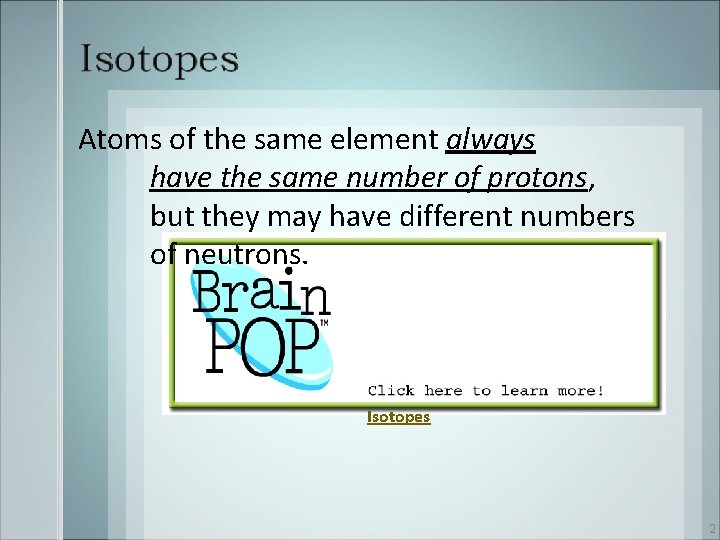
1 Atoms of the same element always have

























![The p. H Scale p. OH scale p. OH = log [OH ] p. The p. H Scale p. OH scale p. OH = log [OH ] p.](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-50.jpg)
![Practice Problem Calculate p. H for a solution that has [H+] = 1. 0 Practice Problem Calculate p. H for a solution that has [H+] = 1. 0](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-51.jpg)
![Practice Problem #2 Calculate p. H for a solution that has [OH-] = 1. Practice Problem #2 Calculate p. H for a solution that has [OH-] = 1.](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-52.jpg)
![Practice Problem #3 Calculate [OH-] for a solution that has a p. H = Practice Problem #3 Calculate [OH-] for a solution that has a p. H =](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-53.jpg)


- Slides: 58

1

Atoms of the same element always have the same number of protons, but they may have different numbers of neutrons. Isotopes 2

3

• The average atomic mass of an element is the weighted average mass of the mixture of an element’s isotopes. 4

Energy from Nuclear Reactions E=mc 2 Energy produced from the Nuclear Reaction Difference in mass between products and reactants Very Large Constant (Speed of Light)2 Because the constant is so large, tiny amounts of mass produce tons of energy Where does the energy come from ? In chemical reactions the energy comes from the bonds, In nuclear reactions the energy comes from the conversion of mass to energy 5

Detection of Radioactivity and the Concept of Half-life • 6 Half-life – time required for half of the original sample of radioactive nuclides to decay

Half-Life Practice l 7 Calculate how long it would take for 120 g of Radioactive Carbon-14 to decay to 15. 0 g if the half-life of Carbon-14 is 1 week (7 days).

Decay Practice 8

Alpha Decay Pra 9

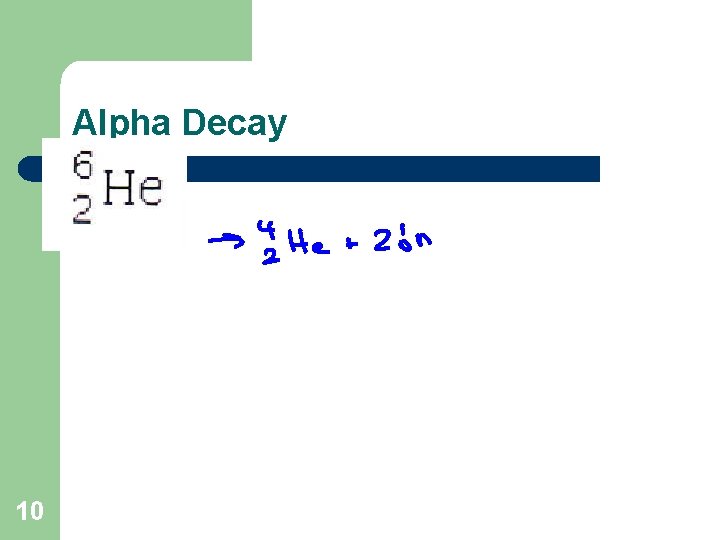
Alpha Decay 10

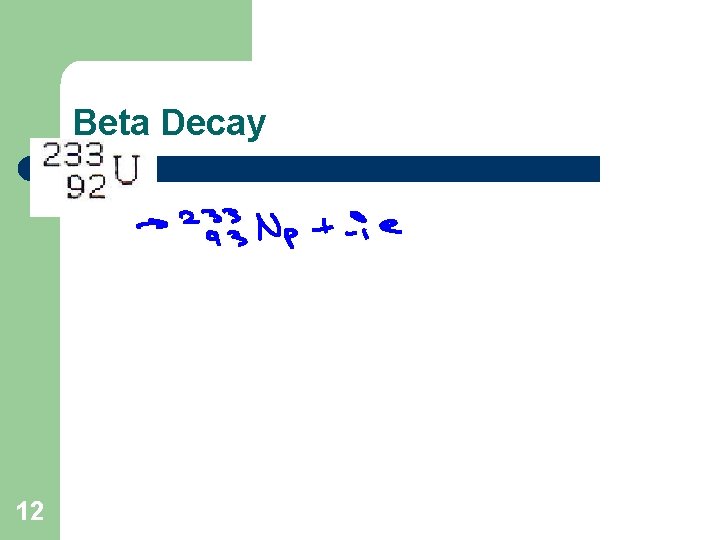
Beta Decay 11

Beta Decay 12

l Which has the highest energy? – 13 Alpha, Beta, Gamma

l 14 Draw a Gamma particle.

Beta Fusion 0/-1 e + 232/91 Pa 15

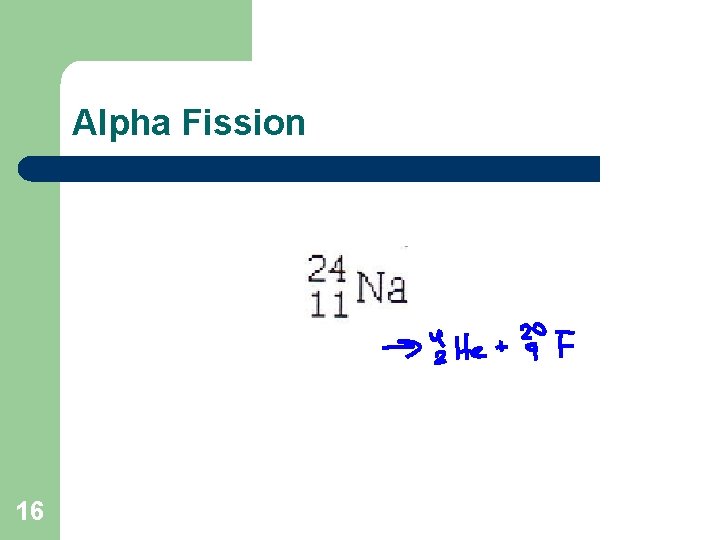
Alpha Fission 16

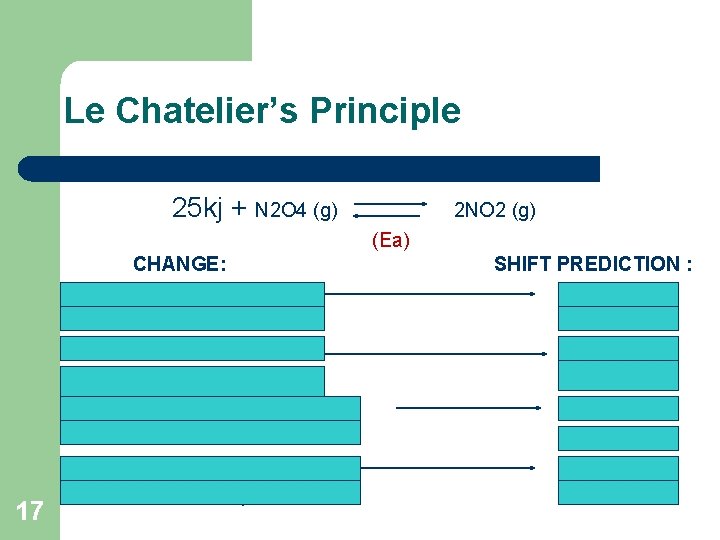
Le Chatelier’s Principle 25 kj + N 2 O 4 (g) 2 NO 2 (g) 1. 2. 3. 4. 5. 6. 7. 17 8. (Ea) CHANGE: Addition of N 2 O 4 Addition of NO 2 Removal of N 2 O 4 Removal of NO 2 Decrease of container volume Increase of temperature Decrease of temperature SHIFT PREDICTION : Right Left Right Left Right Left

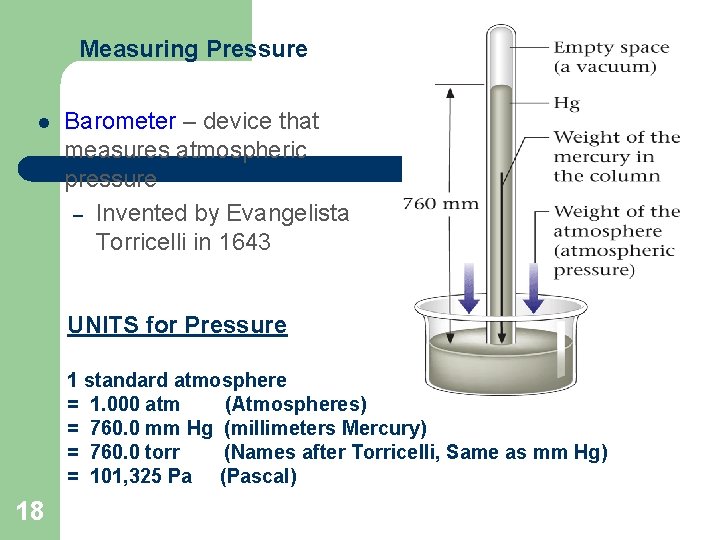
Measuring Pressure l Barometer – device that measures atmospheric pressure – Invented by Evangelista Torricelli in 1643 UNITS for Pressure 1 standard atmosphere = 1. 000 atm (Atmospheres) = 760. 0 mm Hg (millimeters Mercury) = 760. 0 torr (Names after Torricelli, Same as mm Hg) = 101, 325 Pa (Pascal) 18

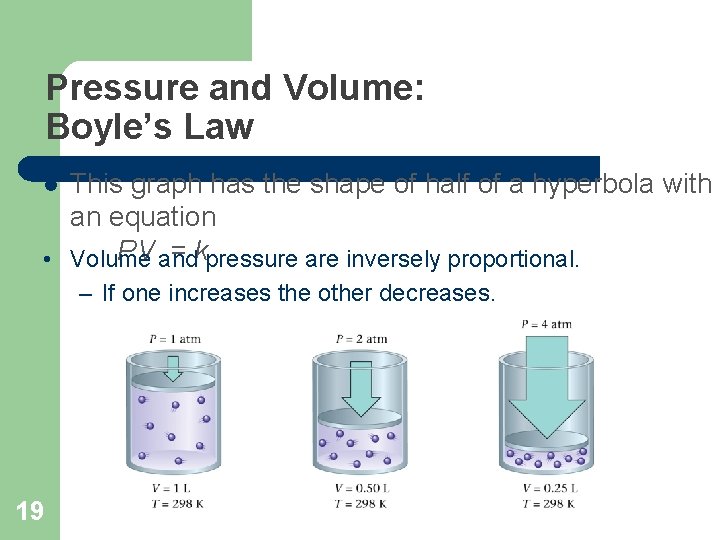
Pressure and Volume: Boyle’s Law This graph has the shape of half of a hyperbola with an equation = k • PV Volume and pressure are inversely proportional. l – If one increases the other decreases. 19

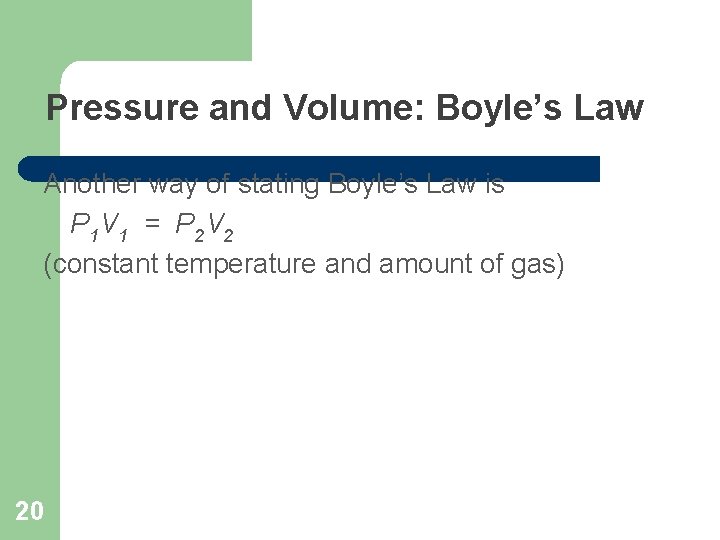
Pressure and Volume: Boyle’s Law Another way of stating Boyle’s Law is P 1 V 1 = P 2 V 2 (constant temperature and amount of gas) 20

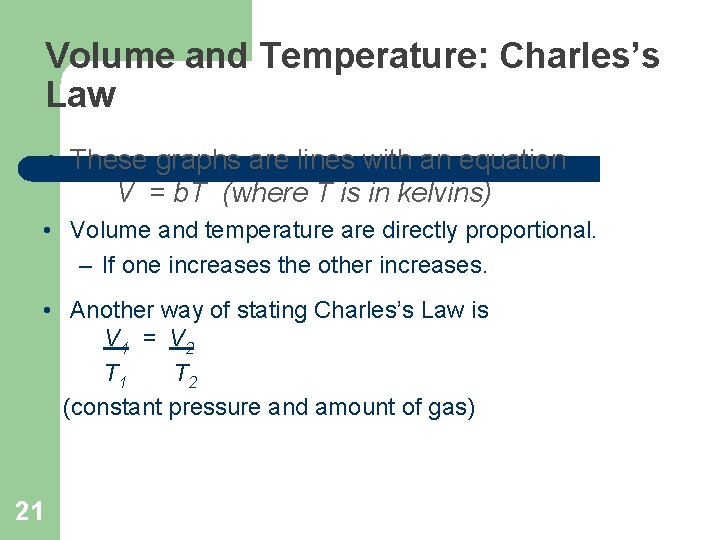
Volume and Temperature: Charles’s Law l These graphs are lines with an equation V = b. T (where T is in kelvins) • Volume and temperature are directly proportional. – If one increases the other increases. • Another way of stating Charles’s Law is V 1 = V 2 T 1 T 2 (constant pressure and amount of gas) 21

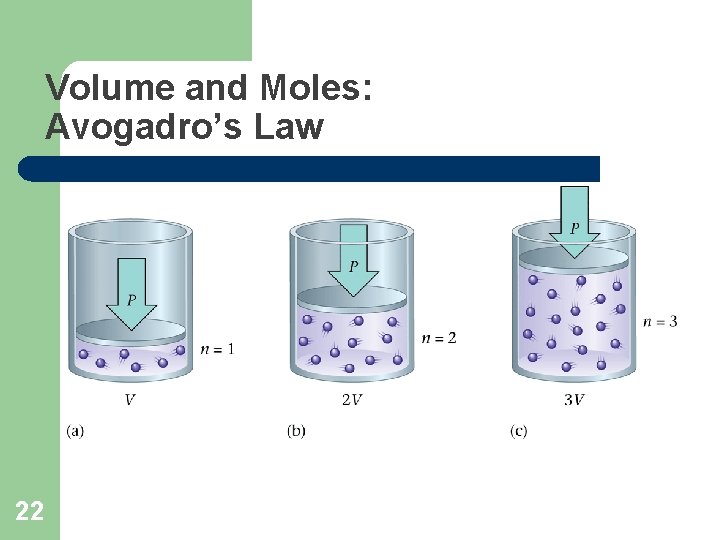
Volume and Moles: Avogadro’s Law 22

Volume and Moles: Avogadro’s Law • Volume and moles are directly proportional. – If one increases the other increases. – V = an – constant temperature and pressure • Another way of stating Avogadro’s Law is V 1 = V 2 n 1 n 2 (constant temperature and pressure) 23

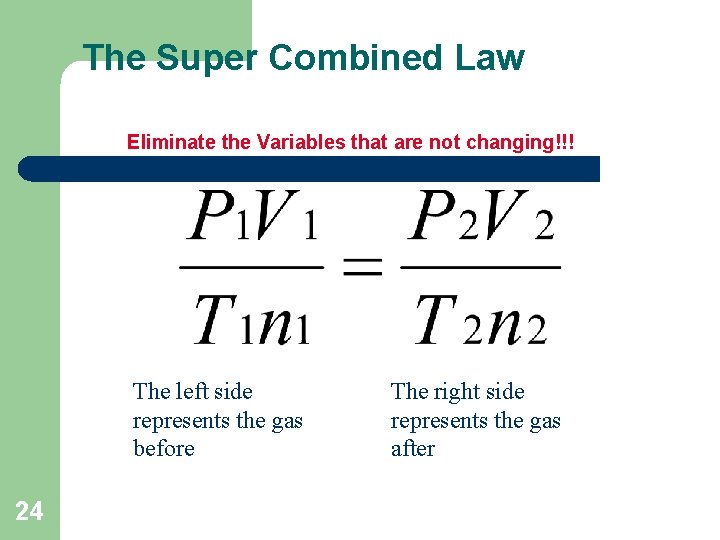
The Super Combined Law Eliminate the Variables that are not changing!!! The left side represents the gas before 24 The right side represents the gas after

We can ignore any part of the equation that remains constant, so If temperature is constant: Boyle’s Law If pressure is constant: Charles’s Law And if volume is constant: 25

1. A gas occupies 12. 3 liters at a pressure of 40. 0 mm. Hg. What is the volume when the pressure is increased to 60. 0 mm. Hg? 26

2. Calculate the decrease in temperature when 2. 00 L at 20. 0 °C is compressed to 1. 00 L. 27

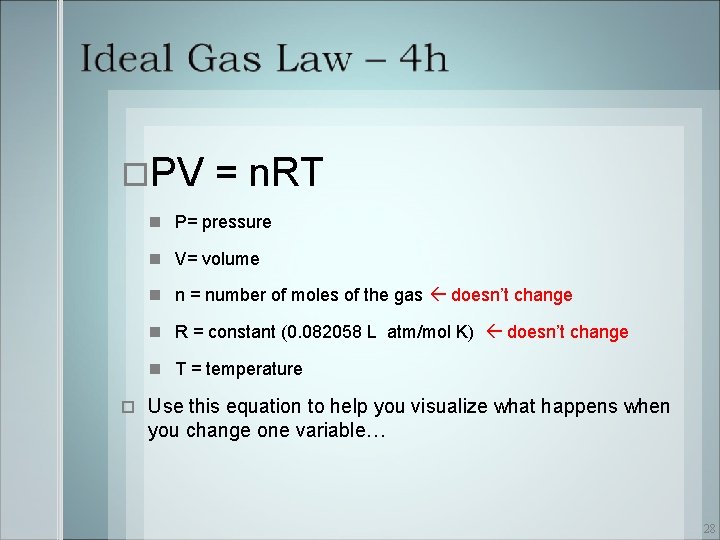
PV = n. RT P= pressure V= volume n = number of moles of the gas doesn’t change R = constant (0. 082058 L atm/mol K) doesn’t change T = temperature Use this equation to help you visualize what happens when you change one variable… 28

Pressure increases? P V = n. RT P V = n. R T or P V = n. R T Temperature must increase or volume decrease (or both) to keep the equation balanced! Temperature decreases? PV = n. RT or PV Pressure and/or volume will decrease. = n. RT 29

Dalton’s Law of Partial Pressures The pressure of the gas is affected by the number of particles. • The pressure is independent of the nature of the particles. 30

The Kinetic Molecular Theory of Gases - describes motion of ideal gases 31

Real Gases At high pressure the volume is decreased Molecule volumes become important Attractions become important 32

Practice At 27. 00 °C a gas has a volume of 6. 00 L. What will the volume be at 327. 0 °C? 33

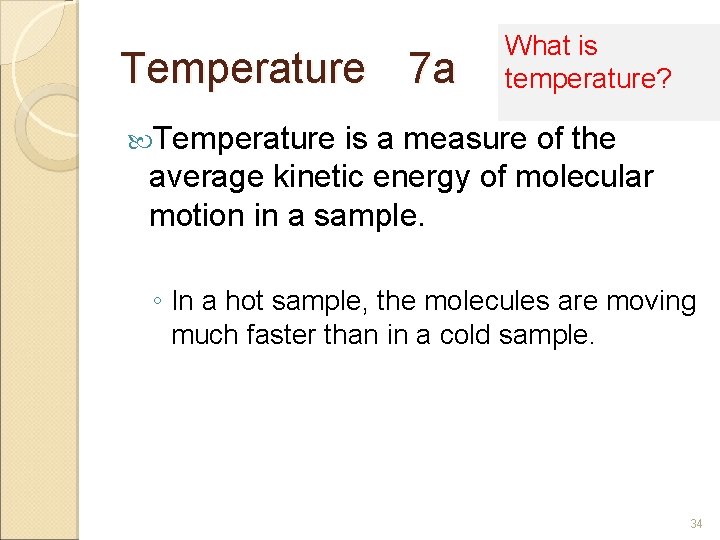
Temperature 7 a What is temperature? Temperature is a measure of the average kinetic energy of molecular motion in a sample. ◦ In a hot sample, the molecules are moving much faster than in a cold sample. 34

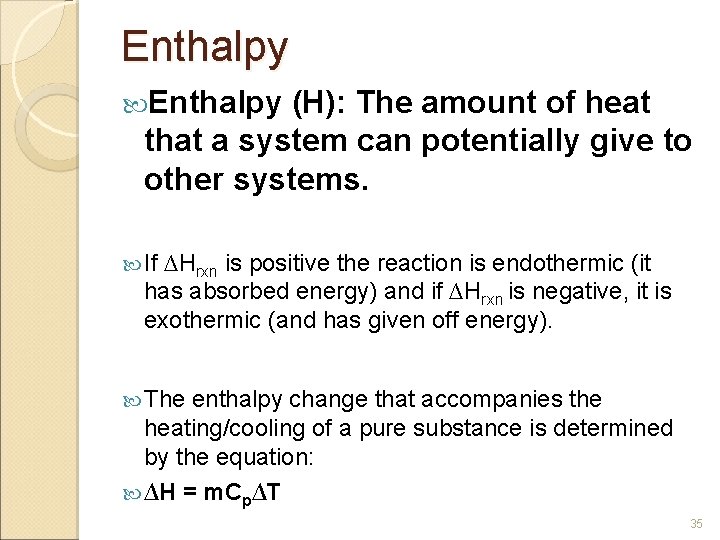
Enthalpy (H): The amount of heat that a system can potentially give to other systems. If ∆Hrxn is positive the reaction is endothermic (it has absorbed energy) and if ∆Hrxn is negative, it is exothermic (and has given off energy). The enthalpy change that accompanies the heating/cooling of a pure substance is determined by the equation: ∆H = m. Cp∆T 35

ΔH – Heat of Reaction(ENTHALPY) Endothermic ◦ The products have more energy than the reactants. ◦ Heat must be put into the reaction, so ΔH is positive. 36

Exothermic ◦ The products have less energy than the reactants. ◦ Heat must be released from the reaction, so ΔH is negative. 37

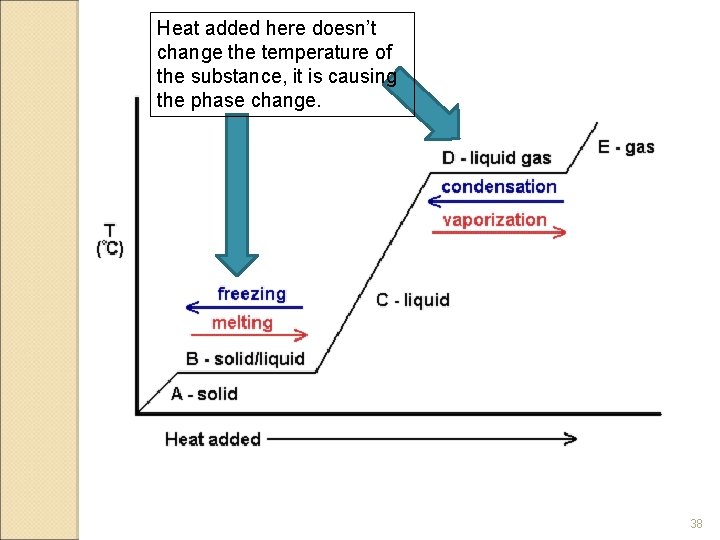
Heat added here doesn’t change the temperature of the substance, it is causing the phase change. 38

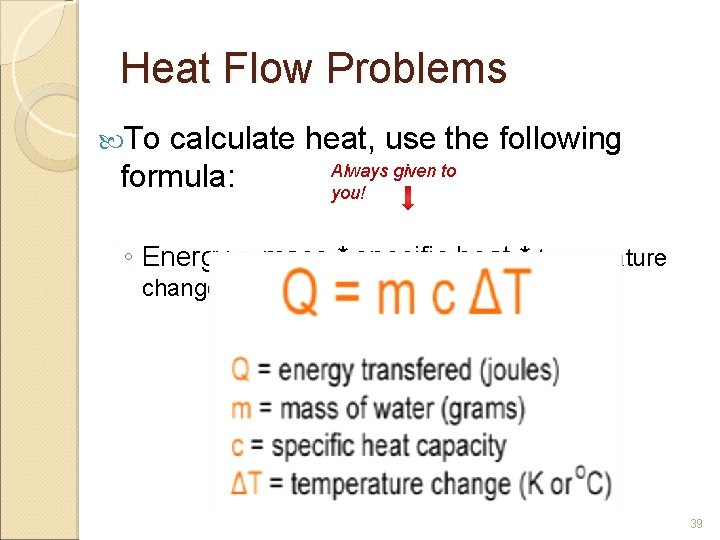
Heat Flow Problems To calculate heat, use the following formula: Always given to you! ◦ Energy = mass * specific heat * temperature change 39

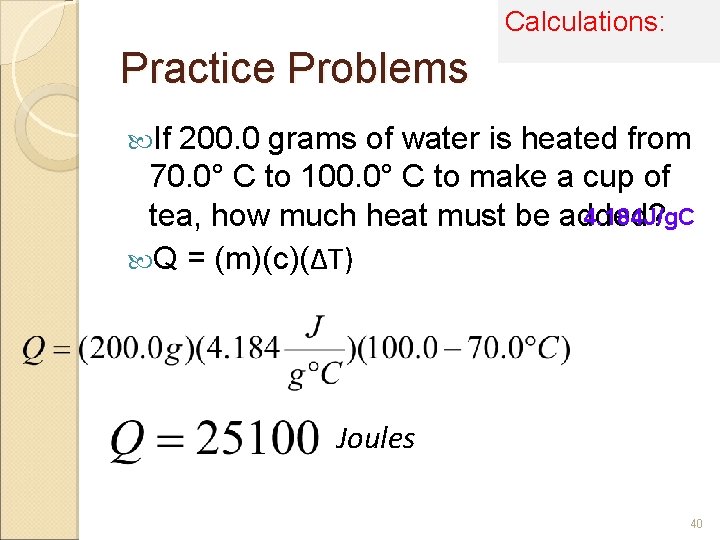
Calculations: Practice Problems If 200. 0 grams of water is heated from 70. 0° C to 100. 0° C to make a cup of 4. 184 J/g. C tea, how much heat must be added? Q = (m)(c)(ΔT) Joules 40

Energy as a Driving Force Entropy, S – function which keeps track of the tendency for the components of the universe to become disordered 41

Entropy is a thermodynamic measure of the randomness in the universe. Phase changes that result in greater molecular freedom have a positive ∆S, and those that result in less molecular freedom have a negative ∆S. Which one favors more Randomness? Example: For melting, ∆S = +, for freezing ∆S 42

Energy as a Driving Force Second law of thermodynamics – The entropy of the universe is always increasing. 43

Entropy Practice Which has a +∆S? ◦ Water Vapor Condensing or water vaporizing �Water Vaporizing – Forming a gas increases randomness Which has a -∆S? ◦ Ice melting or water freezing �Water Freezing – Solids do not move therefore there is a decrease in randomness of the particles 44

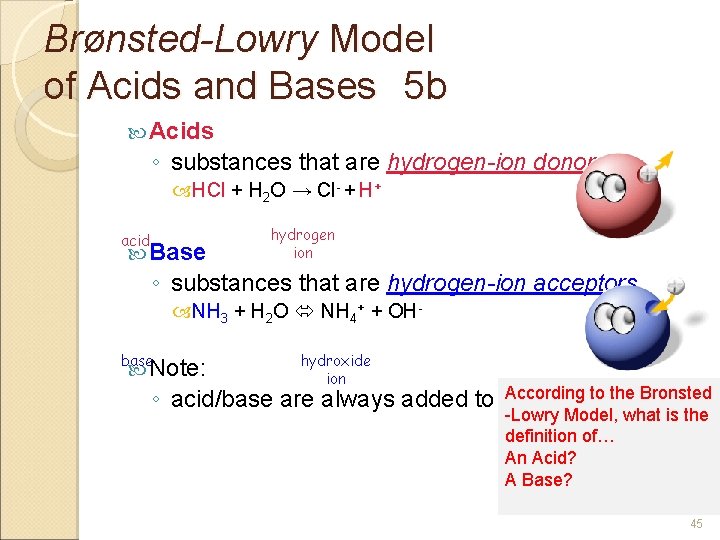
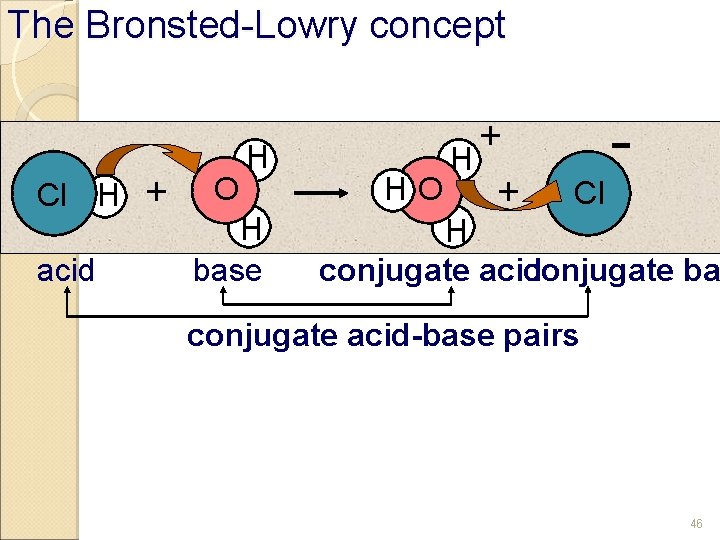
BrØnsted-Lowry Model of Acids and Bases 5 b Acids ◦ substances that are hydrogen-ion donors. HCl + H 2 O → Cl- + H+ acid Base hydrogen ion ◦ substances that are hydrogen-ion acceptors. NH 3 + H 2 O NH 4+ + OHbase Note: hydroxide ion According to the Bronsted ◦ acid/base are always added to water -Lowry Model, what is the definition of… An Acid? A Base? 45

The Bronsted-Lowry concept Cl H acid + O H H base + H HO + Cl H conjugate acid conjugate ba conjugate acid-base pairs 46

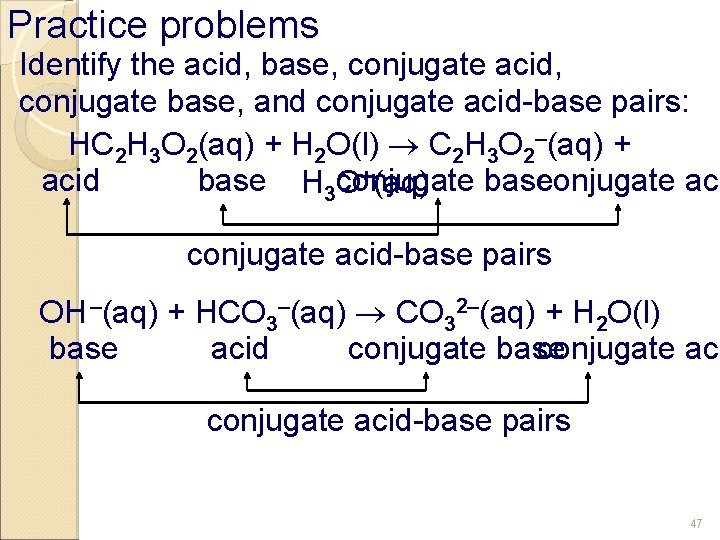
Practice problems Identify the acid, base, conjugate acid, conjugate base, and conjugate acid-base pairs: HC 2 H 3 O 2(aq) + H 2 O(l) C 2 H 3 O 2–(aq) + +(aq) conjugate base conjugate acid base H 3 O conjugate acid-base pairs OH –(aq) + HCO 3–(aq) CO 32–(aq) + H 2 O(l) base acid conjugate base conjugate acid-base pairs 47

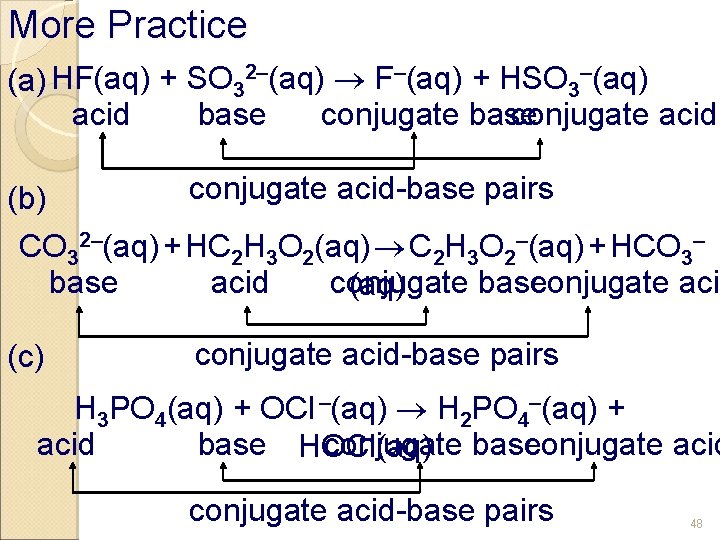
More Practice (a) HF(aq) + SO 32–(aq) F–(aq) + HSO 3–(aq) conjugate base conjugate acid-base pairs (b) CO 32–(aq) + HC 2 H 3 O 2(aq) C 2 H 3 O 2–(aq) + HCO 3– base acid conjugate base conjugate acid (aq) (c) conjugate acid-base pairs H 3 PO 4(aq) + OCl –(aq) H 2 PO 4–(aq) + conjugate base conjugate acid base HOCl(aq) conjugate acid-base pairs 48

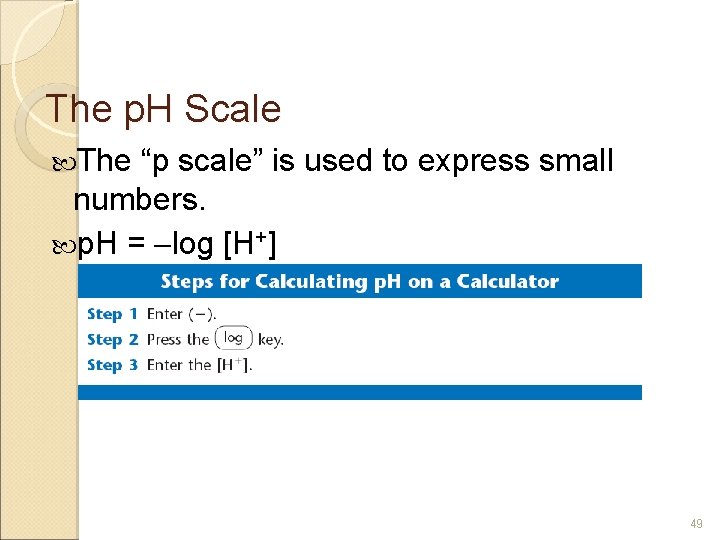
The p. H Scale The “p scale” is used to express small numbers. p. H = log [H+] 49
![The p H Scale p OH scale p OH log OH p The p. H Scale p. OH scale p. OH = log [OH ] p.](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-50.jpg)
The p. H Scale p. OH scale p. OH = log [OH ] p. H + p. OH = 14. 00 50
![Practice Problem Calculate p H for a solution that has H 1 0 Practice Problem Calculate p. H for a solution that has [H+] = 1. 0](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-51.jpg)
Practice Problem Calculate p. H for a solution that has [H+] = 1. 0 x 10 -9 M Step 1) [H+] = 1. 0 x 10 -9 M Step 2) -log (1. 0 x 10 -9 )= 9. 00 Step 3) p. H = 9. 00 51
![Practice Problem 2 Calculate p H for a solution that has OH 1 Practice Problem #2 Calculate p. H for a solution that has [OH-] = 1.](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-52.jpg)
Practice Problem #2 Calculate p. H for a solution that has [OH-] = 1. 0 x 10 -6 M. 1) Kw = [H+] [OH-] = 1. 0 x 10 -14 2) [H+] = 1. 0 x 10 -14 / [OH-] 3) [H+] = 1. 0 x 10 -14 / 1. 0 x 10 -6 = 1. 0 x 10 -8 M 4) -log 1. 0 x 10 -8 = 8. 00 5) p. H = 8. 00 52
![Practice Problem 3 Calculate OH for a solution that has a p H Practice Problem #3 Calculate [OH-] for a solution that has a p. H =](https://slidetodoc.com/presentation_image_h/ad5b8959850bd31d335cc0242fb2d59f/image-53.jpg)
Practice Problem #3 Calculate [OH-] for a solution that has a p. H = 6. 20 [OH-] = 1. 6 x 10 -8 53

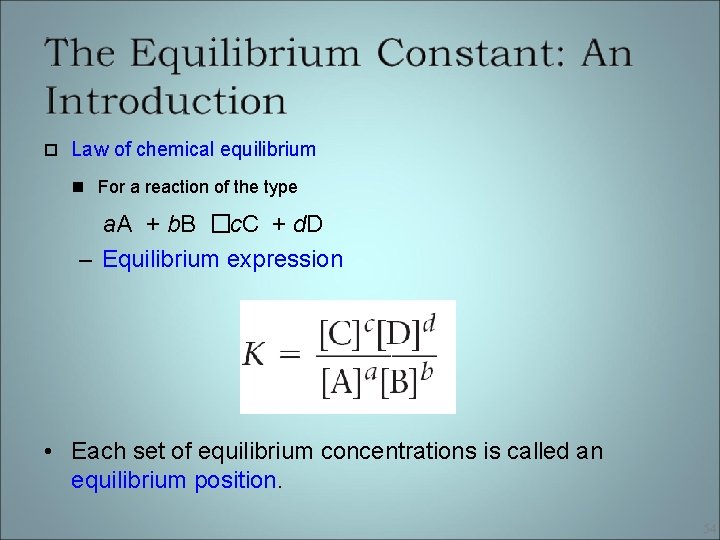
Law of chemical equilibrium For a reaction of the type a. A + b. B � c. C + d. D – Equilibrium expression • Each set of equilibrium concentrations is called an equilibrium position. 54

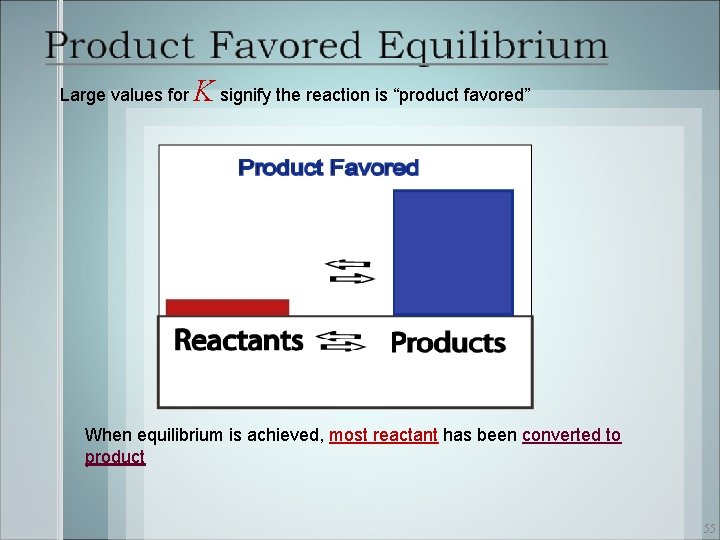
Large values for K signify the reaction is “product favored” When equilibrium is achieved, most reactant has been converted to product 55

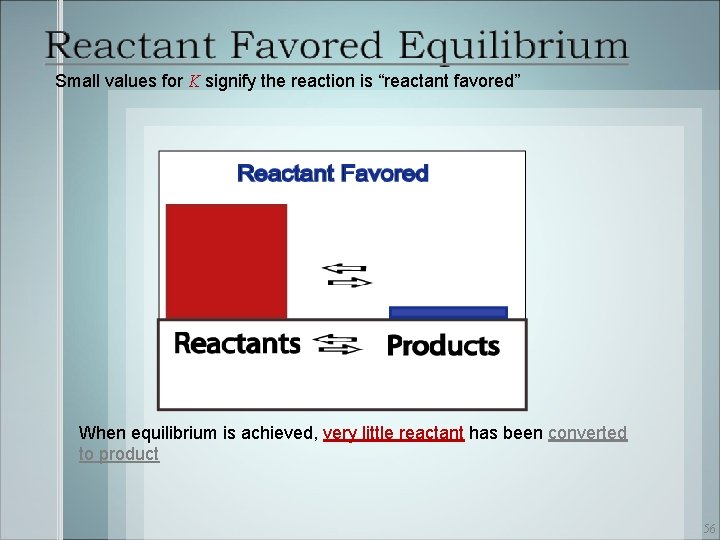
Small values for K signify the reaction is “reactant favored” When equilibrium is achieved, very little reactant has been converted to product 56

For the reaction: For the reaction 2 A + 3 B 4 C + 2 D Where K is the equilibrium constant, and is unitless 57

The Meaning of K – K > 1 the equilibrium position is far to the right – K < 1 the equilibrium position is far to the left 58