Same Element Different Atom Isotopes All atoms of


- Slides: 10

Same Element Different Atom. Isotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes)

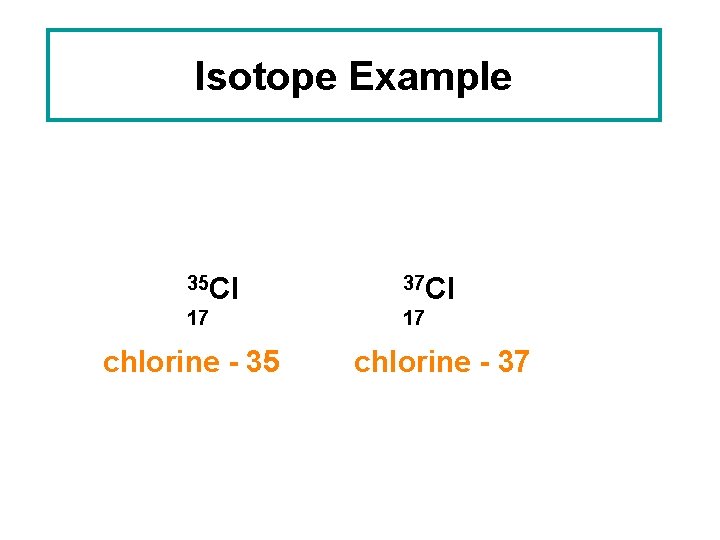
Isotope Example 35 Cl 37 Cl 17 17 chlorine - 35 chlorine - 37

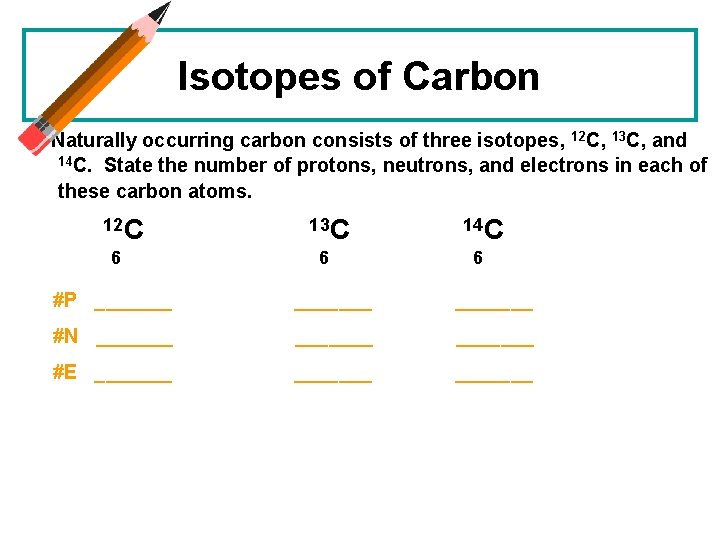
Isotopes of Carbon Naturally occurring carbon consists of three isotopes, 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12 C 6 13 C 6 14 C 6 #P _______ #N _______ #E _______

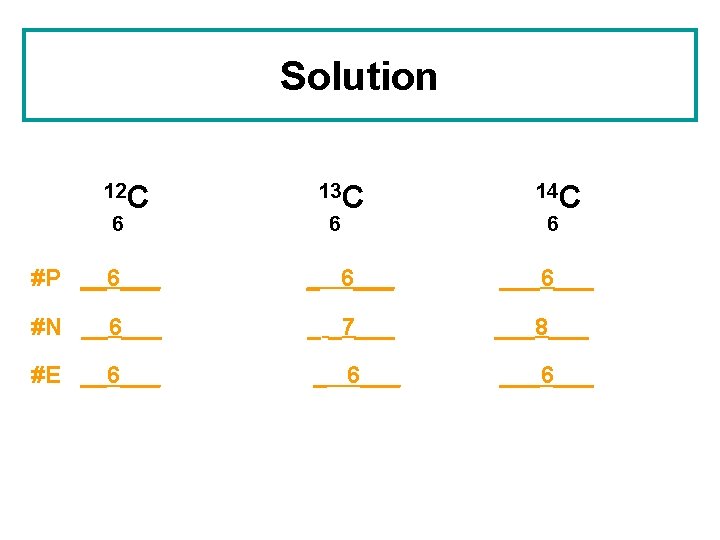
Solution 12 C 6 13 C 6 14 C 6 #P __6___ ___6___ #N __6___ _ _7___ ___8___ #E __6___ ___6___

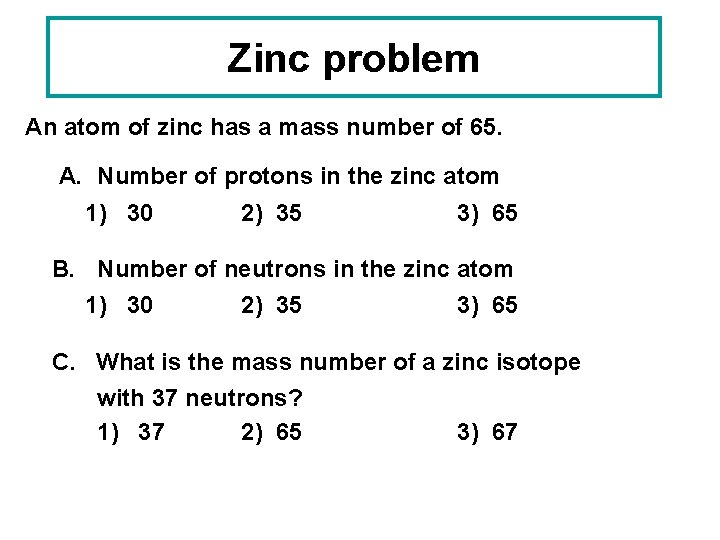
Zinc problem An atom of zinc has a mass number of 65. A. Number of protons in the zinc atom 1) 30 2) 35 3) 65 B. Number of neutrons in the zinc atom 1) 30 2) 35 3) 65 C. What is the mass number of a zinc isotope with 37 neutrons? 1) 37 2) 65 3) 67

Solution to Zinc Problem An atom of zinc has a mass number of 65. A. Number of protons in the zinc atom 1) 30 B. Number of neutrons in the zinc atom 2) 35 C. What is the mass number of a zinc isotope with 37 neutrons? 3) 67

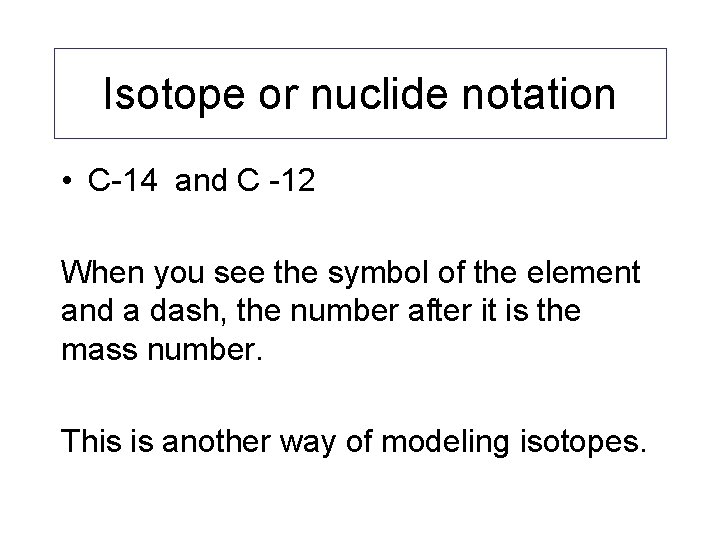
Isotope or nuclide notation • C-14 and C -12 When you see the symbol of the element and a dash, the number after it is the mass number. This is another way of modeling isotopes.

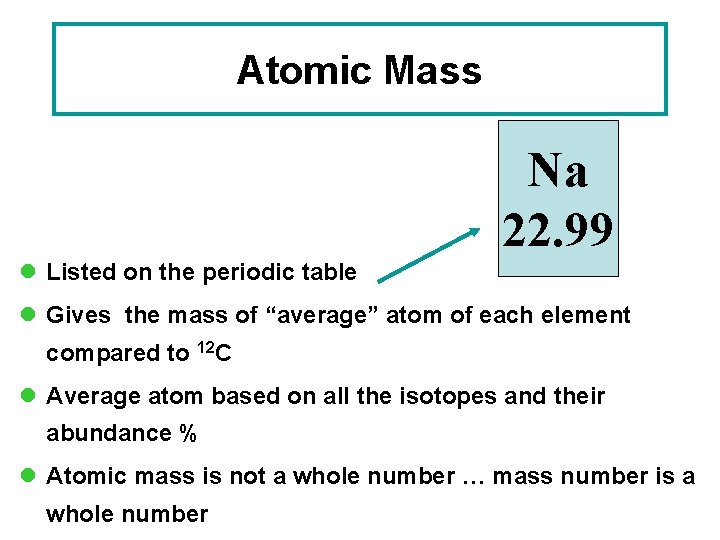
Atomic Mass Na 22. 99 l Listed on the periodic table l Gives the mass of “average” atom of each element compared to 12 C l Average atom based on all the isotopes and their abundance % l Atomic mass is not a whole number … mass number is a whole number

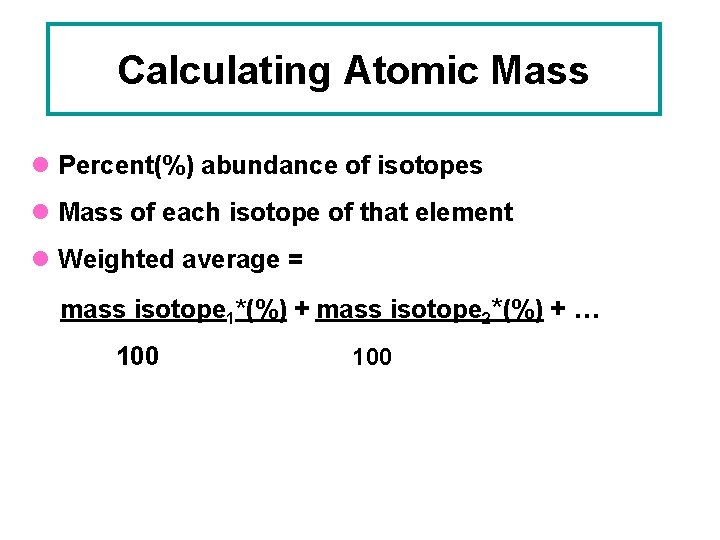
Calculating Atomic Mass l Percent(%) abundance of isotopes l Mass of each isotope of that element l Weighted average = mass isotope 1*(%) + mass isotope 2*(%) + … 100

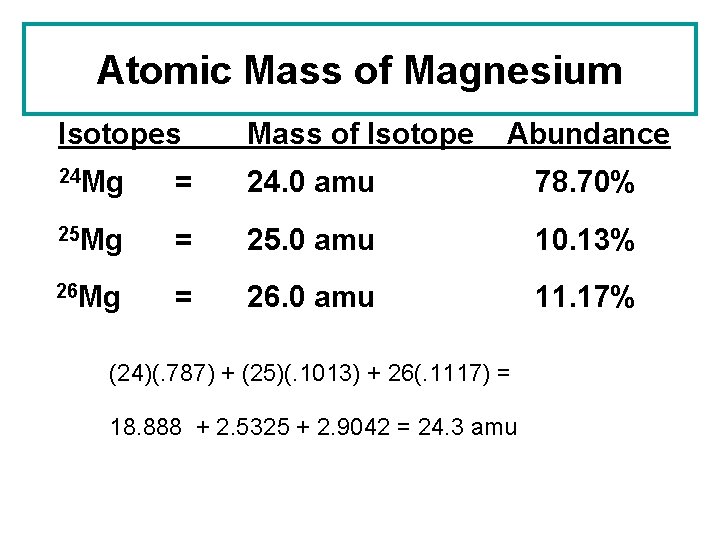
Atomic Mass of Magnesium Isotopes Mass of Isotope Abundance 24 Mg = 24. 0 amu 78. 70% 25 Mg = 25. 0 amu 10. 13% 26 Mg = 26. 0 amu 11. 17% (24)(. 787) + (25)(. 1013) + 26(. 1117) = 18. 888 + 2. 5325 + 2. 9042 = 24. 3 amu