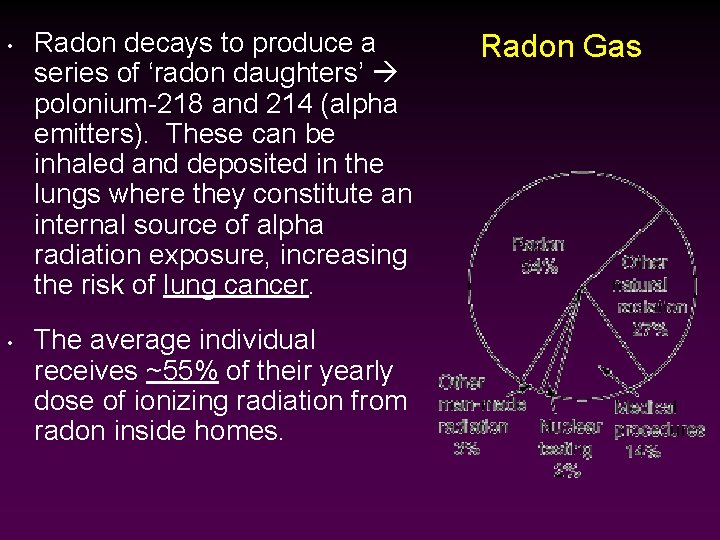
• • Radon decays to produce a series of ‘radon daughters’ polonium-218 and 214 (alpha emitters). These can be inhaled and deposited in the lungs where they constitute an internal source of alpha radiation exposure, increasing the risk of lung cancer. The average individual receives ~55% of their yearly dose of ionizing radiation from radon inside homes. Radon Gas

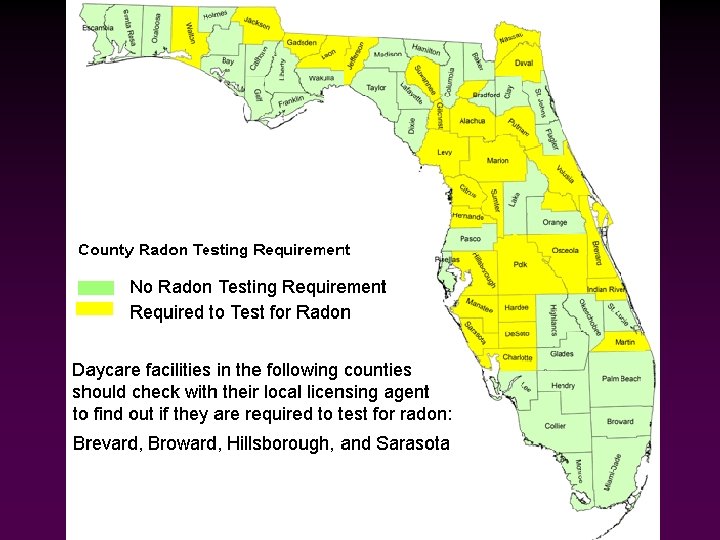
Radon Gas • • Outdoor radon concentrations measure ~0. 4 p. Ci/L, while the average indoor concentration is ~1. 3 p. Ci/L. The EPA regards 4. 0 p. Ci/L as the action level the point at which homeowners are advised to take action. The EPA estimates that 1 in 15 homes are above the action level. The EPA recommends that all homes and schools be tested for radon.


Formaldehyde • • Known to cause skin and respiratory irritation; suspected carcinogen. A variety of household products contain formaldehyde: v v v • particleboard plywood some floor coverings and textiles (draperies, permanent-press fabrics) Formaldehyde levels are likely to be high in mobile homes - residents frequently complain of rashes, reparatory irritation, nausea, headaches, dizziness, lethargy

Biological Pollutants • • Bacteria and fungal spores can enter structures via air handling systems and occasionally cause disease outbreaks (Legionnaires’ Disease episode in Philadelphia in 1976). Many allergies are associated with exposure to household dust which may contain fungal spores, bacteria, animal dander, and feces of roaches or mites.

Biological Pollutants • • Perhaps the most significant in stimulating allergic reactions are live dust mites found in enormous numbers on bedsheets and blankets where they feed on sloughed-off scales of skin. Humid conditions stimulate dust mite populations.

Asbestos • • About 2/3 of the asbestos used in the US is used in building materials, brake linings, textiles, and insulation. It was also used in paints, plastics, caulking compounds, floor tiles, cement, roofing paper, radiator covers, filters in gas masks, conveyor belts, potholders, ironing board covers, theater curtains, fake fireplace ash, etc.

Asbestos-Related Diseases • Asbestosis: v v a chronic disease characterized by scarring of the lungs. irreversible, progressively worsening disease. it takes 20 years or more for symptoms to appear. synergistic effects of cigarette smoking

Asbestos-Related Diseases • Lung cancer: v v v leading cause of asbestos related death 20 -25% of asbestos workers die of lung cancer (compared to 4 -5% of the general population) synergistic effects of cigarette smoking

Asbestos-Related Diseases • Mesothelioma: v v v rare cancer of the lung or stomach lining kills 5% of all asbestos workers symptoms appear 25 -40 years after initial exposure to low levels of asbestos can result in mesothelioma (some children of asbestos workers eventually developed mesothelioma after they were exposed to their father’s work clothes) the only known causative agent of mesothelioma is exposure to asbestos (marker disease) death generally occurs within 2 years of diagnosis.

Asbestos-Related Diseases • Gastroinstestinal cancers: v cancers of the colon, rectum, esophagus, and stomach occurs at higher rate among asbestos workers than in the general population

Asbestos • The risk of asbestos-related disease depends on: v v v level and duration of exposure time since exposure occurred age at which exposure occurred personal history of cigarette smoking type and size of asbestos fibers

Asbestos Problems in Public Buildings • • • During the 1946 -73, asbestos-containing fireproofing materials were extensively used in constructing or renovating schools throughout the country. In 1973 the EPA banned all spray applications of asbestos in insulating and fireproofing materials. In 1986, President Reagan signed into law the ‘Asbestos Hazard Emergency Response Act’ (AHERA), requiring that all schools be inspected for the presence of asbestos; if asbestos was found, the school district must file and carry out an asbestos abatement plan.

Asbestos abatement options • • • Encapsulation - asbestos is coated with a polymer sealant Enclosure - building a nonpermeable barrier Removal

Gaither High School Asbestos Abatement Plan In accordance with 40 CFR Part 763. 84 (c) and Subpart E, Asbestos Hazard Emergency Response Act (AHERA) , We would like to notify you of the availability of the Asbestos Management Plan for Gaither High School. Management plans for Gaither High School are available during normal business hours for review in the main office, in the office of The Assistant Principal For Administration. Copies of the plan can be made for a nominal price per copy. The plan is also available for review by employees; without cost or restriction, before work begins in any area of the school or building. Information provided in the management plan includes the recent facility survey, response actions planned or taken, and post periodic surveillance and re-inspections activities.


Sick-Building Syndrome (SBS) • • • Building occupants experience acute discomfort (eye irritation, scratchiness of the throat, dry cough, headache, itchy skin, fatigue, difficulty in concentrating, nausea, and dizziness) but no causative agent can be found. These symptoms generally vanish soon after sufferers leave the building. Sick-building syndrome is most often traced to poor ventilation. Energy efficient buildings tend to be poorly ventilated.


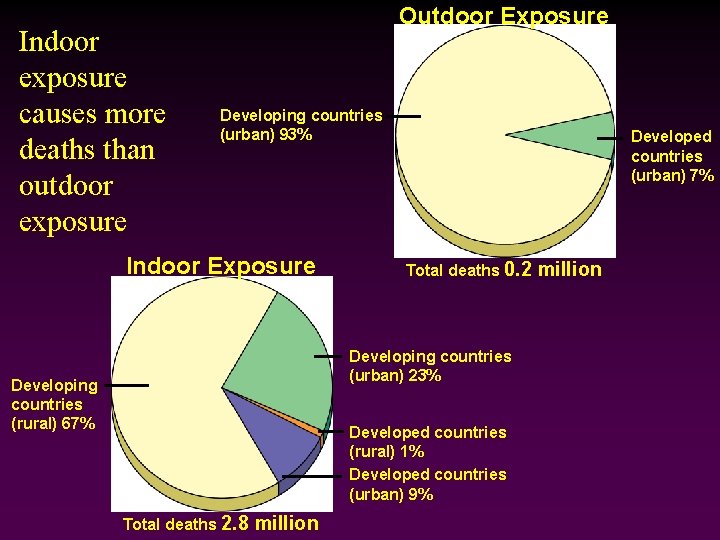
Indoor exposure causes more deaths than outdoor exposure Outdoor Exposure Developing countries (urban) 93% Indoor Exposure Developed countries (urban) 7% Total deaths 0. 2 Developing countries (urban) 23% Developing countries (rural) 67% Developed countries (rural) 1% Developed countries (urban) 9% Total deaths 2. 8 million

EFFECTS OF AIR POLLUTION • Human Health v EPA estimates each year 50, 000 people die prematurely from illnesses related to air pollution. - Likelihood of suffering ill health is related to intensity and duration of exposure. Ø Inhalation is the most common route, but absorption through the skin and consumption via food can also occur. 103