Zumdahl De Coste World of CHEMISTRY Topic 9







- Slides: 23

Zumdahl • De. Coste World of CHEMISTRY

Topic 9 -2008 Properties of Gases

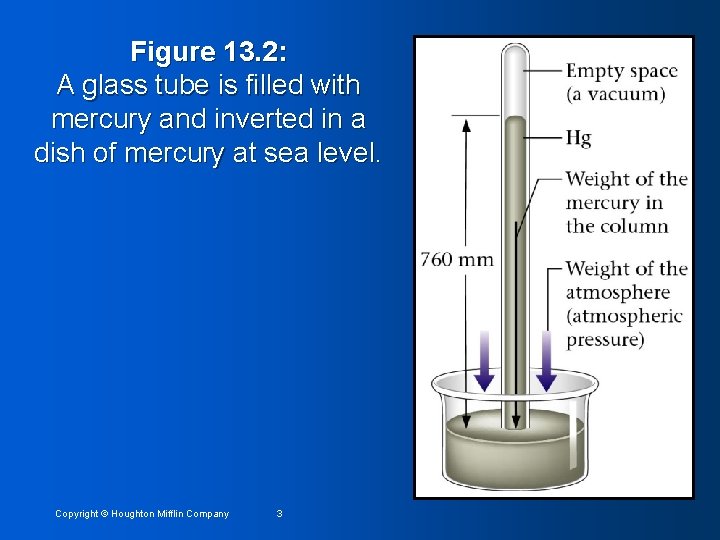
Figure 13. 2: A glass tube is filled with mercury and inverted in a dish of mercury at sea level. Copyright © Houghton Mifflin Company 3

When water at 25ºC is heated and changes to a gas at 110ºC, the water molecules A. become more organized. B. move farther apart. C. stop moving. D. move closer together. E. move more slowly. Copyright © Houghton Mifflin Company 4

6. A sample of liquid ammonia (NH 3) is completely evaporated (changed to a gas) in a closed container as shown: Which of the following diagrams best represents what you would “see” in the same area of the magnified view of the vapor? Copyright © Houghton Mifflin Company 5

7. A diagram representing carbon dioxide molecules in the solid phase, also known as dry ice, is shown below. Which of these molecular diagrams best shows what dry ice would look like after it sublimates (solid into a gas)? Copyright © Houghton Mifflin Company 6

Comparing Gases Helium Gas Argon Gas 10 m. L 1. How do the radii of these types of atoms compare? 2. How will the mass of these two gases compare? 3. How will the number of atoms compare? Copyright © Houghton Mifflin Company 7

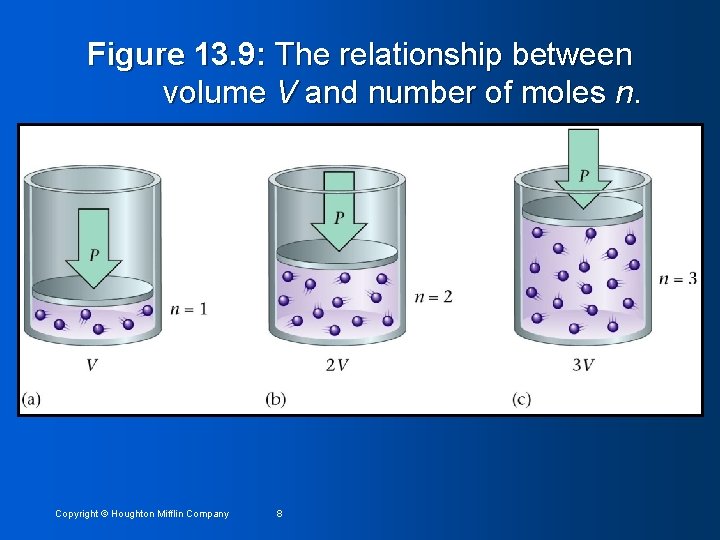
Figure 13. 9: The relationship between volume V and number of moles n. Copyright © Houghton Mifflin Company 8

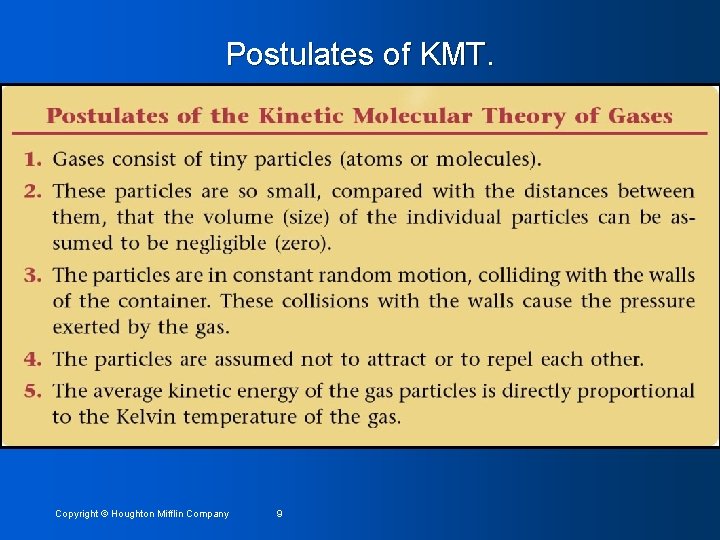
Postulates of KMT. Copyright © Houghton Mifflin Company 9

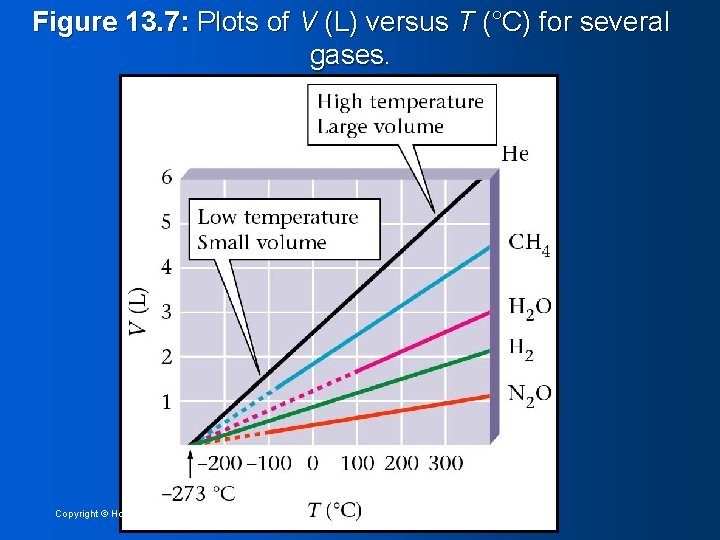
Figure 13. 7: Plots of V (L) versus T (°C) for several gases. Copyright © Houghton Mifflin Company 10

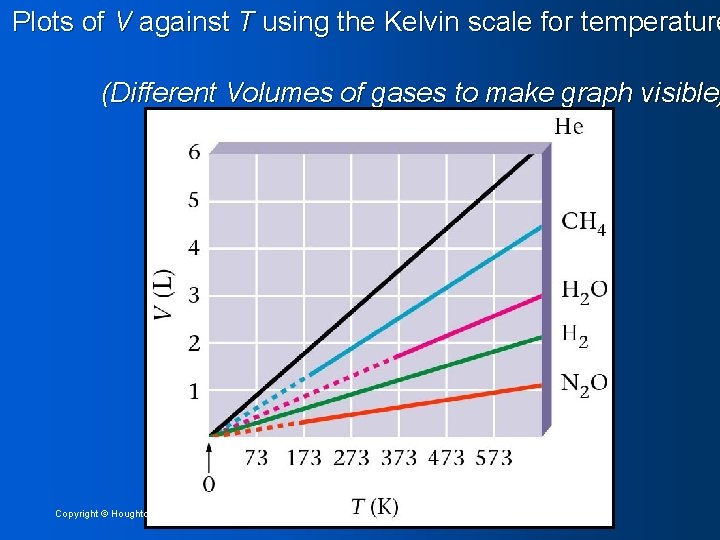
Plots of V against T using the Kelvin scale for temperature (Different Volumes of gases to make graph visible) Copyright © Houghton Mifflin Company 11

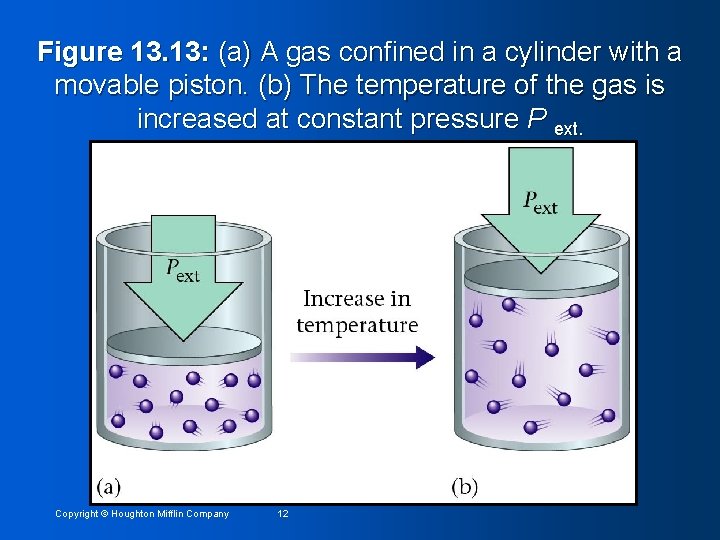
Figure 13. 13: (a) A gas confined in a cylinder with a movable piston. (b) The temperature of the gas is increased at constant pressure P ext. Copyright © Houghton Mifflin Company 12

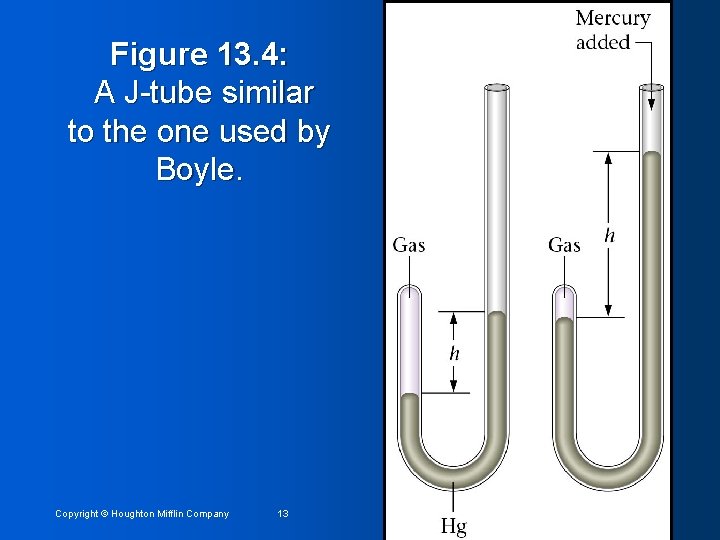
Figure 13. 4: A J-tube similar to the one used by Boyle. Copyright © Houghton Mifflin Company 13

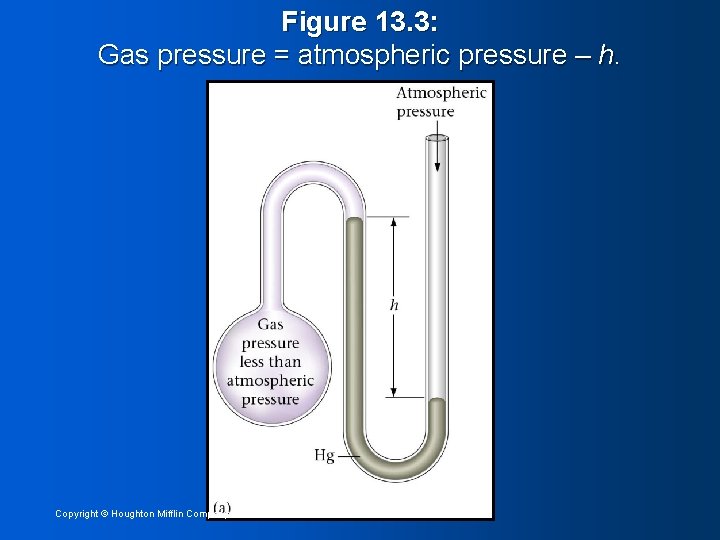
Figure 13. 3: Gas pressure = atmospheric pressure – h. Copyright © Houghton Mifflin Company 14

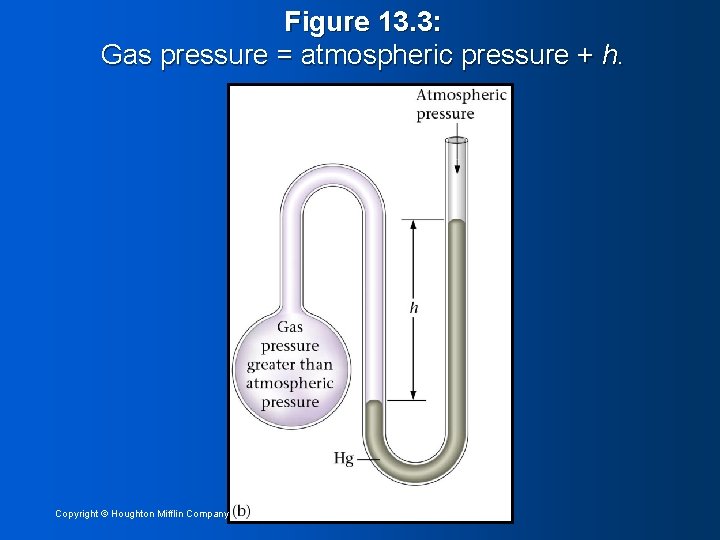
Figure 13. 3: Gas pressure = atmospheric pressure + h. Copyright © Houghton Mifflin Company 15

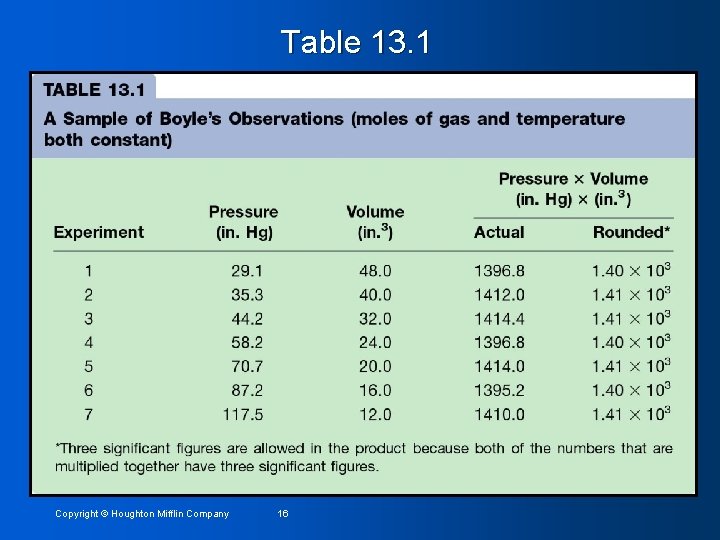
Table 13. 1 Copyright © Houghton Mifflin Company 16

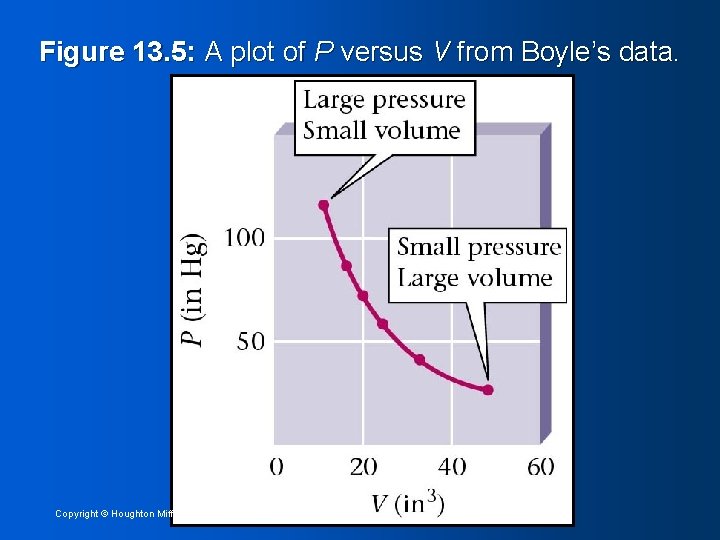
Figure 13. 5: A plot of P versus V from Boyle’s data. Copyright © Houghton Mifflin Company 17

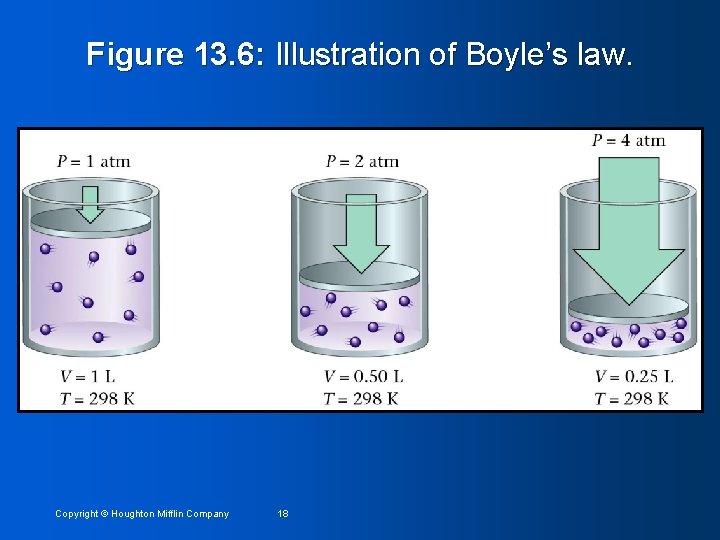
Figure 13. 6: Illustration of Boyle’s law. Copyright © Houghton Mifflin Company 18

“Vacuum-packed Students” Increasing the external pressure reduces the volume. Copyright © Houghton Mifflin Company 19

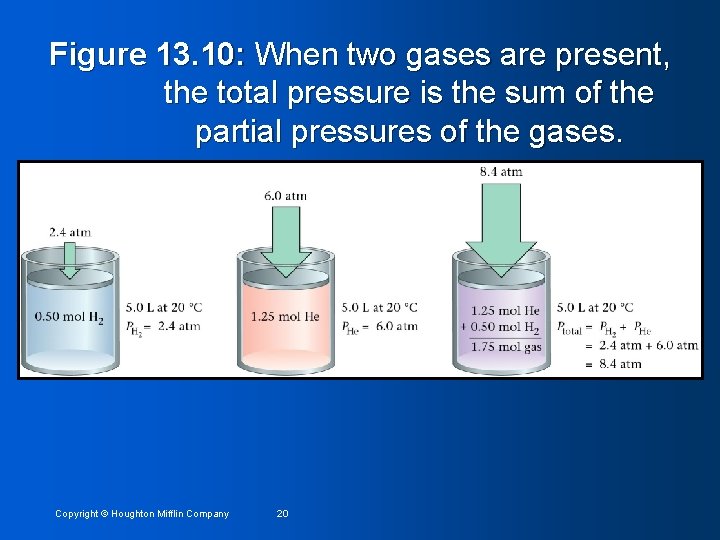
Figure 13. 10: When two gases are present, the total pressure is the sum of the partial pressures of the gases. Copyright © Houghton Mifflin Company 20

Collecting Gas over Water Pressure. Total = PO 2 + PH 2 O

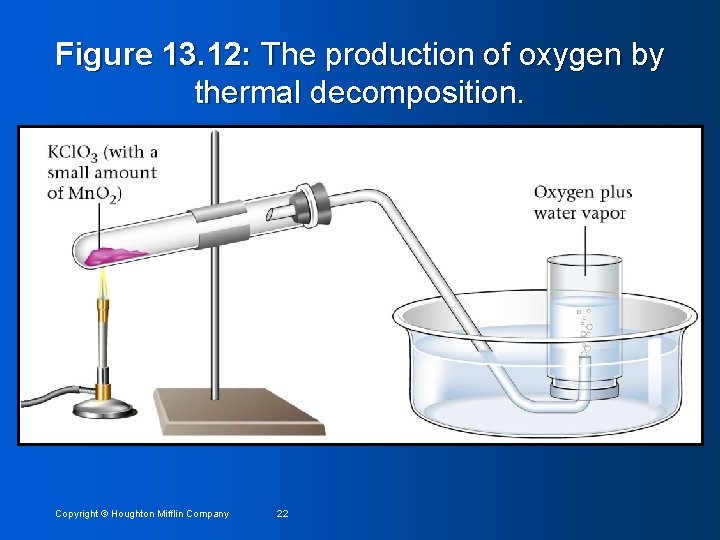
Figure 13. 12: The production of oxygen by thermal decomposition. Copyright © Houghton Mifflin Company 22

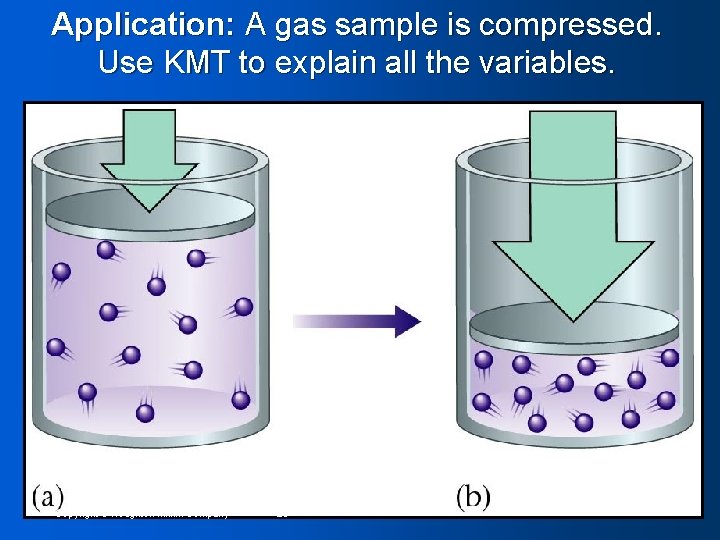
Application: A gas sample is compressed. Use KMT to explain all the variables. Copyright © Houghton Mifflin Company 23
 Ap chemistry notes zumdahl
Ap chemistry notes zumdahl C#lambda
C#lambda Zumdahl chemistry
Zumdahl chemistry Intermolecular forces lesson
Intermolecular forces lesson Electron cloud model
Electron cloud model Metodo uniforme
Metodo uniforme Coste explícito
Coste explícito Coste marginal
Coste marginal Activos financieros a coste
Activos financieros a coste Il mare di baffin lambisce le coste
Il mare di baffin lambisce le coste Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Example of a clincher
Example of a clincher /topic/ down
/topic/ down Topic 14 ib chemistry
Topic 14 ib chemistry Organic chemistry topic 11
Organic chemistry topic 11 Hess's law ib chemistry
Hess's law ib chemistry Topic 2 chemistry
Topic 2 chemistry Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Primate traits
Primate traits Are oranges old world or new world
Are oranges old world or new world Real world vs digital world
Real world vs digital world The world of ideas plato
The world of ideas plato