Chapter 4 Isotopes Atoms of the same element





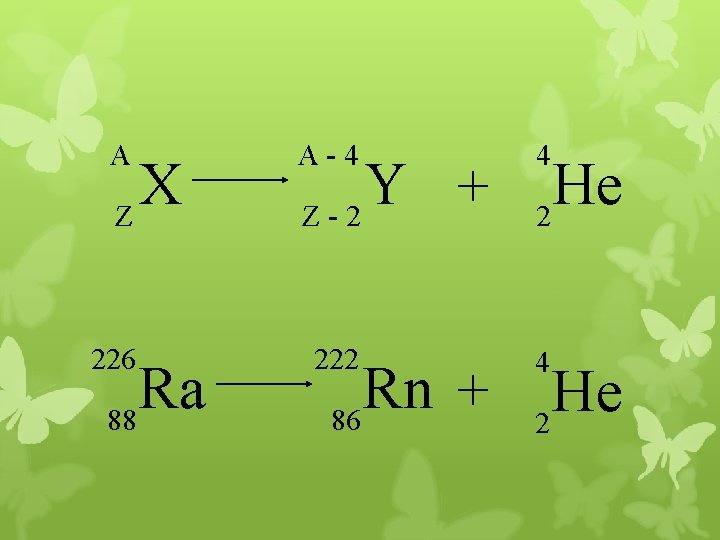
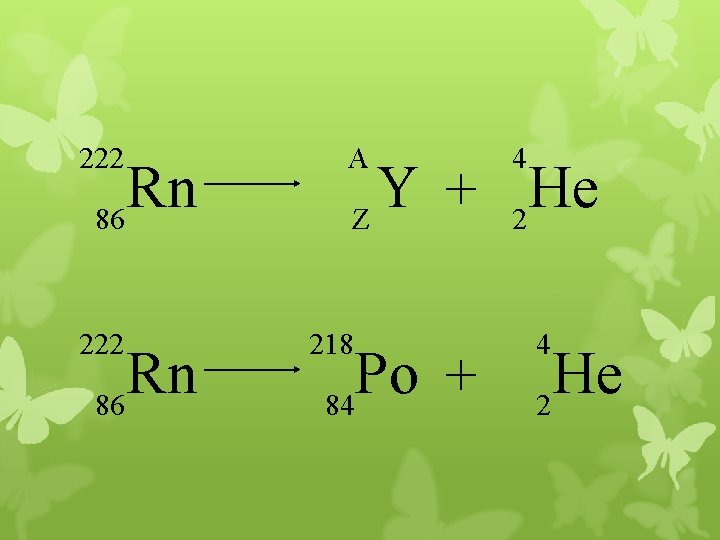
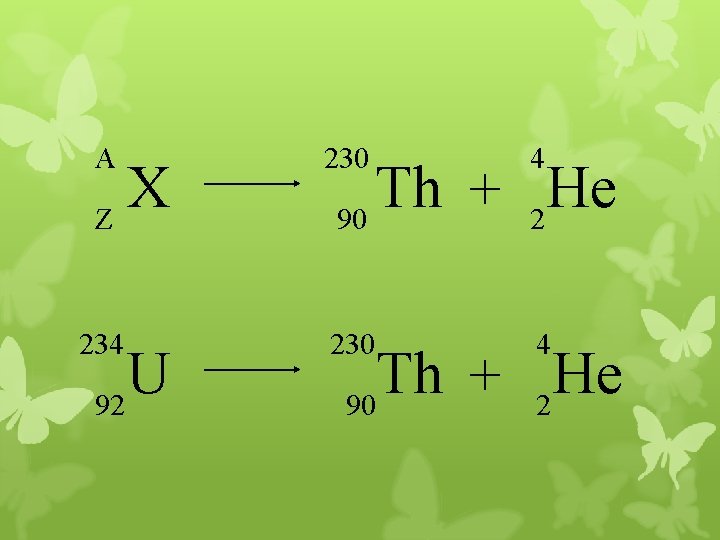
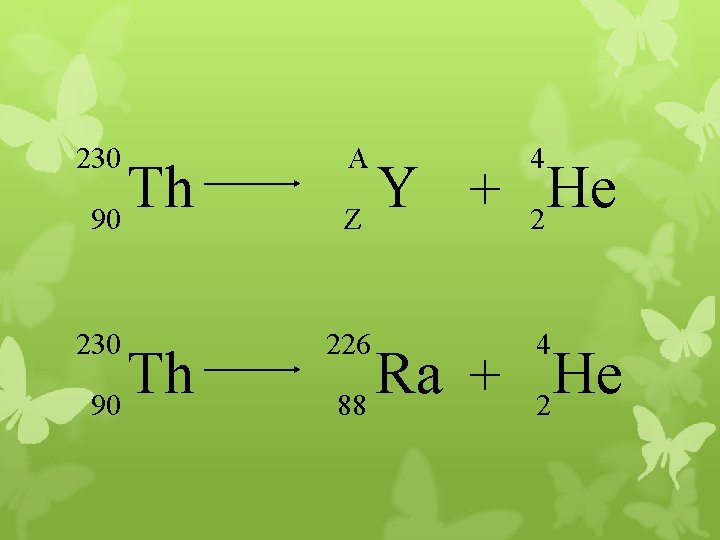
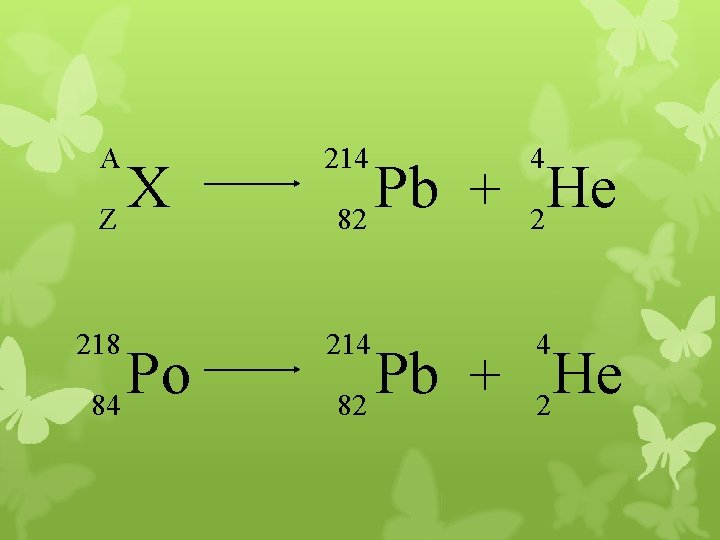
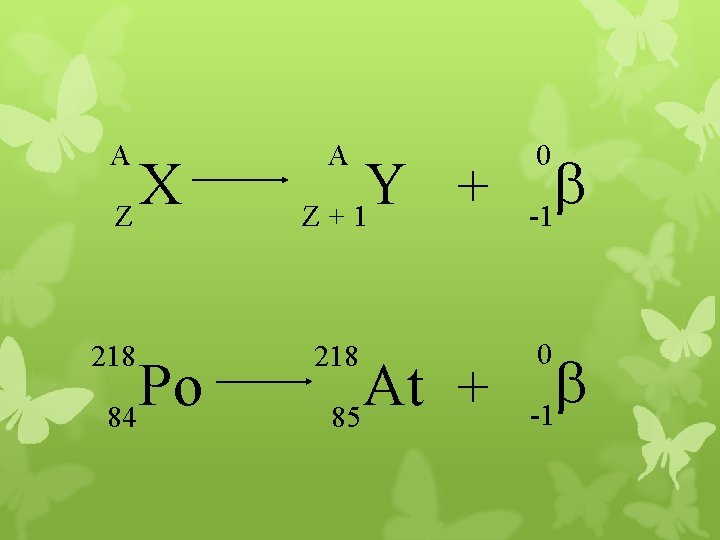
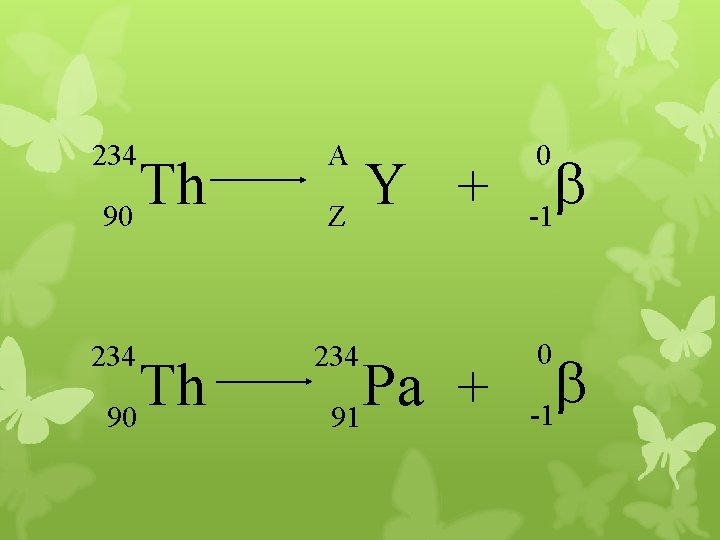
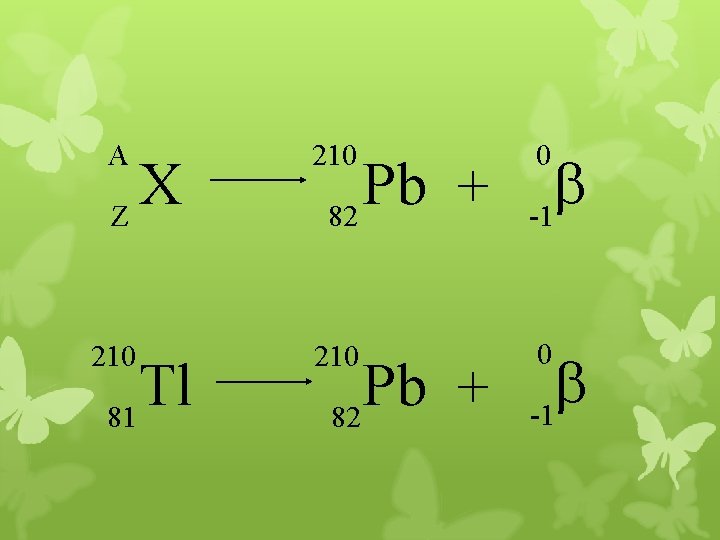
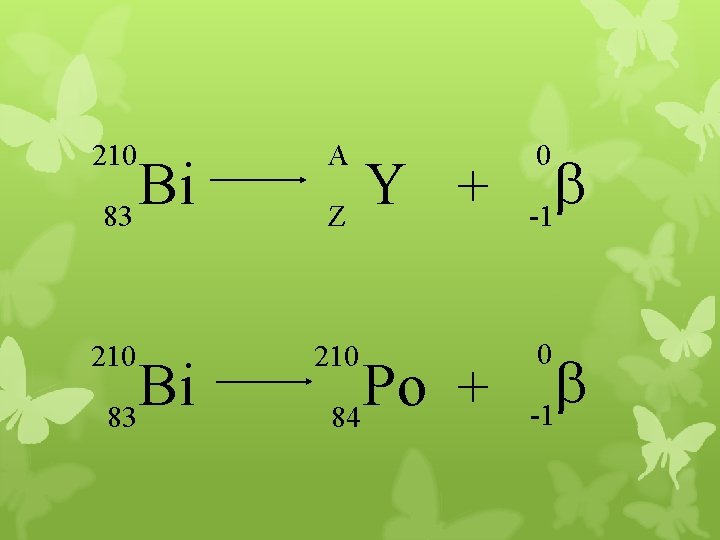
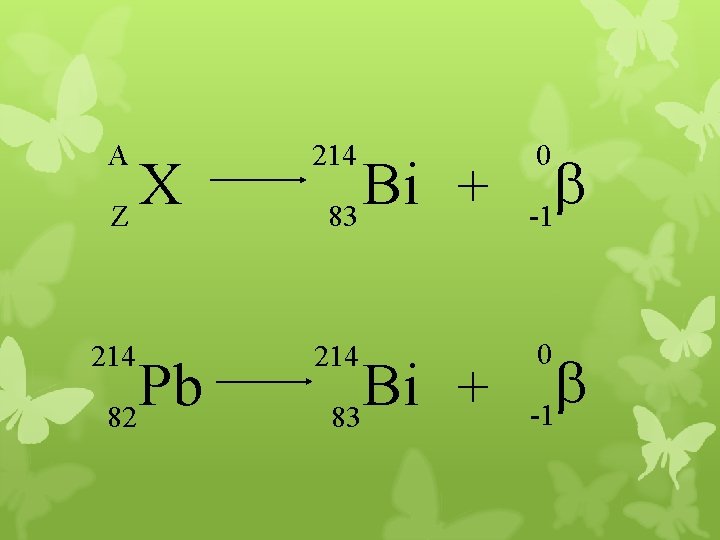






- Slides: 33

Chapter 4

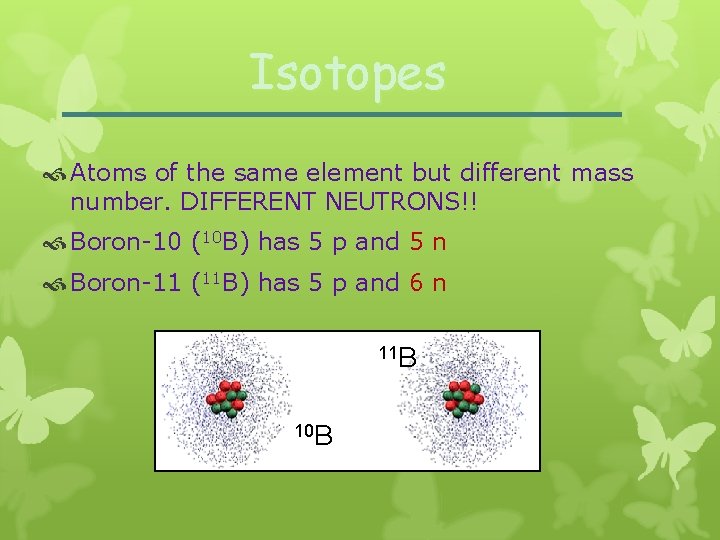
Isotopes Atoms of the same element but different mass number. DIFFERENT NEUTRONS!! Boron-10 (10 B) has 5 p and 5 n Boron-11 (11 B) has 5 p and 6 n 11 B 10 B

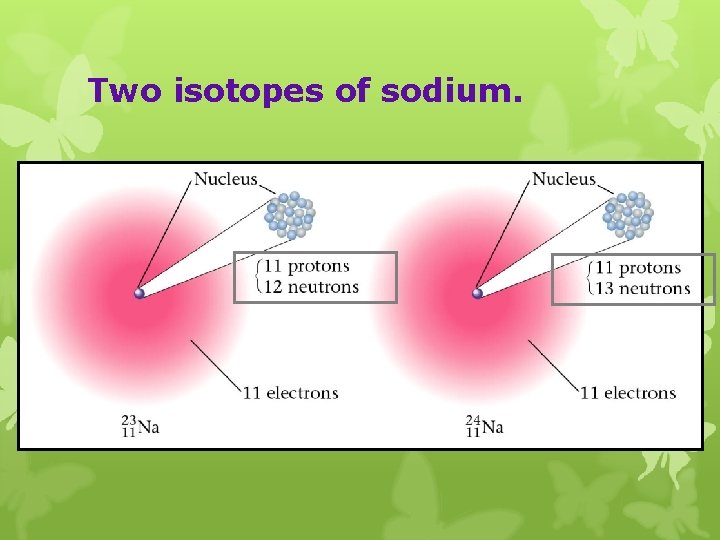
Two isotopes of sodium.

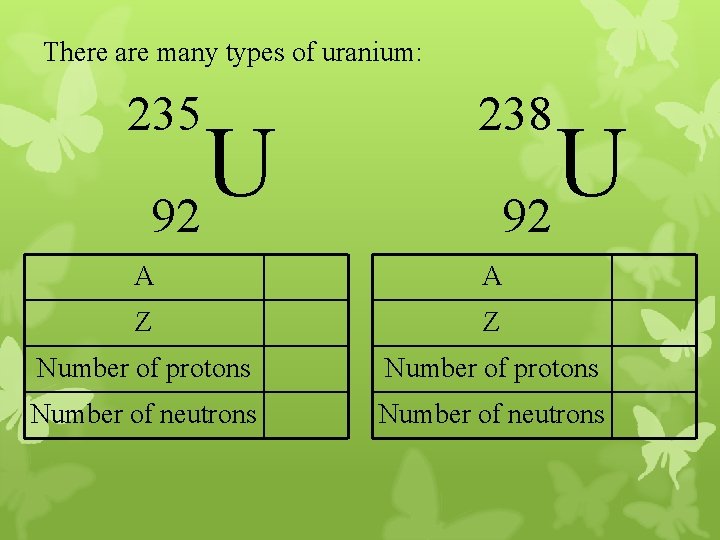
There are many types of uranium: 235 238 A A Z Z Number of protons Number of neutrons U 92

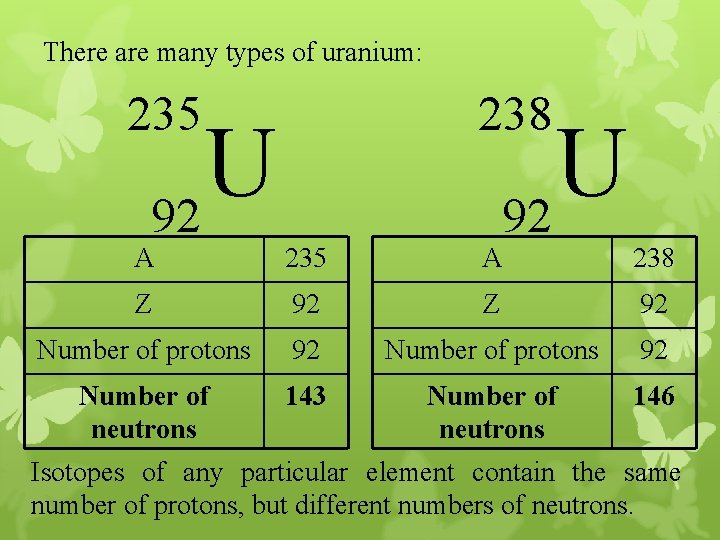
There are many types of uranium: 238 235 U 92 A 235 A 238 Z 92 Number of protons 92 Number of neutrons 143 Number of neutrons 146 Isotopes of any particular element contain the same number of protons, but different numbers of neutrons.

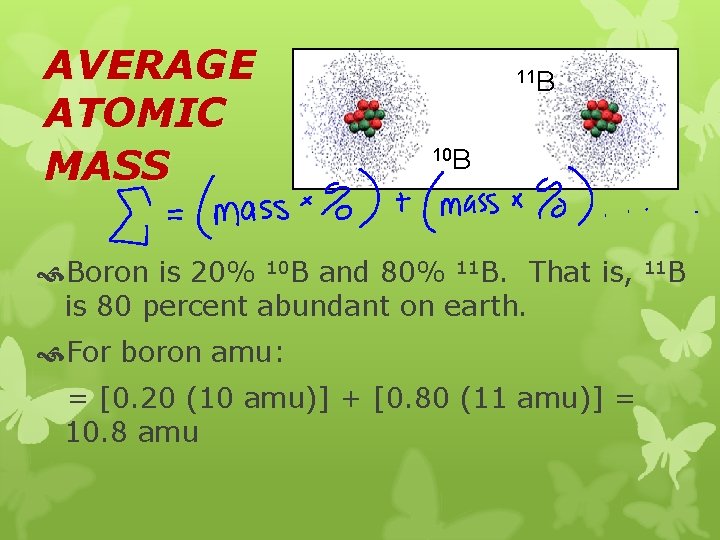
AVERAGE ATOMIC MASS 11 B 10 B Boron is 20% 10 B and 80% 11 B. That is, is 80 percent abundant on earth. For boron amu: = [0. 20 (10 amu)] + [0. 80 (11 amu)] = 10. 8 amu 11 B

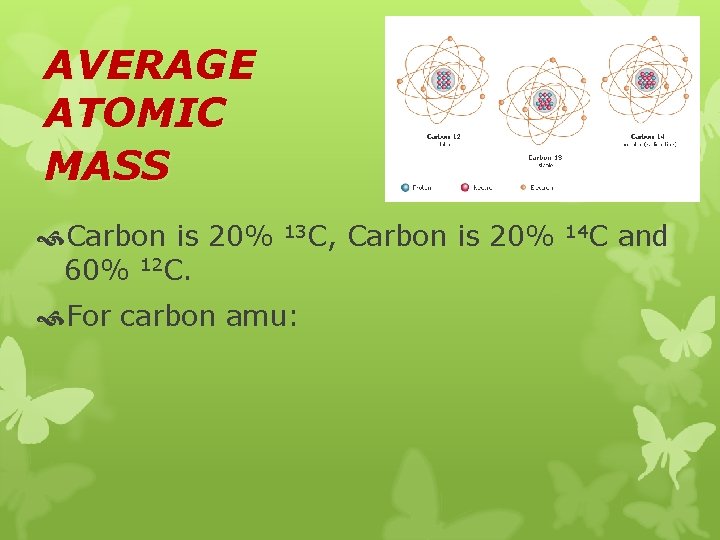
AVERAGE ATOMIC MASS Carbon is 20% 60% 12 C. 13 C, For carbon amu: Carbon is 20% 14 C and

STOP Isotope Practice Problems

Chapter 4. 4 Radioactive Decay

Radioactivity Atoms decay into other atoms The rays/particles emitted are called radiation Three common types of radiation: Alpha (α) Beta (β) Gamma (γ) Decay as a random event, independent of influences

Alpha Particles/Radiation • An alpha particle (α) is the same as a helium nucleus—two protons and two neutrons— radiation is like losing a helium Property Composition Description Charge Alpha Particles Helium 2 4 2 He Penetrating Power Blocked by paper

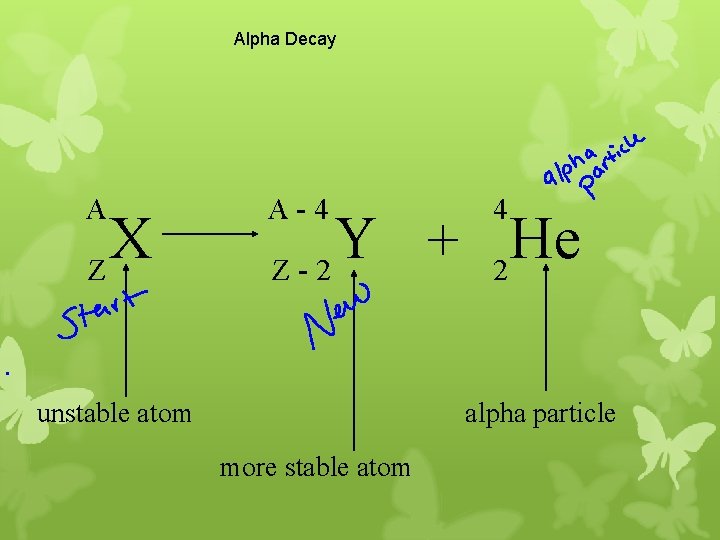
Alpha Decay A X Z A-4 4 Y He + Z-2 2 unstable atom alpha particle more stable atom

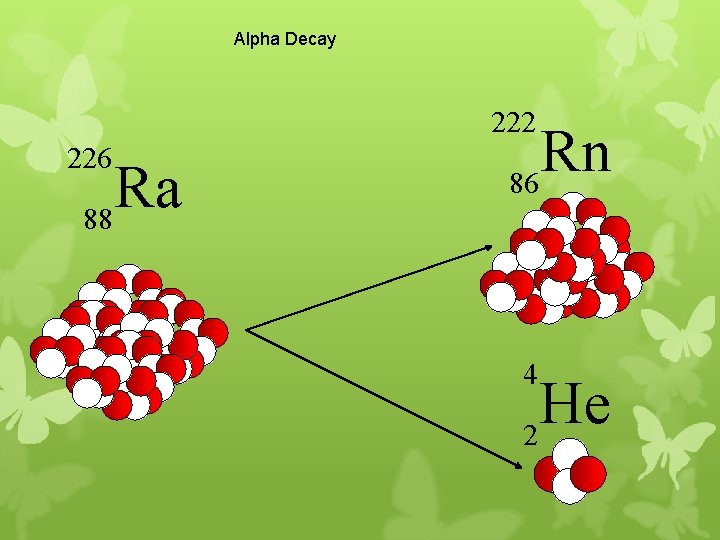
Alpha Decay 222 226 Ra 88 Rn 86 4 He 2
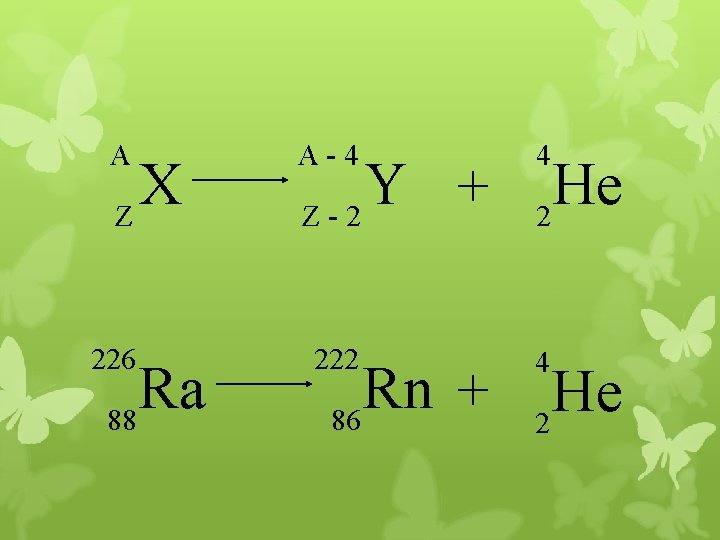
A A-4 4 226 222 4 X Z Ra 88 Y + Z-2 Rn + 86 He 2
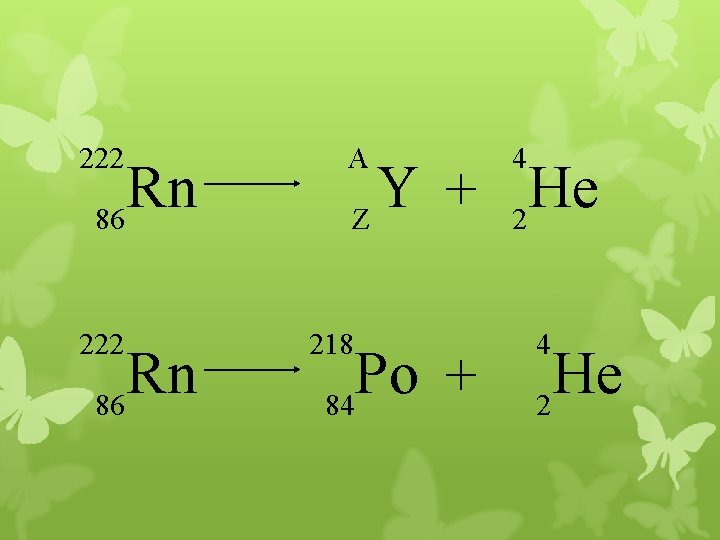
222 Rn 86 A 4 Y He + Z 2 218 Po + 84 4 He 2
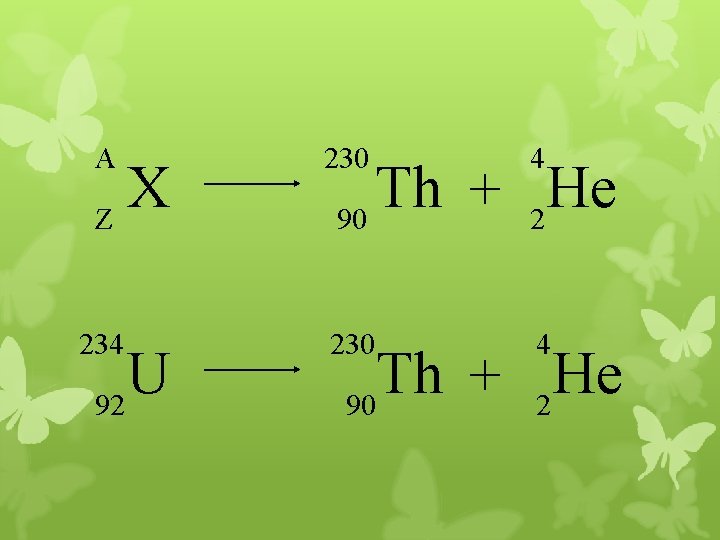
A 230 4 X Z U 92 Th He + 90 2
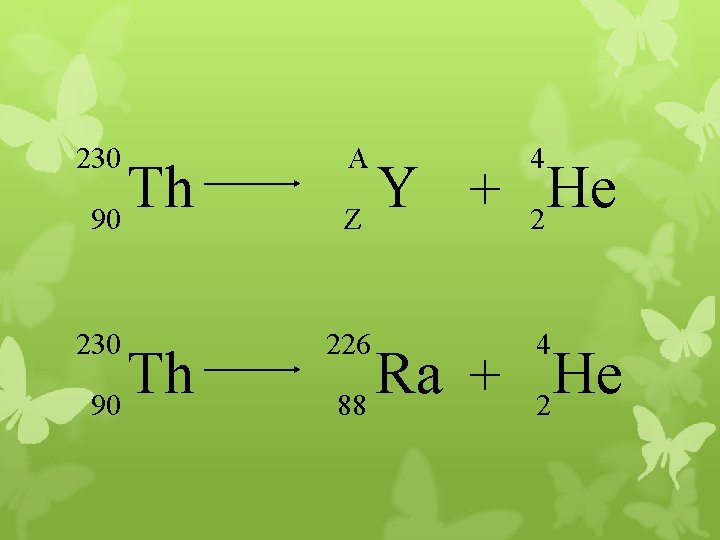
230 Th 90 A 4 226 4 Y He + Z 2 Ra He + 88 2
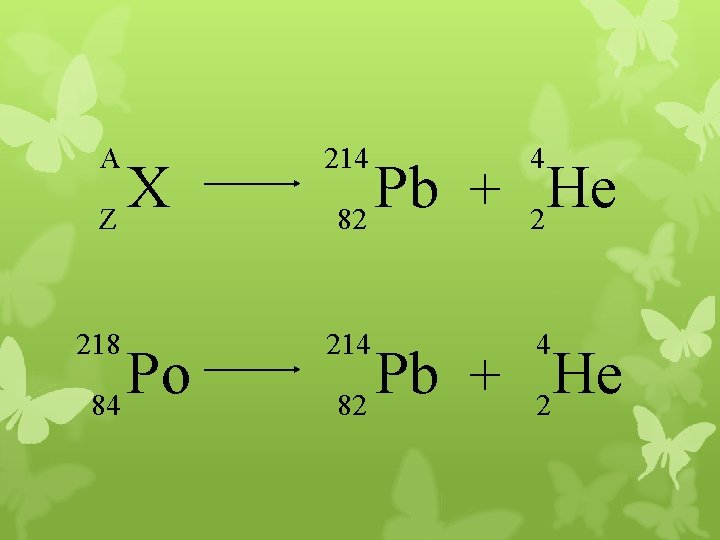
A 214 4 218 214 4 X Z Po 84 Pb He + 82 2

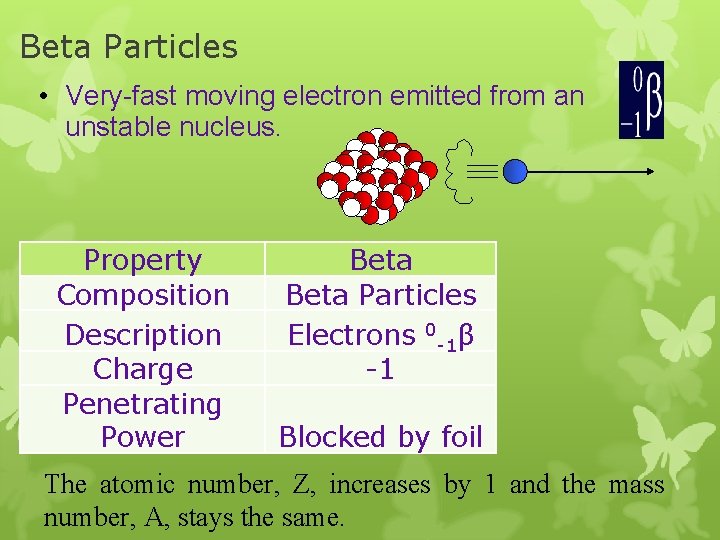
Beta Particles • Very-fast moving electron emitted from an unstable nucleus. Property Composition Description Charge Penetrating Power Beta Particles Electrons 0 -1β -1 Blocked by foil The atomic number, Z, increases by 1 and the mass number, A, stays the same.

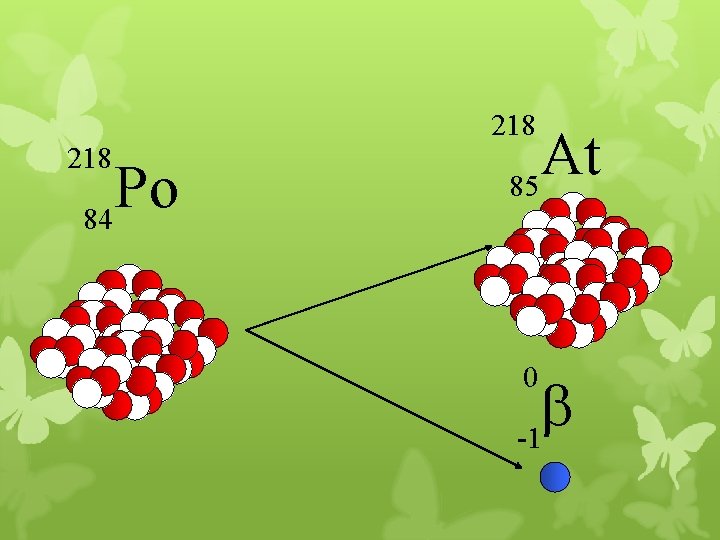
218 Po 84 At 85 0 b -1
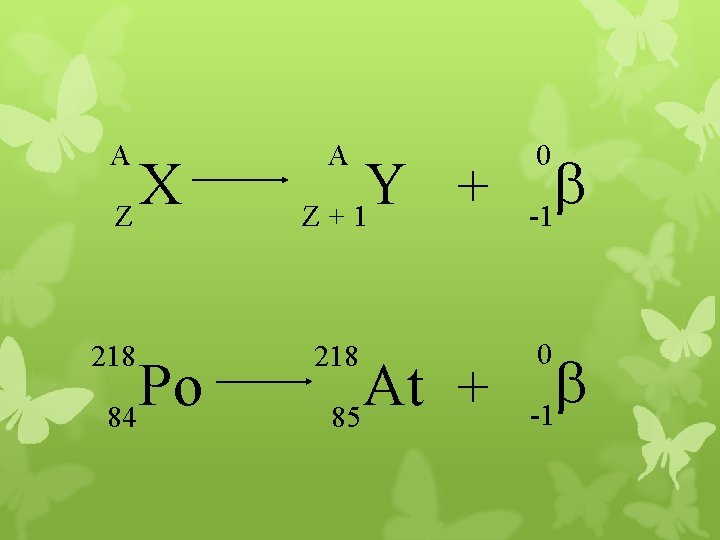
A X Z 218 Po 84 A Y + Z+1 218 At + 85 0 b -1
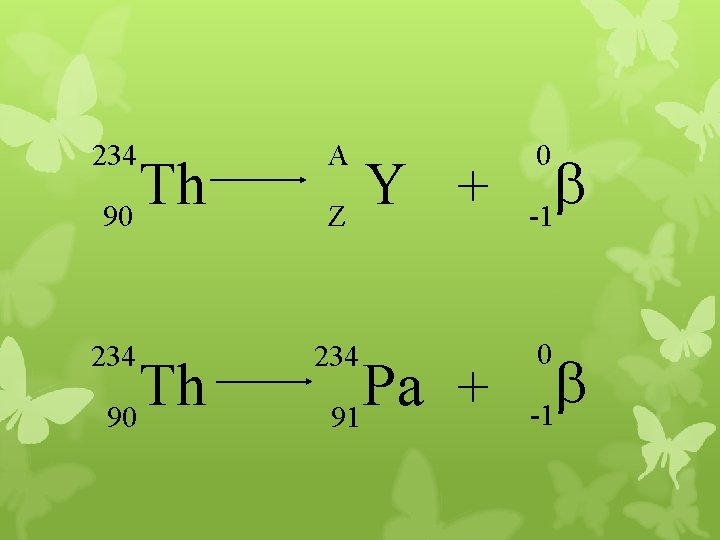
234 A 234 Th 90 Y + Z Pa + 91 0 b -1
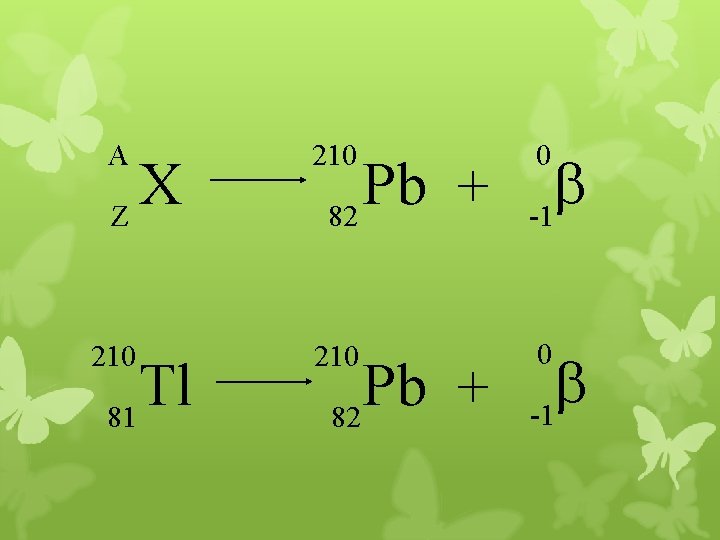
A 210 b -1 210 0 X Z Tl 81 Pb + 82 0 b -1
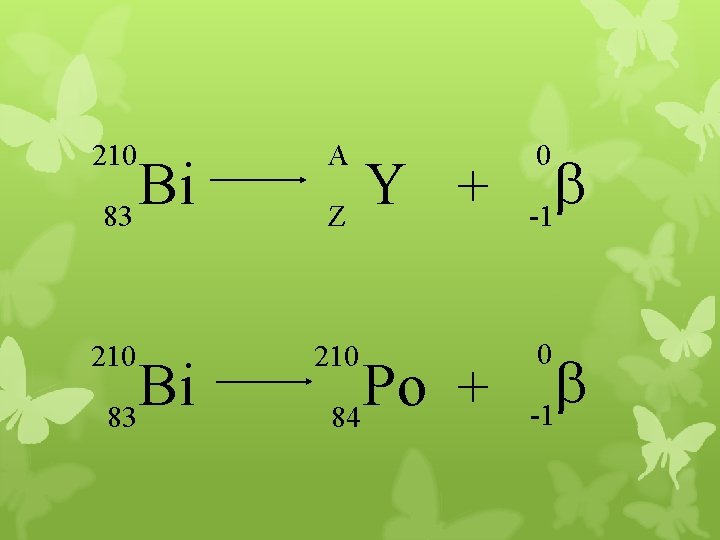
210 A 210 Bi 83 Y + Z Po + 84 0 b -1
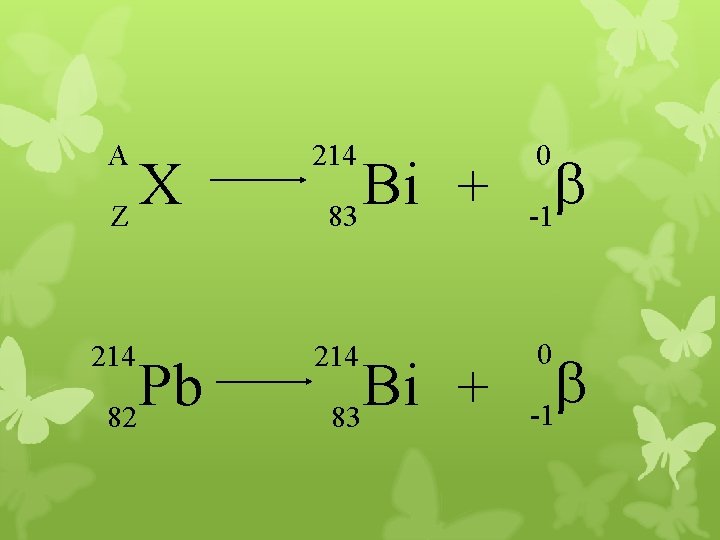
A 214 b -1 214 0 X Z Pb 82 Bi + 83 0 b -1

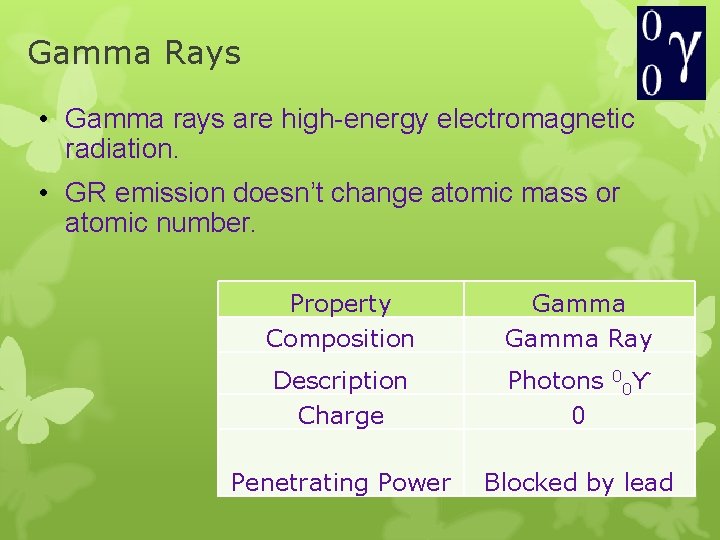
Gamma Rays • Gamma rays are high-energy electromagnetic radiation. • GR emission doesn’t change atomic mass or atomic number. Property Composition Gamma Ray Description Charge Photons 0 Penetrating Power 0 0ϒ Blocked by lead

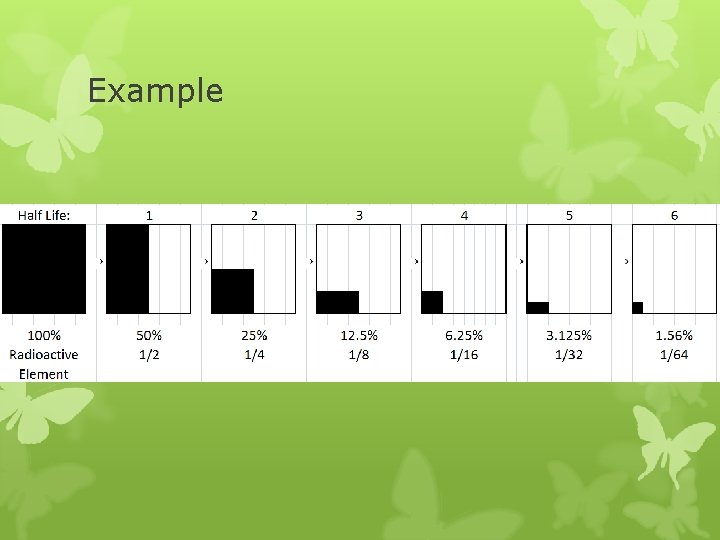
Half-Life Amount of time it takes for one half of a sample of radioactive atoms to decay Amount remaining = (Initial amount)(1/2)n

Example

Half-Life Calculation #1 You have 400 mg of a radioisotope with a halflife of 5 minutes. How much will be left after 30 minutes?

Half-Life Calculation #2 Suppose you have a 100 mg sample of Au-191, which has a half-life of 3. 4 hours. How much will remain after 10. 2 hours?

Half-Life Calculation # 3 Cobalt-60 is a radioactive isotope used in cancer treatment. Co-60 has a halflife of 5 years. If a hospital starts with a 1000 mg supply, how many mg will need to be purchased after 10 years to replenish the original supply?

Answers to Half-Life Calculations Half-Life Calculation #1 6. 25 mg Half-Life Calculation #2 12. 5 mg Half-Life Calculation #3 750 mg

THE END