Zumdahl De Coste World of CHEMISTRY Chapter 20



- Slides: 19

Zumdahl De. Coste World of CHEMISTRY

Chapter 20 Organic Chemistry Copyright© by Houghton Mifflin Company. All rights reserved.

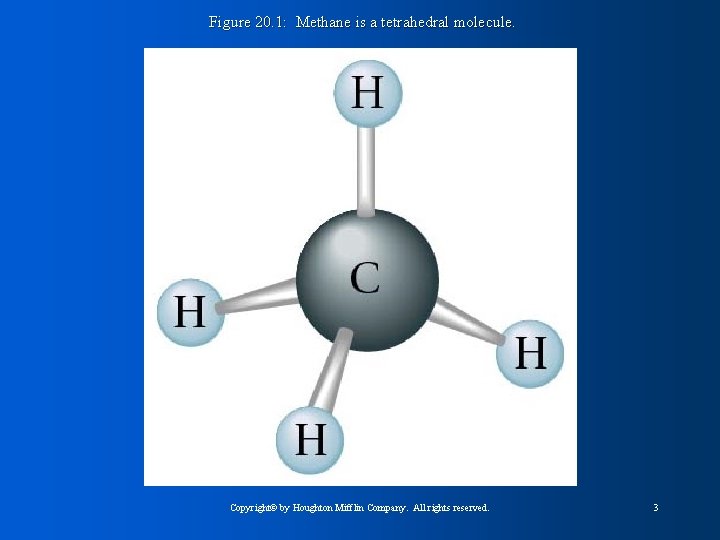
Figure 20. 1: Methane is a tetrahedral molecule. Copyright© by Houghton Mifflin Company. All rights reserved. 3

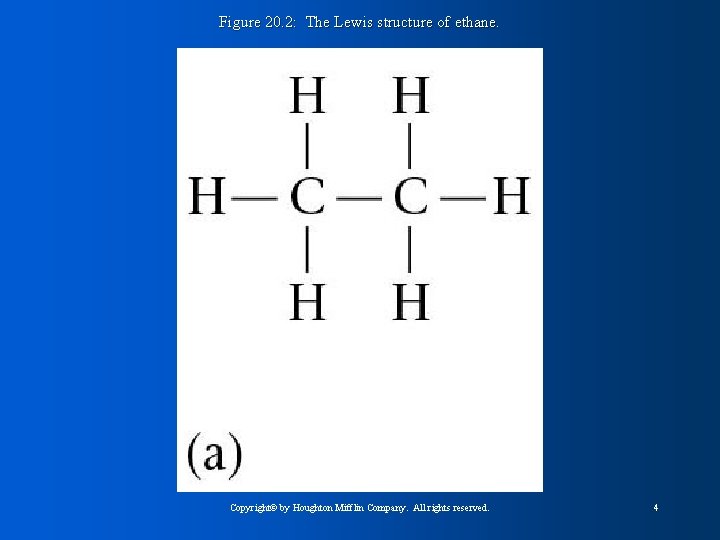
Figure 20. 2: The Lewis structure of ethane. Copyright© by Houghton Mifflin Company. All rights reserved. 4

Figure 20. 2: A space-filling model of ethane. Copyright© by Houghton Mifflin Company. All rights reserved. 5

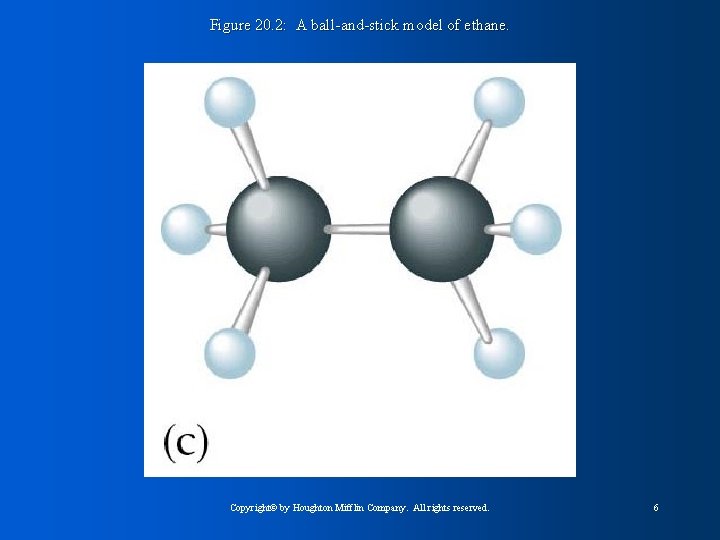
Figure 20. 2: A ball-and-stick model of ethane. Copyright© by Houghton Mifflin Company. All rights reserved. 6

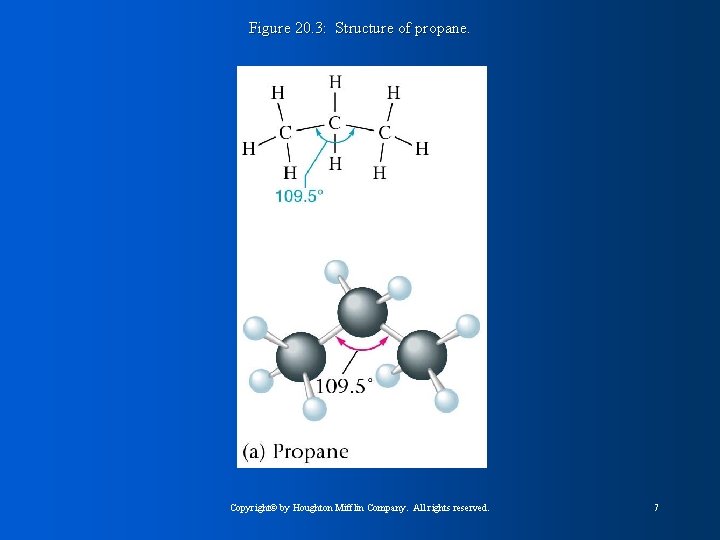
Figure 20. 3: Structure of propane. Copyright© by Houghton Mifflin Company. All rights reserved. 7

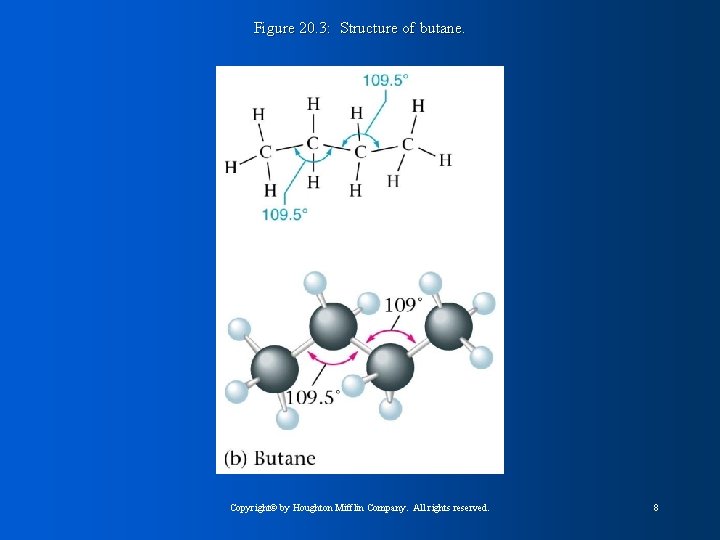
Figure 20. 3: Structure of butane. Copyright© by Houghton Mifflin Company. All rights reserved. 8

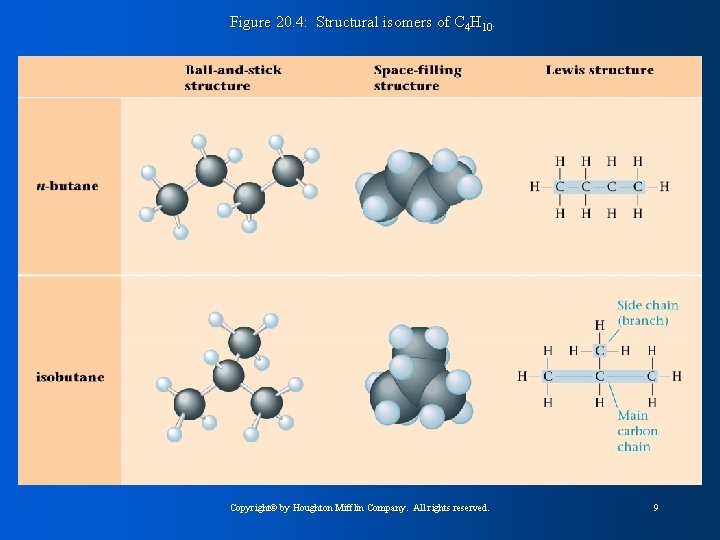
Figure 20. 4: Structural isomers of C 4 H 10. Copyright© by Houghton Mifflin Company. All rights reserved. 9

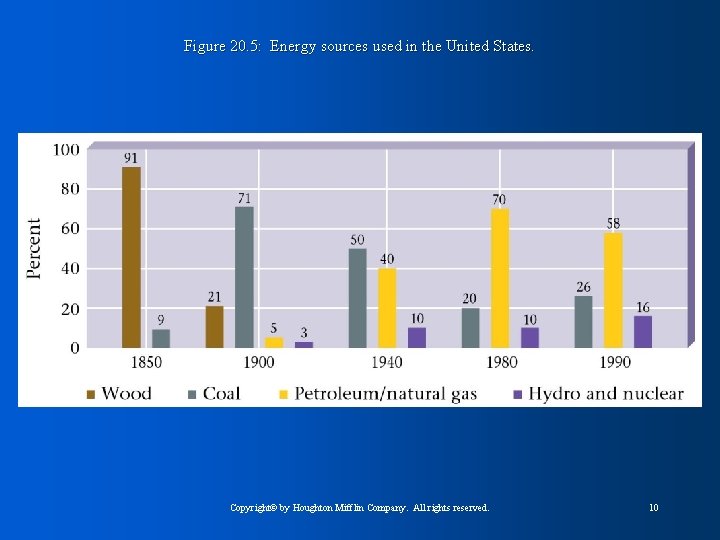
Figure 20. 5: Energy sources used in the United States. Copyright© by Houghton Mifflin Company. All rights reserved. 10

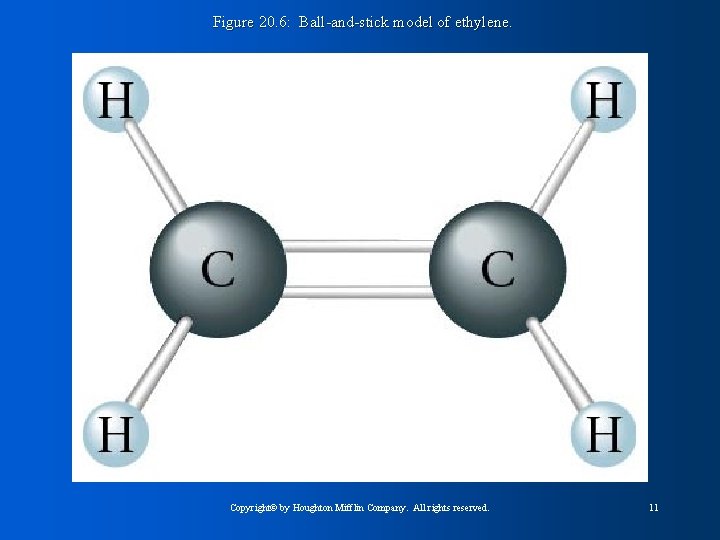
Figure 20. 6: Ball-and-stick model of ethylene. Copyright© by Houghton Mifflin Company. All rights reserved. 11

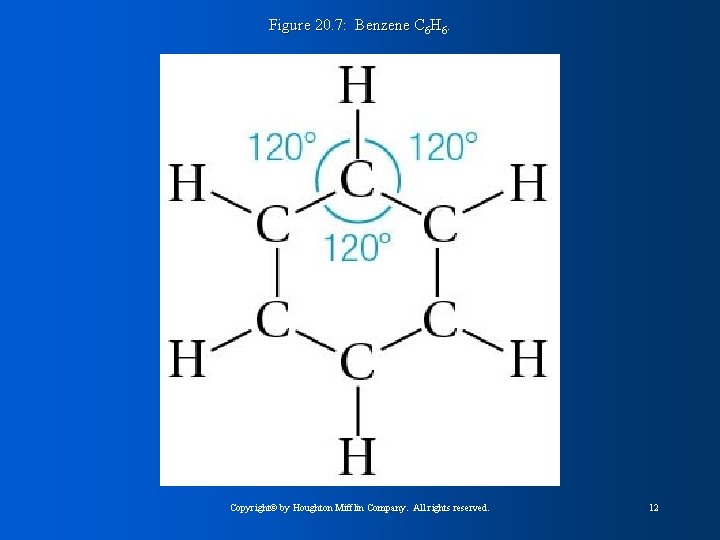
Figure 20. 7: Benzene C 6 H 6. Copyright© by Houghton Mifflin Company. All rights reserved. 12

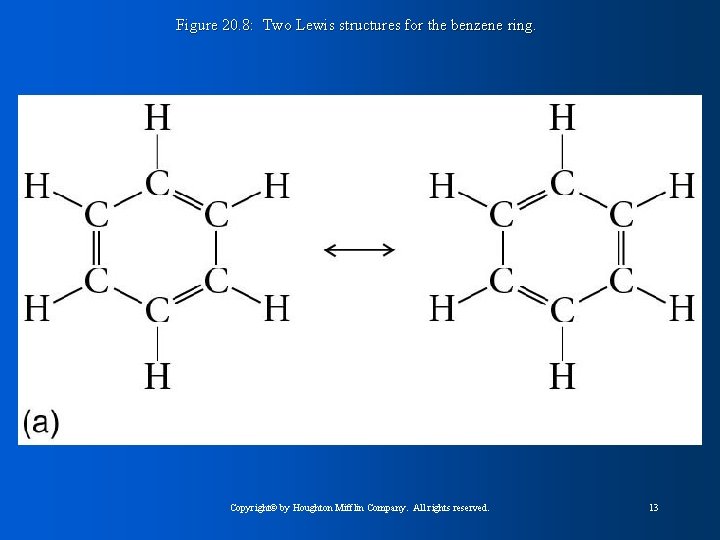
Figure 20. 8: Two Lewis structures for the benzene ring. Copyright© by Houghton Mifflin Company. All rights reserved. 13

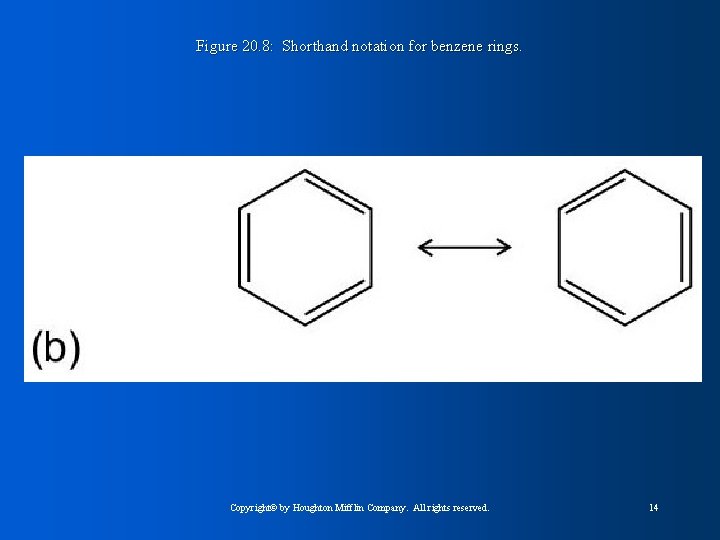
Figure 20. 8: Shorthand notation for benzene rings. Copyright© by Houghton Mifflin Company. All rights reserved. 14

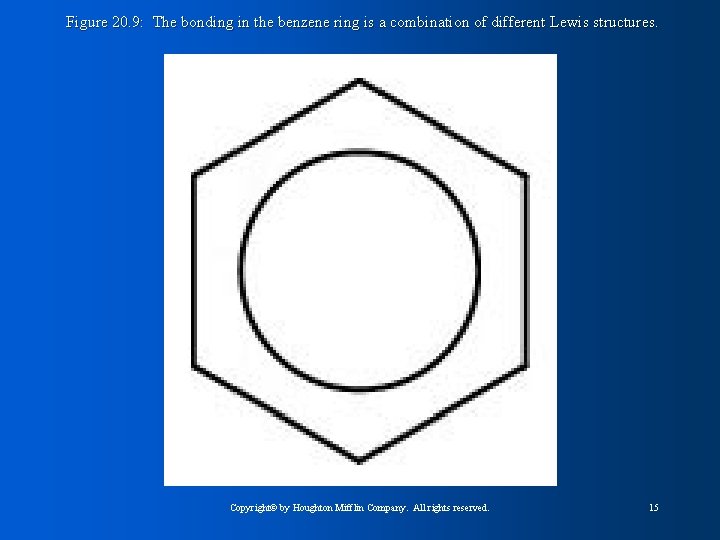
Figure 20. 9: The bonding in the benzene ring is a combination of different Lewis structures. Copyright© by Houghton Mifflin Company. All rights reserved. 15

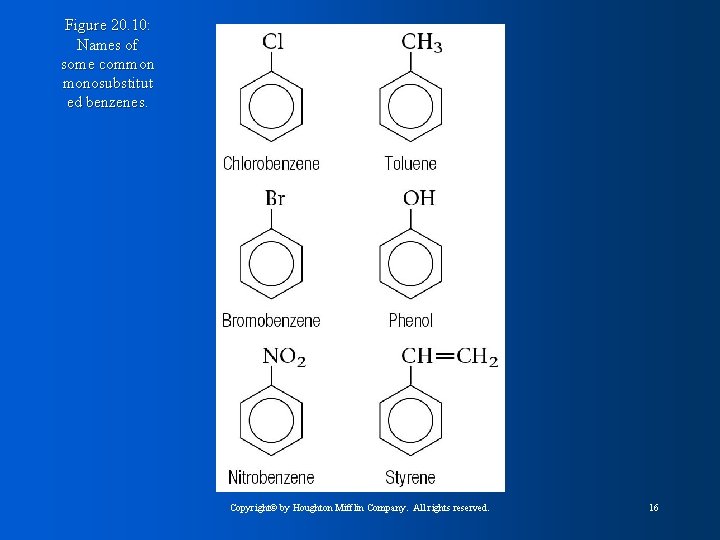
Figure 20. 10: Names of some common monosubstitut ed benzenes. Copyright© by Houghton Mifflin Company. All rights reserved. 16

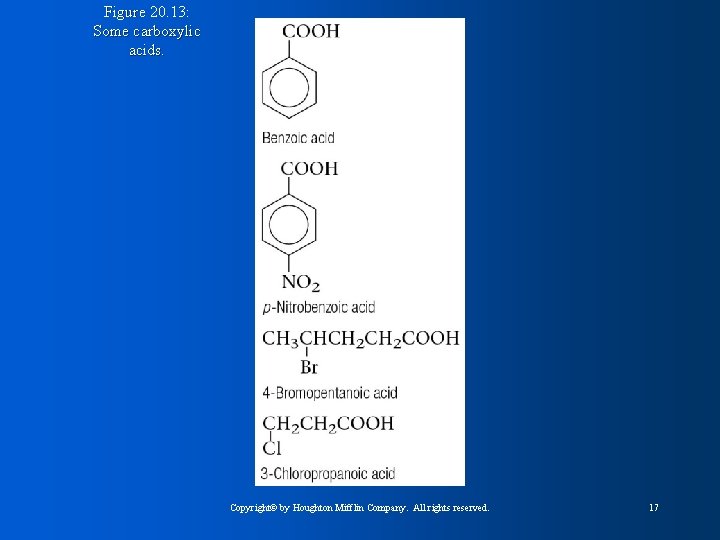
Figure 20. 13: Some carboxylic acids. Copyright© by Houghton Mifflin Company. All rights reserved. 17

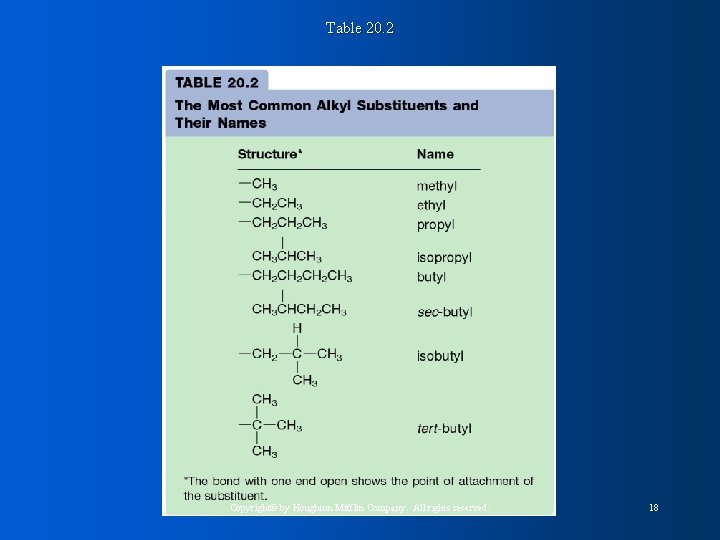
Table 20. 2 Copyright© by Houghton Mifflin Company. All rights reserved. 18

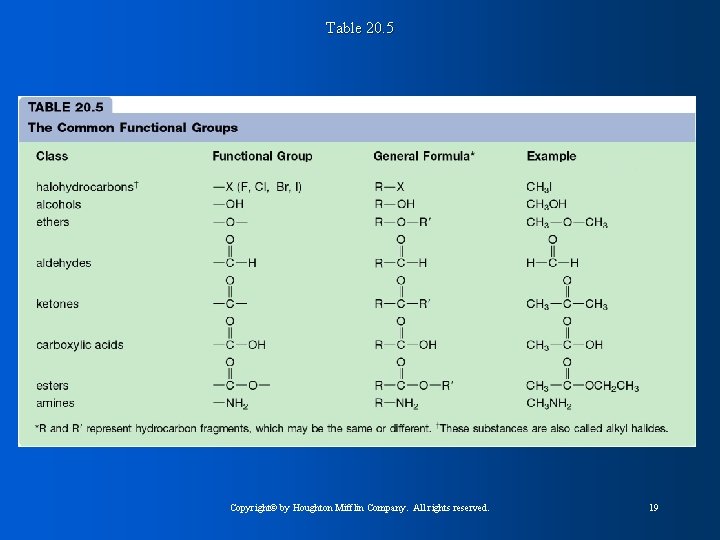
Table 20. 5 Copyright© by Houghton Mifflin Company. All rights reserved. 19
 Ap chemistry notes zumdahl
Ap chemistry notes zumdahl C/lamba
C/lamba De broglie hypothesis
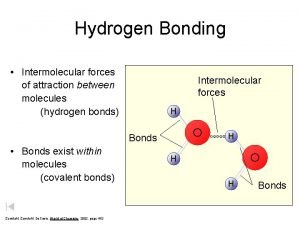
De broglie hypothesis Hydrogen bonding intermolecular forces
Hydrogen bonding intermolecular forces Electron cloud model
Electron cloud model Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Costo uniforme
Costo uniforme Coste explícito
Coste explícito Coste marginal
Coste marginal Activos financieros a coste
Activos financieros a coste Il mare di baffin lambisce le coste
Il mare di baffin lambisce le coste Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Ap world history chapter 25 africa and the atlantic world
Ap world history chapter 25 africa and the atlantic world Old world monkeys vs new world monkeys
Old world monkeys vs new world monkeys New world to old world columbian exchange
New world to old world columbian exchange Real world vs digital world
Real world vs digital world The world of the forms
The world of the forms The changing world output and world trade picture
The changing world output and world trade picture