Zumdahl De Coste World of CHEMISTRY Chapter 12


- Slides: 24

Zumdahl De. Coste World of CHEMISTRY

Chapter 12 Chemical Bonding Copyright© by Houghton Mifflin Company. All rights reserved.

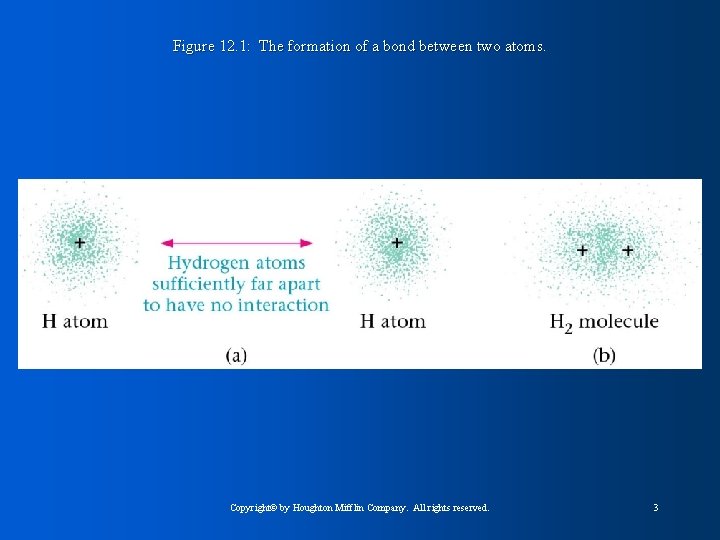
Figure 12. 1: The formation of a bond between two atoms. Copyright© by Houghton Mifflin Company. All rights reserved. 3

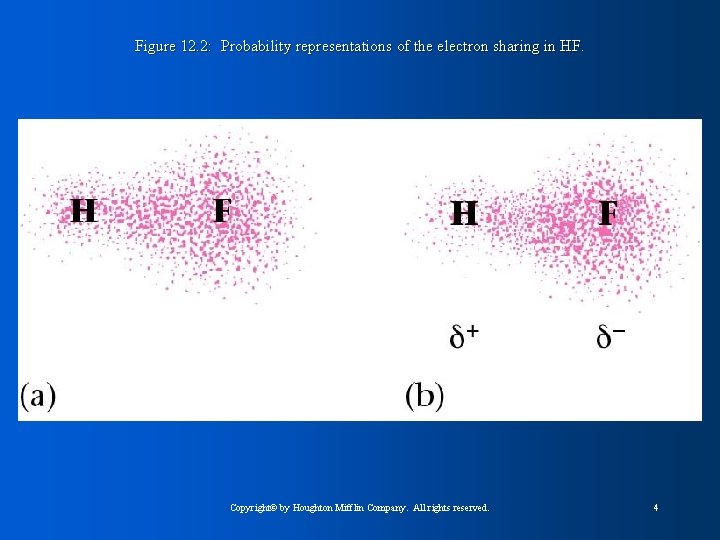
Figure 12. 2: Probability representations of the electron sharing in HF. Copyright© by Houghton Mifflin Company. All rights reserved. 4

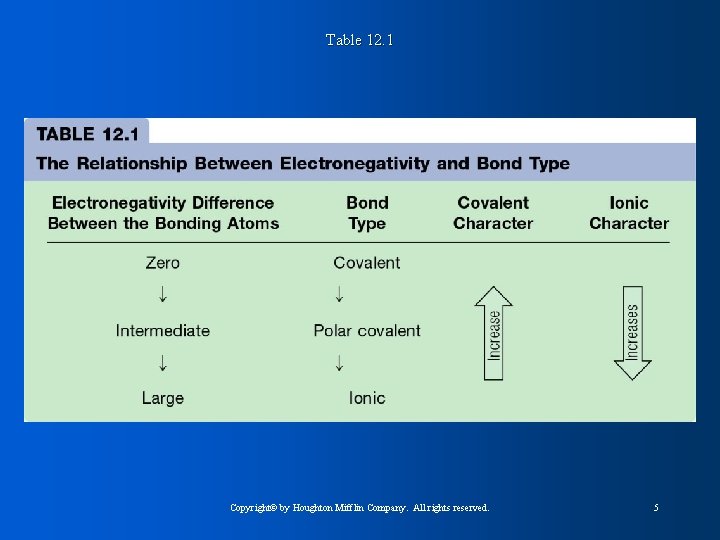
Table 12. 1 Copyright© by Houghton Mifflin Company. All rights reserved. 5

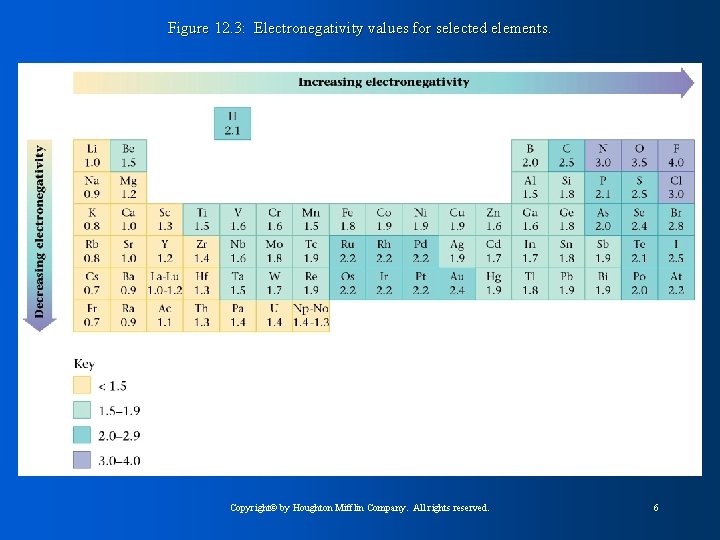
Figure 12. 3: Electronegativity values for selected elements. Copyright© by Houghton Mifflin Company. All rights reserved. 6

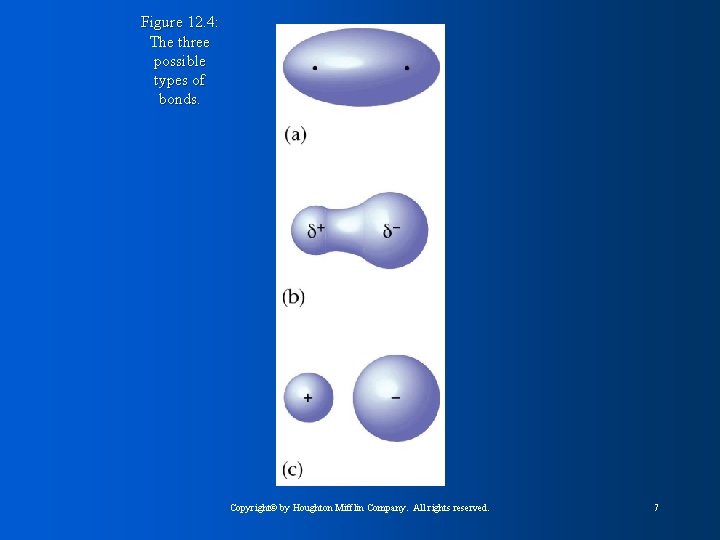
Figure 12. 4: The three possible types of bonds. Copyright© by Houghton Mifflin Company. All rights reserved. 7

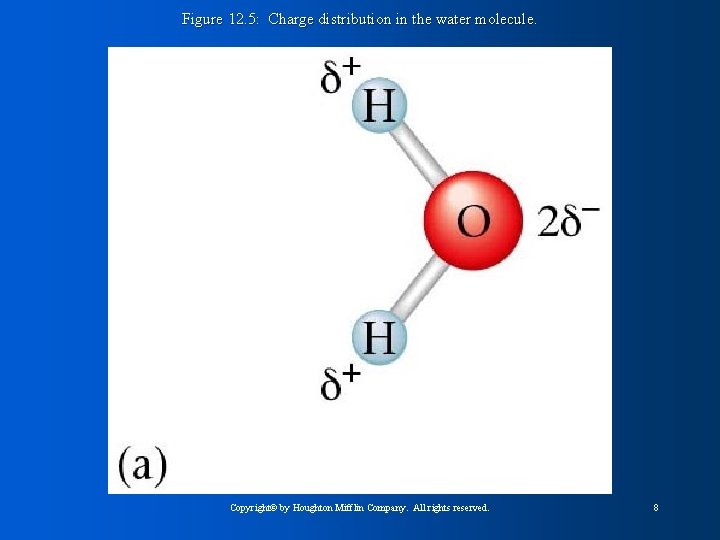
Figure 12. 5: Charge distribution in the water molecule. Copyright© by Houghton Mifflin Company. All rights reserved. 8

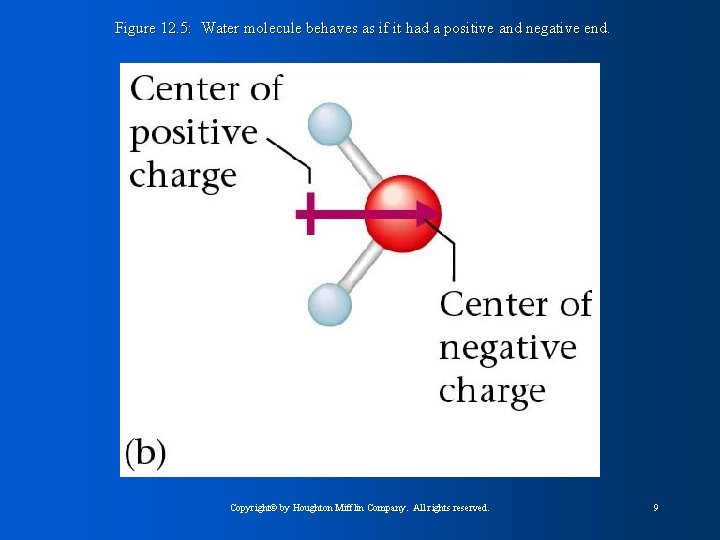
Figure 12. 5: Water molecule behaves as if it had a positive and negative end. Copyright© by Houghton Mifflin Company. All rights reserved. 9

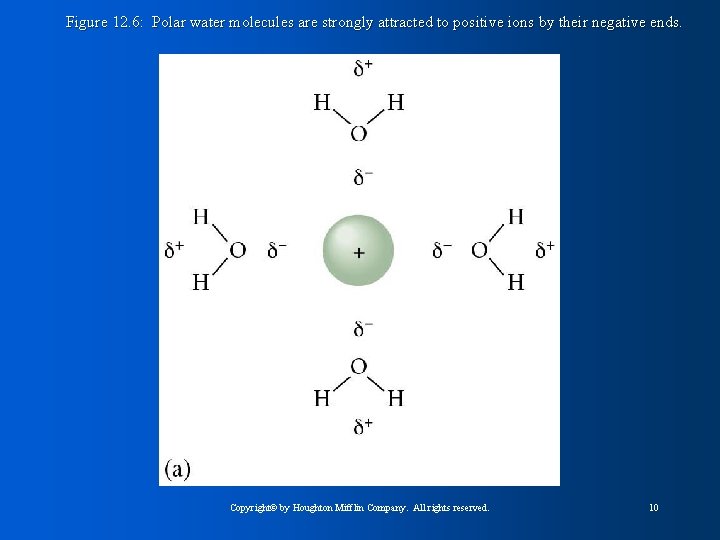
Figure 12. 6: Polar water molecules are strongly attracted to positive ions by their negative ends. Copyright© by Houghton Mifflin Company. All rights reserved. 10

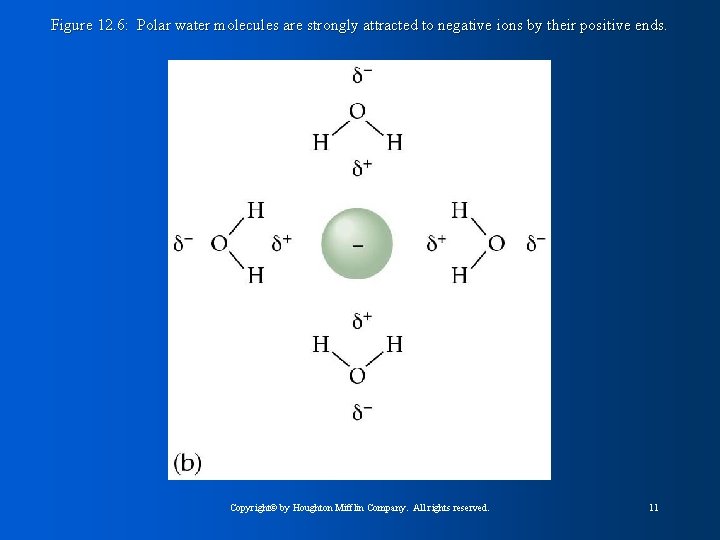
Figure 12. 6: Polar water molecules are strongly attracted to negative ions by their positive ends. Copyright© by Houghton Mifflin Company. All rights reserved. 11

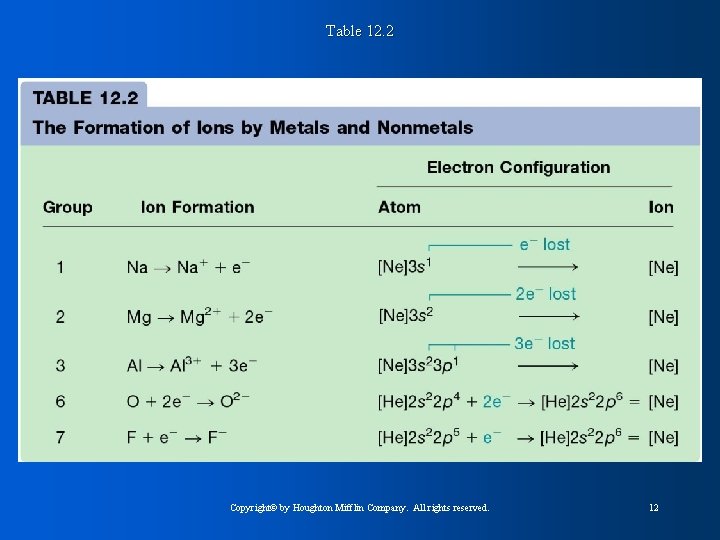
Table 12. 2 Copyright© by Houghton Mifflin Company. All rights reserved. 12

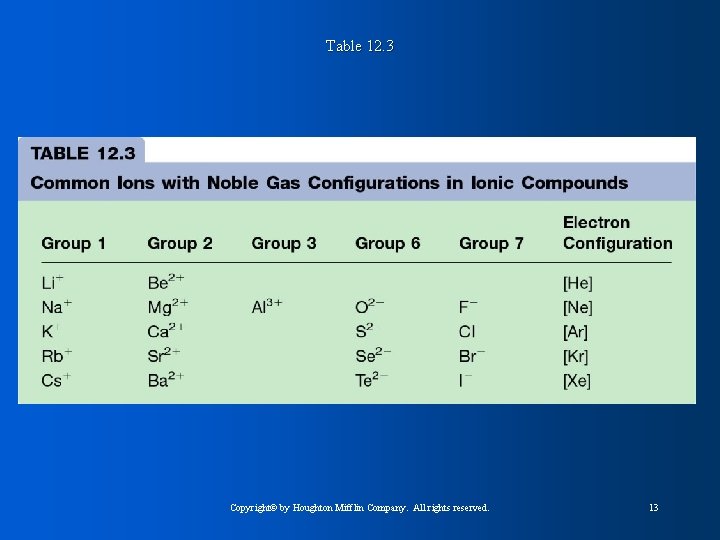
Table 12. 3 Copyright© by Houghton Mifflin Company. All rights reserved. 13

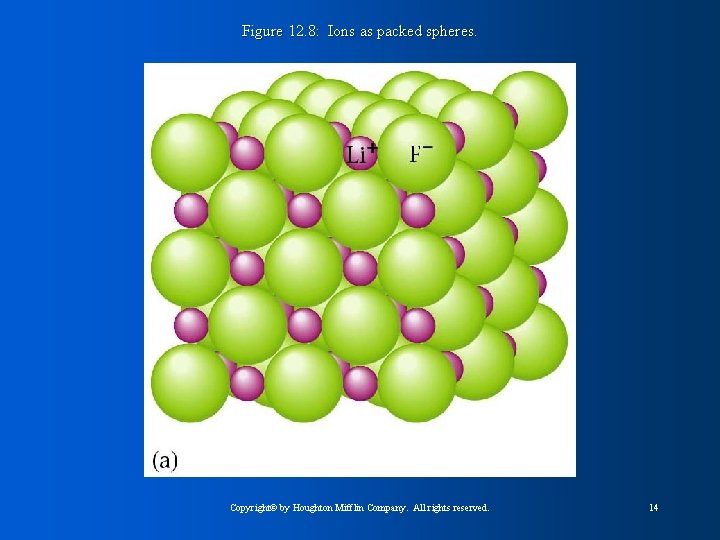
Figure 12. 8: Ions as packed spheres. Copyright© by Houghton Mifflin Company. All rights reserved. 14

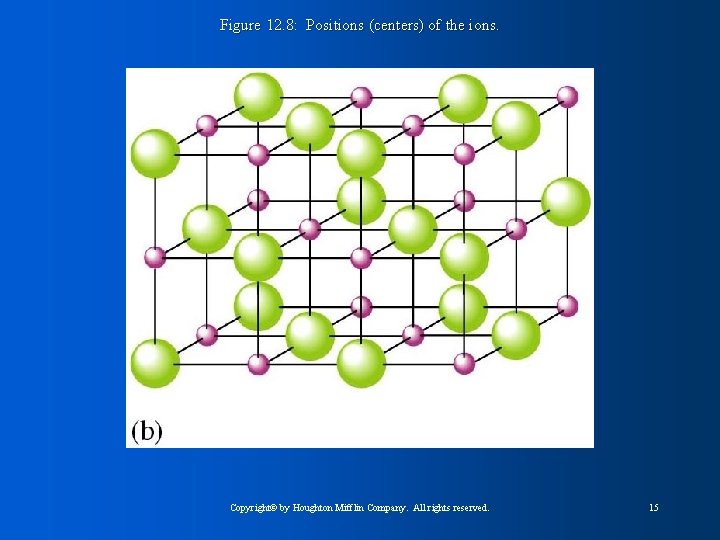
Figure 12. 8: Positions (centers) of the ions. Copyright© by Houghton Mifflin Company. All rights reserved. 15

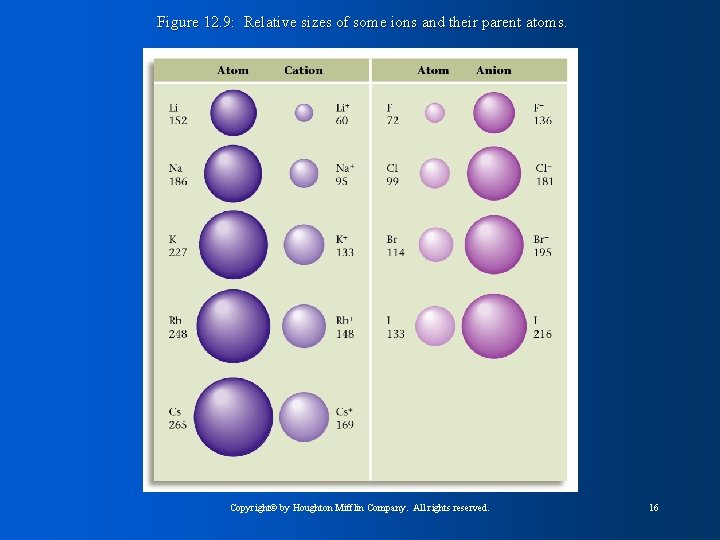
Figure 12. 9: Relative sizes of some ions and their parent atoms. Copyright© by Houghton Mifflin Company. All rights reserved. 16

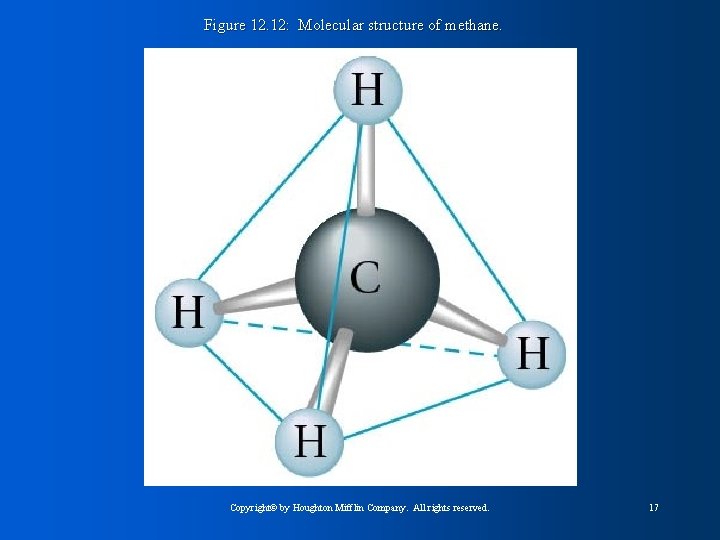
Figure 12. 12: Molecular structure of methane. Copyright© by Houghton Mifflin Company. All rights reserved. 17

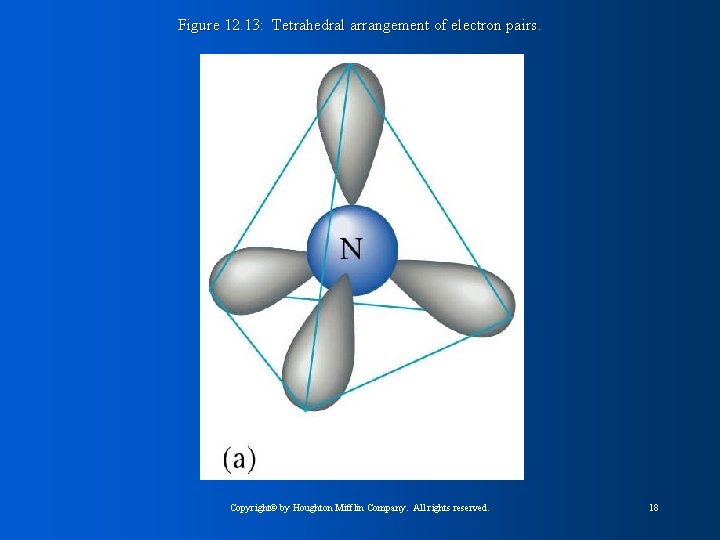
Figure 12. 13: Tetrahedral arrangement of electron pairs. Copyright© by Houghton Mifflin Company. All rights reserved. 18

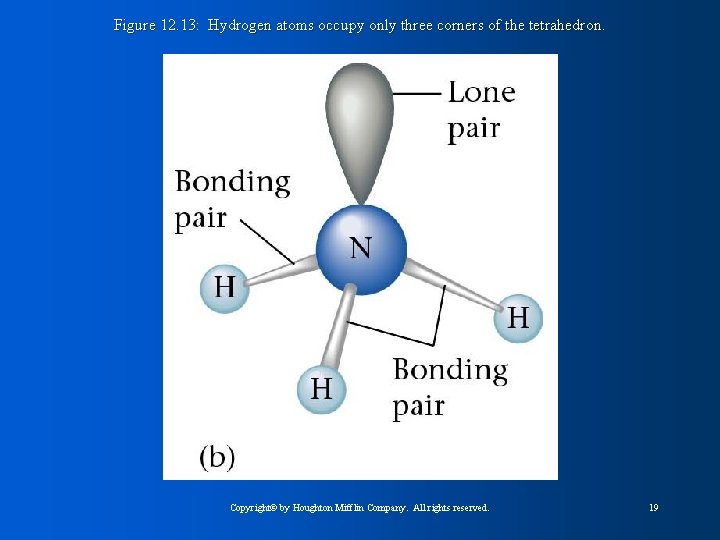
Figure 12. 13: Hydrogen atoms occupy only three corners of the tetrahedron. Copyright© by Houghton Mifflin Company. All rights reserved. 19

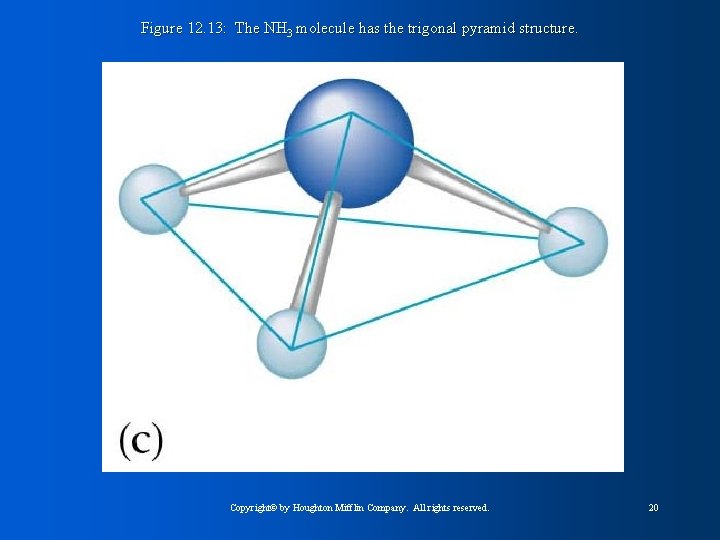
Figure 12. 13: The NH 3 molecule has the trigonal pyramid structure. Copyright© by Houghton Mifflin Company. All rights reserved. 20

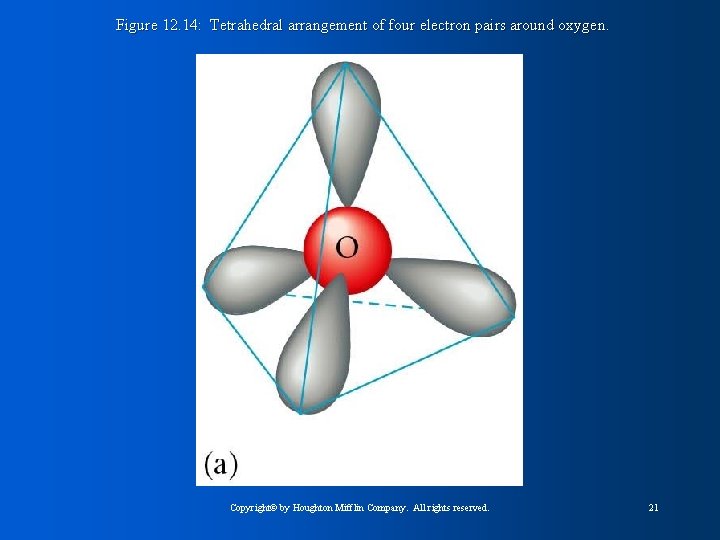
Figure 12. 14: Tetrahedral arrangement of four electron pairs around oxygen. Copyright© by Houghton Mifflin Company. All rights reserved. 21

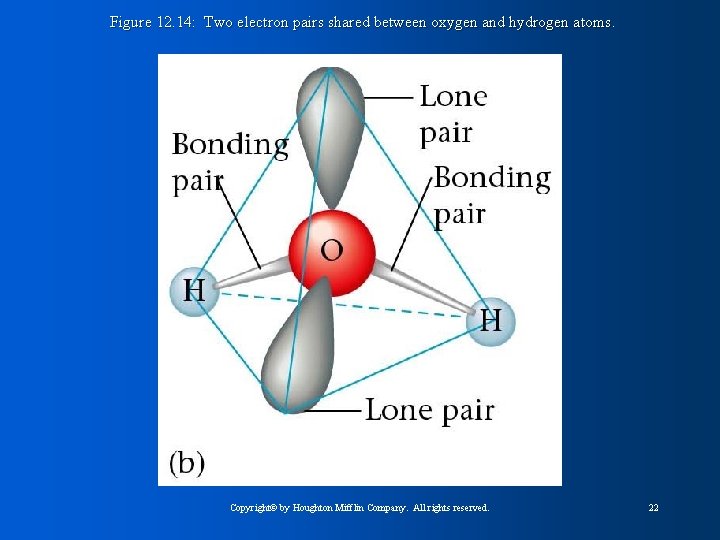
Figure 12. 14: Two electron pairs shared between oxygen and hydrogen atoms. Copyright© by Houghton Mifflin Company. All rights reserved. 22

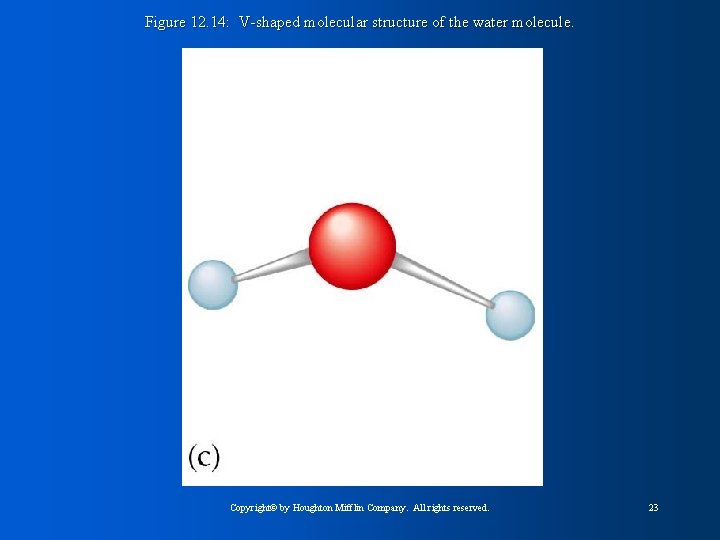
Figure 12. 14: V-shaped molecular structure of the water molecule. Copyright© by Houghton Mifflin Company. All rights reserved. 23

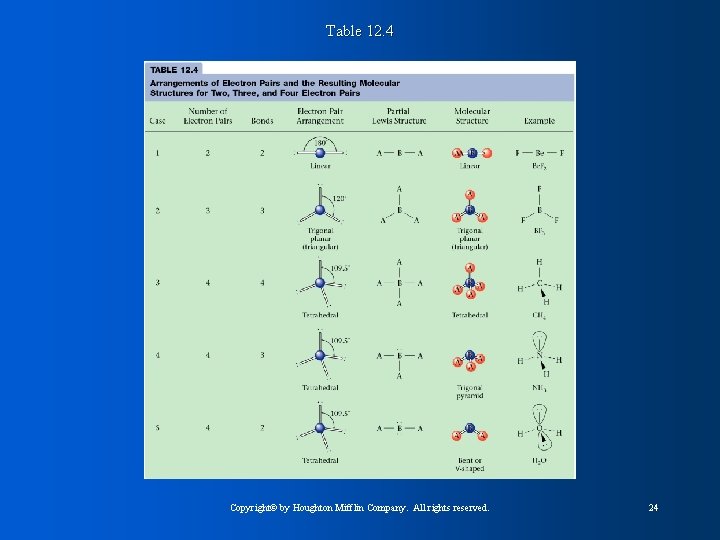
Table 12. 4 Copyright© by Houghton Mifflin Company. All rights reserved. 24
 Ap chemistry notes zumdahl
Ap chemistry notes zumdahl C=lambda/f
C=lambda/f De broglie hypothesis
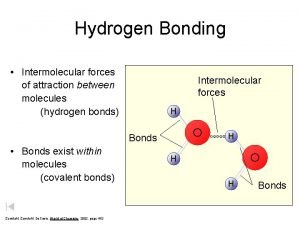
De broglie hypothesis Intermolecular forces hydrogen bonding
Intermolecular forces hydrogen bonding Electron cloud model
Electron cloud model Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Busqueda de coste uniforme
Busqueda de coste uniforme Coste explícito
Coste explícito Coste marginal
Coste marginal Mural uv
Mural uv Il mare di baffin lambisce le coste
Il mare di baffin lambisce le coste Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Ap world history chapter 25 africa and the atlantic world
Ap world history chapter 25 africa and the atlantic world Site:slidetodoc.com
Site:slidetodoc.com New world to old world columbian exchange
New world to old world columbian exchange Real world vs digital world
Real world vs digital world The world of the forms
The world of the forms The changing world output and world trade picture
The changing world output and world trade picture Dangerous world tour history world tour - hockenheimring
Dangerous world tour history world tour - hockenheimring What does shakespeare head symbolises
What does shakespeare head symbolises Cloudless at dawn figure of speech
Cloudless at dawn figure of speech Unit 9 english in the world looking back
Unit 9 english in the world looking back The changing world output and world trade picture
The changing world output and world trade picture