Zumdahl De Coste World of CHEMISTRY Chapter 14


















- Slides: 38

Zumdahl De. Coste World of CHEMISTRY

Chapter 14 Liquids and Solids Copyright© by Houghton Mifflin Company. All rights reserved.

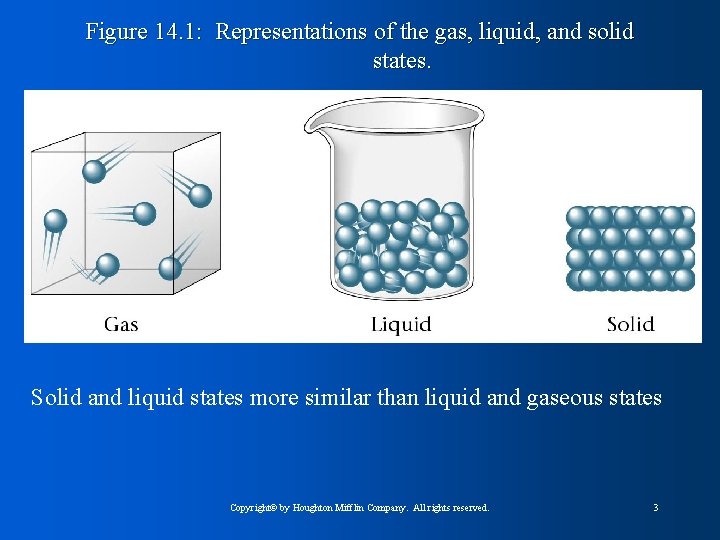
Figure 14. 1: Representations of the gas, liquid, and solid states. Solid and liquid states more similar than liquid and gaseous states Copyright© by Houghton Mifflin Company. All rights reserved. 3

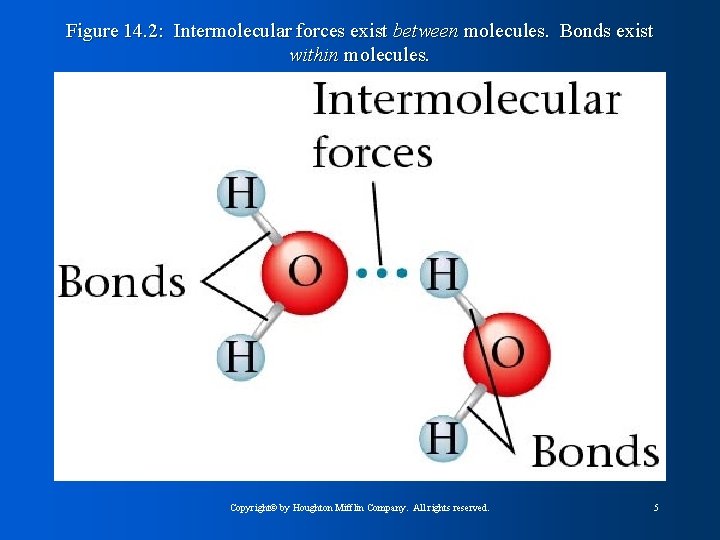
Intermolecular Forces Explain why water is liquid at normal temperature and pressure l Forces that occur between molecules l – Dipole-dipole attraction l l 1% as strong as bonds Weaken as distance increases – Hydrogen bonding (type of dipole-dipole force) l Hydrogen bound to highly electronegative atom – London dispersion forces l l l Exist in noble gases and nonpolar molecules Temporary dipole moments form producing attraction Intramolecular forces: forces within molecule itself (bonds) Copyright© by Houghton Mifflin Company. All rights reserved. 4

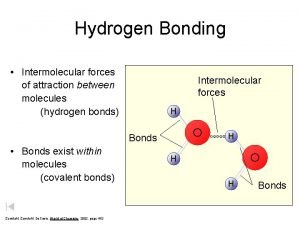
Figure 14. 2: Intermolecular forces exist between molecules. Bonds exist within molecules. Copyright© by Houghton Mifflin Company. All rights reserved. 5

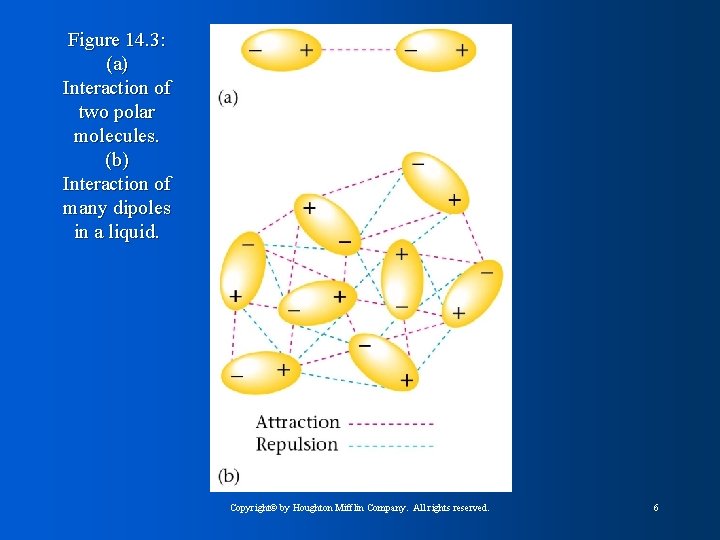
Figure 14. 3: (a) Interaction of two polar molecules. (b) Interaction of many dipoles in a liquid. Copyright© by Houghton Mifflin Company. All rights reserved. 6

Figure 14. 4: Polar water molecules. Copyright© by Houghton Mifflin Company. All rights reserved. 7

Figure 14. 4: Hydrogen bonding among water molecules. Copyright© by Houghton Mifflin Company. All rights reserved. 8

Figure 14. 5: The boiling points of covalent hydrides. Copyright© by Houghton Mifflin Company. All rights reserved. 9

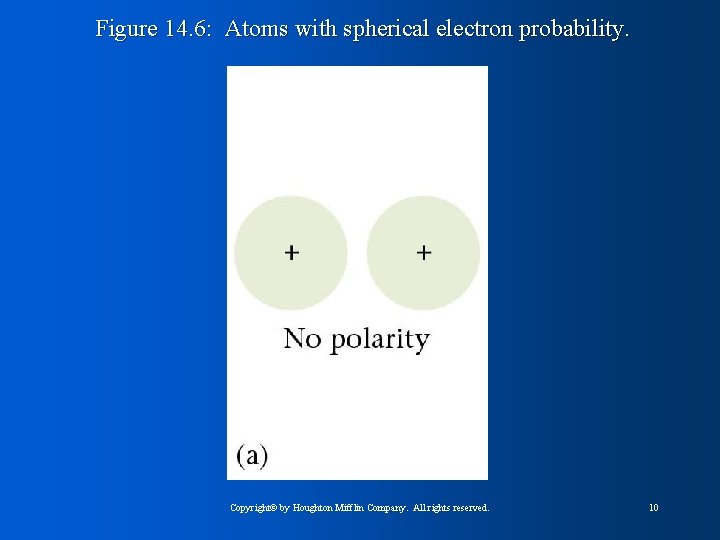
Figure 14. 6: Atoms with spherical electron probability. Copyright© by Houghton Mifflin Company. All rights reserved. 10

14. 6: The atom on the left develops an instantaneous dipole. Copyright© by Houghton Mifflin Company. All rights reserved. 11

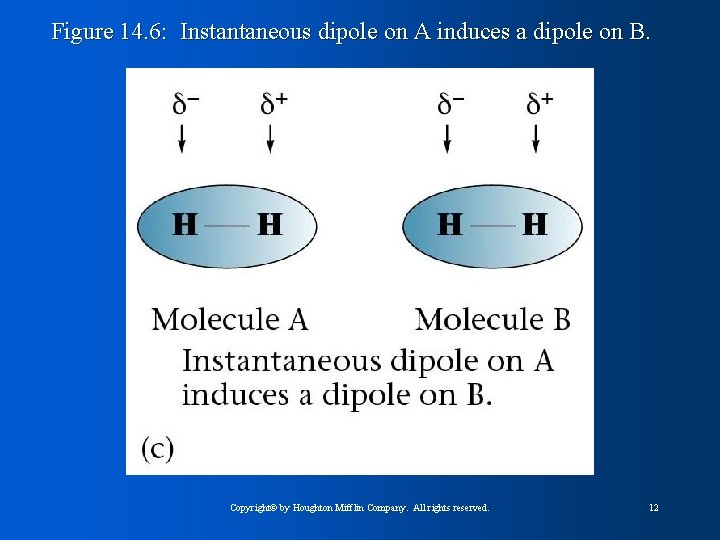
Figure 14. 6: Instantaneous dipole on A induces a dipole on B. Copyright© by Houghton Mifflin Company. All rights reserved. 12

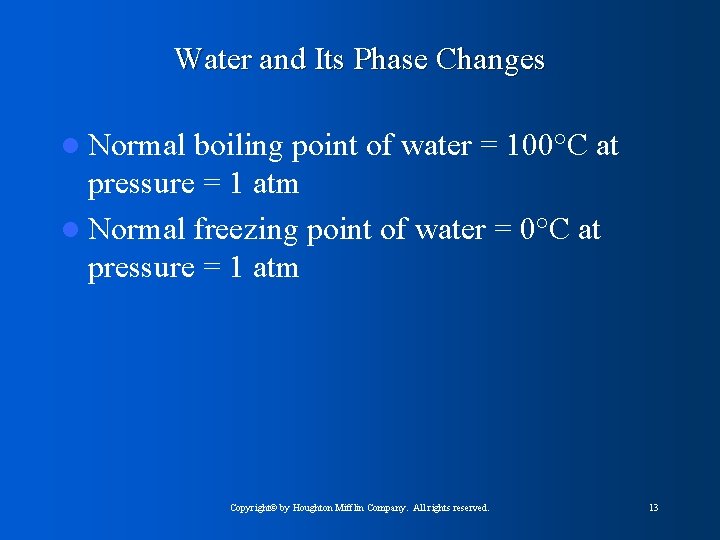
Water and Its Phase Changes l Normal boiling point of water = 100°C at pressure = 1 atm l Normal freezing point of water = 0°C at pressure = 1 atm Copyright© by Houghton Mifflin Company. All rights reserved. 13

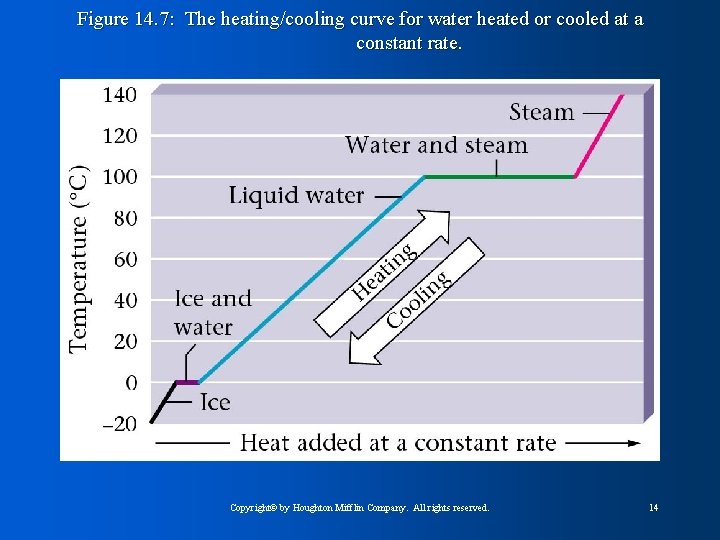
Figure 14. 7: The heating/cooling curve for water heated or cooled at a constant rate. Copyright© by Houghton Mifflin Company. All rights reserved. 14

Energy Requirements for Change of State l Extra energy needed to change state (solid to liquid, liquid to vapor) – need to overcome intermolecular forces l Molar heat of fusion (water) = 6. 02 k. J/mol l Molar heat of vaporization = 40. 6 k. J/mol Copyright© by Houghton Mifflin Company. All rights reserved. 15

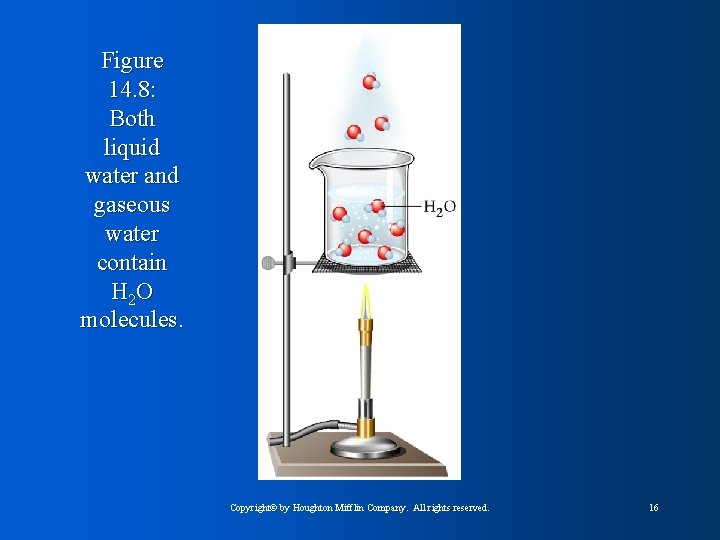
Figure 14. 8: Both liquid water and gaseous water contain H 2 O molecules. Copyright© by Houghton Mifflin Company. All rights reserved. 16

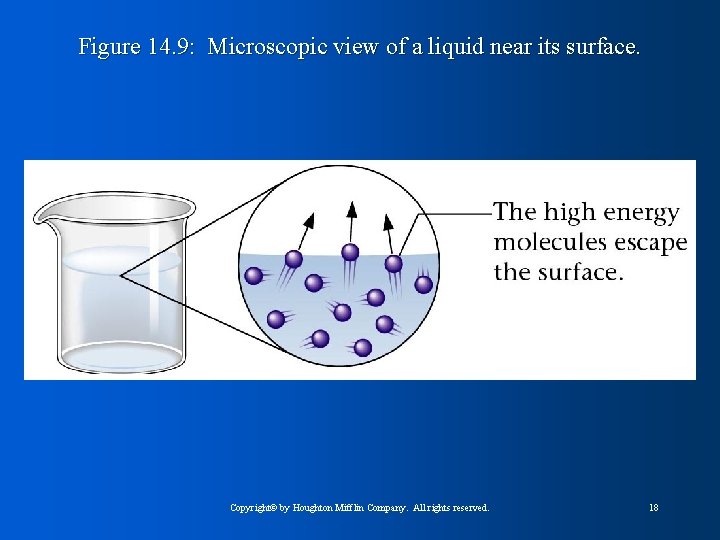
Evaporation and Vapor Pressure l l l Evaporation/vaporization: requires energy to overcome intermolecular forces Endothermic process Condensation: vapor molecules form a liquid Eventually (in closed container) the rate of vaporization = rate of condensation; system is in equilibrium Molecules are still vaporizing/condensing, but there is no net change because they balance each other out Copyright© by Houghton Mifflin Company. All rights reserved. 17

Figure 14. 9: Microscopic view of a liquid near its surface. Copyright© by Houghton Mifflin Company. All rights reserved. 18

Figure 14. 10: Behavior of a liquid in a closed container. Copyright© by Houghton Mifflin Company. All rights reserved. 19

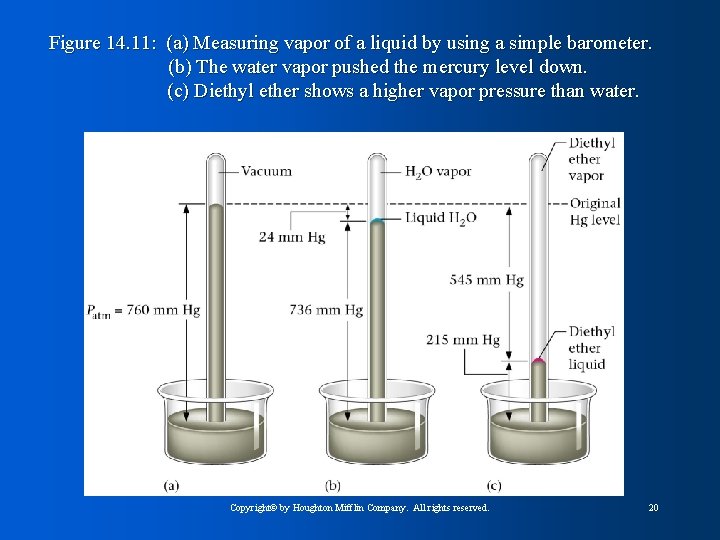
Figure 14. 11: (a) Measuring vapor of a liquid by using a simple barometer. (b) The water vapor pushed the mercury level down. (c) Diethyl ether shows a higher vapor pressure than water. Copyright© by Houghton Mifflin Company. All rights reserved. 20

Vapor Pressure l Liquids with high vapor pressures are said to be volatile: they evaporate rapidly l Determined by intermolecular forces – Large intermolecular forces = low vapor pressure Copyright© by Houghton Mifflin Company. All rights reserved. 21

Figure 14. 12: Water rapidly boiling on a stove. Bubbles form on interior of liquid, then rise to surface and POP! Copyright© by Houghton Mifflin Company. All rights reserved. 22

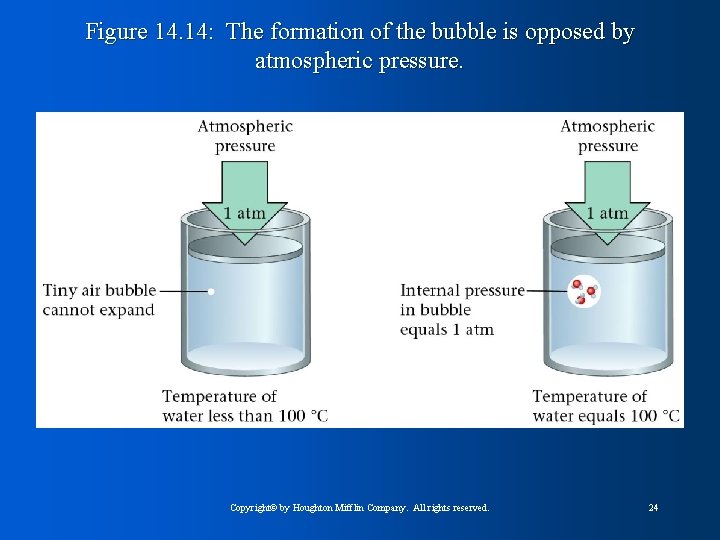
Figure 14. 13: Bubble expands as H 2 O molecules enter. • Vapor pressure must equal atmospheric pressure for boiling to occur • Bubbles must have enough energy to sustain pressure in bubble • Boiling point decreases with altitude because atmospheric pressure is less Copyright© by Houghton Mifflin Company. All rights reserved. 23

Figure 14. 14: The formation of the bubble is opposed by atmospheric pressure. Copyright© by Houghton Mifflin Company. All rights reserved. 24

Types of Solids l Crystalline solids: have regular arrangements of their components – highly ordered arrangement produces beautiful, regularly shaped crystals Copyright© by Houghton Mifflin Company. All rights reserved. 25

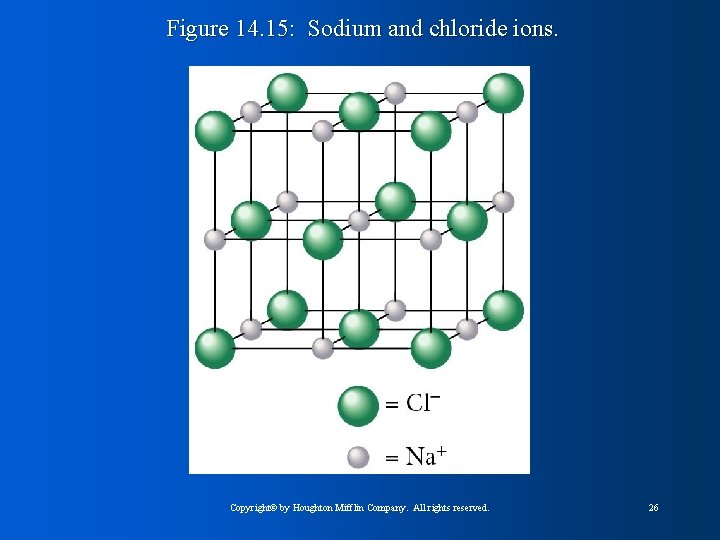
Figure 14. 15: Sodium and chloride ions. Copyright© by Houghton Mifflin Company. All rights reserved. 26

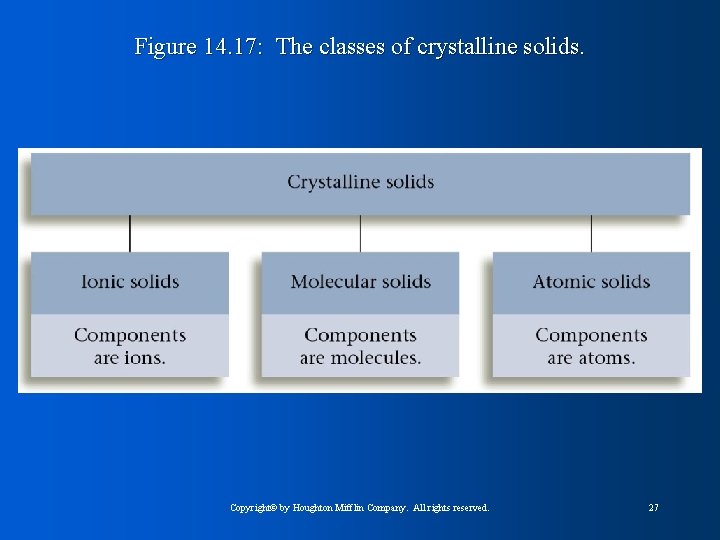
Figure 14. 17: The classes of crystalline solids. Copyright© by Houghton Mifflin Company. All rights reserved. 27

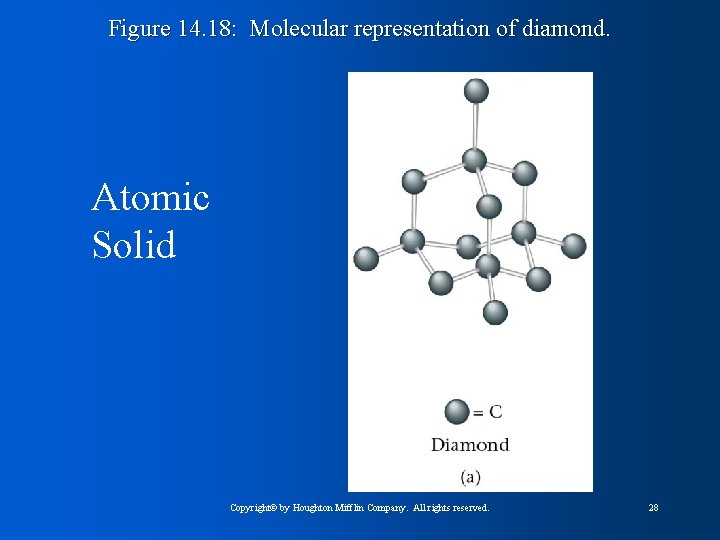
Figure 14. 18: Molecular representation of diamond. Atomic Solid Copyright© by Houghton Mifflin Company. All rights reserved. 28

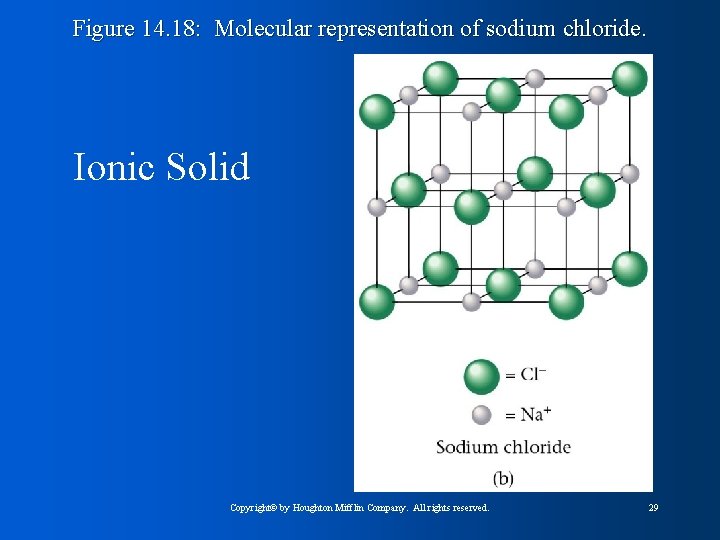
Figure 14. 18: Molecular representation of sodium chloride. Ionic Solid Copyright© by Houghton Mifflin Company. All rights reserved. 29

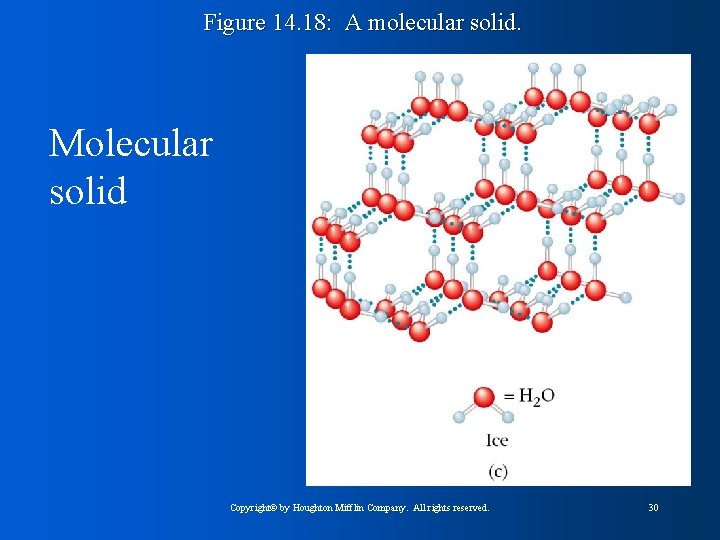
Figure 14. 18: A molecular solid. Molecular solid Copyright© by Houghton Mifflin Company. All rights reserved. 30

Bonding in Solids Copyright© by Houghton Mifflin Company. All rights reserved. 31

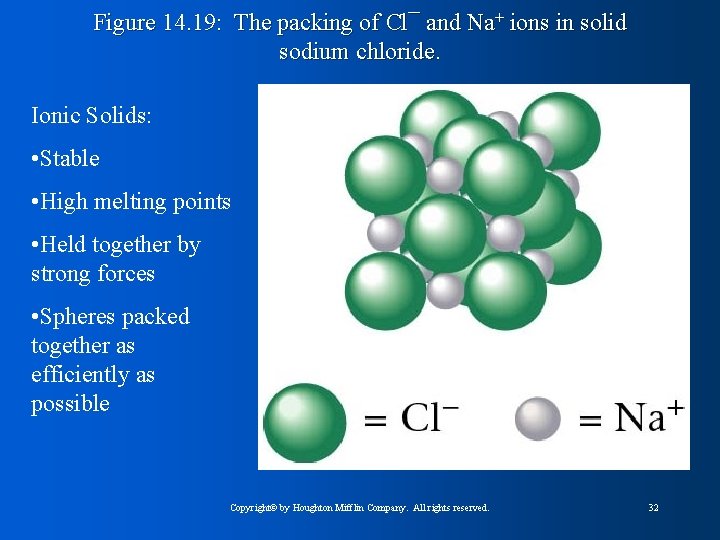
Figure 14. 19: The packing of Cl¯ and Na+ ions in solid sodium chloride. Ionic Solids: • Stable • High melting points • Held together by strong forces • Spheres packed together as efficiently as possible Copyright© by Houghton Mifflin Company. All rights reserved. 32

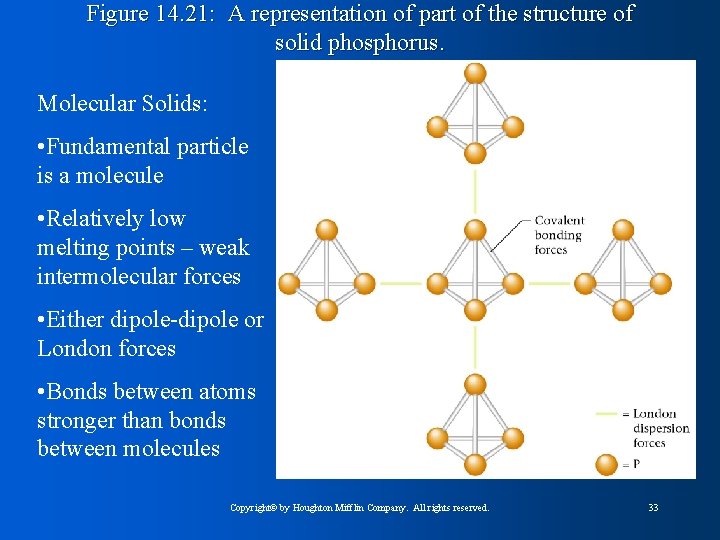
Figure 14. 21: A representation of part of the structure of solid phosphorus. Molecular Solids: • Fundamental particle is a molecule • Relatively low melting points – weak intermolecular forces • Either dipole-dipole or London forces • Bonds between atoms stronger than bonds between molecules Copyright© by Houghton Mifflin Company. All rights reserved. 33

Atomic Solids l Properties vary greatly because of different ways fundamental particles interact with each other – Group 8: low melting points, filled shells, weak London dispersion forces – Diamond: one of hardest substances known, extremely high melting point, very strong covalent carbon-carbon bonds Copyright© by Houghton Mifflin Company. All rights reserved. 34

Bonding in Metals l Shape can be changed easily, but durable with high melting points l Bonding is strong but nondirectional l Electron sea model: array of metal atoms in a “sea” of valence electrons that are shared among the atoms – Electrons can conduct heat and electricity and atoms can move easily Copyright© by Houghton Mifflin Company. All rights reserved. 35

Alloys Alloy: substance that contains a mixture of elements and has metallic properties Because of nature of metallic crystal, other substances can be easily introduced Copyright© by Houghton Mifflin Company. All rights reserved. 36

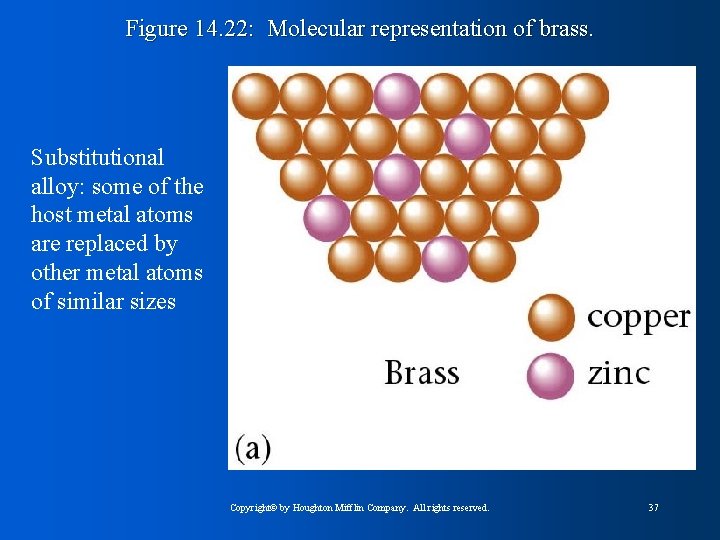
Figure 14. 22: Molecular representation of brass. Substitutional alloy: some of the host metal atoms are replaced by other metal atoms of similar sizes Copyright© by Houghton Mifflin Company. All rights reserved. 37

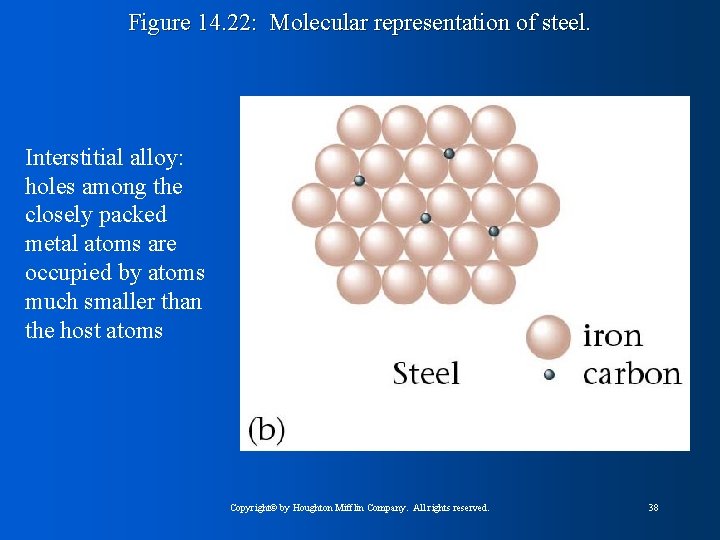
Figure 14. 22: Molecular representation of steel. Interstitial alloy: holes among the closely packed metal atoms are occupied by atoms much smaller than the host atoms Copyright© by Houghton Mifflin Company. All rights reserved. 38
 Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes C / lambda
C / lambda Zumdahl chemistry
Zumdahl chemistry Zumdahl chemistry
Zumdahl chemistry Electron cloud model
Electron cloud model Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Busqueda costo uniforme
Busqueda costo uniforme Coste explícito
Coste explícito Coste marginal
Coste marginal Mural uv
Mural uv Il mare di baffin lambisce le coste
Il mare di baffin lambisce le coste Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Ap world history chapter 25 africa and the atlantic world
Ap world history chapter 25 africa and the atlantic world Site:slidetodoc.com
Site:slidetodoc.com Are oranges old world or new world
Are oranges old world or new world Real world vs digital world
Real world vs digital world Plato example
Plato example The changing world output and world trade picture
The changing world output and world trade picture Bad dangerous tour
Bad dangerous tour Sour cream walls is an example of pun
Sour cream walls is an example of pun Endless night figure of speech
Endless night figure of speech World world
World world The changing world output and world trade picture
The changing world output and world trade picture Is chalk natural or manmade
Is chalk natural or manmade Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Intro to organic chemistry
Intro to organic chemistry Modern chemistry chapter 9
Modern chemistry chapter 9 Chapter 7 review modern chemistry answers
Chapter 7 review modern chemistry answers Modern chemistry chapter 15 review answers
Modern chemistry chapter 15 review answers Chapter 14 review acids and bases
Chapter 14 review acids and bases Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Chapter 12 review solutions answer key
Chapter 12 review solutions answer key Chemistry chapter 11 gases test
Chemistry chapter 11 gases test Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 the mole study guide
Chapter 10 the mole study guide