Zumdahl De Coste World of CHEMISTRY Chapter 18












- Slides: 27

Zumdahl De. Coste World of CHEMISTRY

Chapter 18 Oxidation-Reduction Reactions and Electrochemistry Copyright© by Houghton Mifflin Company. All rights reserved.

Overview Metal/Nonmetal oxidation reduction reactions n Assign oxidation states n What are oxidation and reduction n Identify oxidizing and reducing agents n

Oxidation-Reduction Examples Forest fire n Rusting steel n Internal combustion engine n Food metabolism n Most reactions that provide energy & batteries n

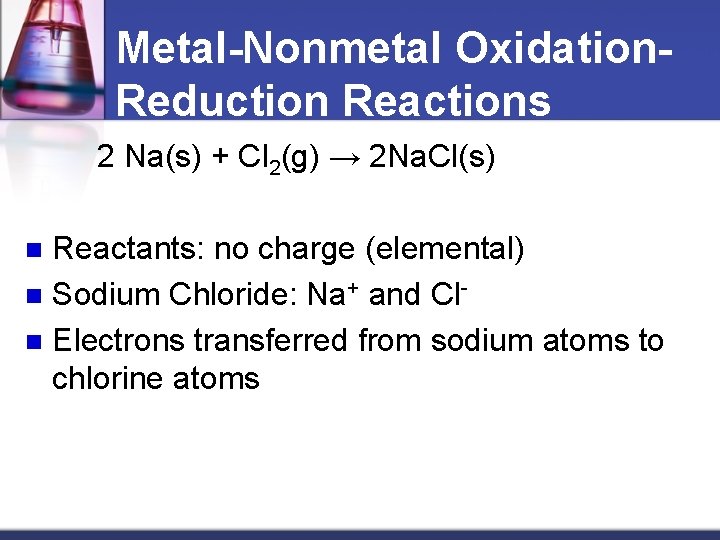
Metal-Nonmetal Oxidation. Reduction Reactions 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) Reactants: no charge (elemental) n Sodium Chloride: Na+ and Cln Electrons transferred from sodium atoms to chlorine atoms n

Definitions n Oxidation-reduction reaction: reaction in which one or more electrons are transferred n Also called redox reactions Oxidation: loss of electrons n Reduction: gain of electrons n

2 Na(s) + Cl 2(g) → 2 Na. Cl(s) Sodium atom loses electron = sodium is oxidized n Chlorine atom gains electron = chlorine is reduced n Whenever a metal and nonmetal react to form an ionic compound, oxidationreduction reaction occurs n Metal is oxidized (loses electrons) n Nonmetal is reduced (gains electrons) n

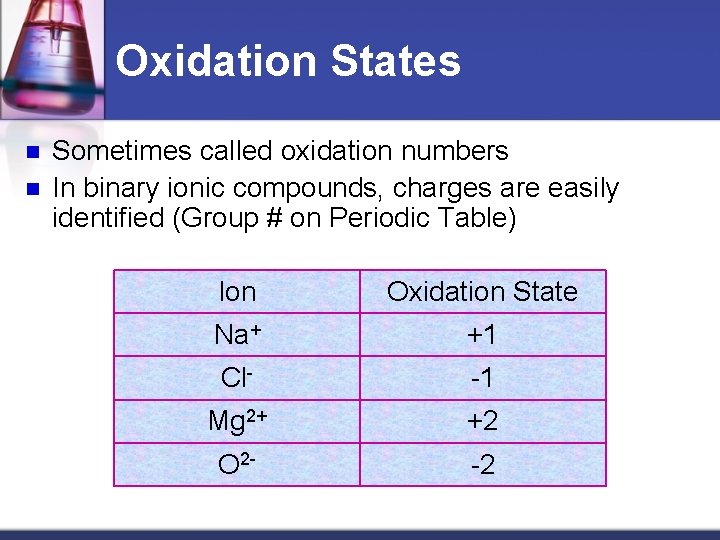
Oxidation States Uncombined element = no charge (neutral) Na metal = neutral Cl 2 gas = neutral Oxidation state = 0

Oxidation States n n Sometimes called oxidation numbers In binary ionic compounds, charges are easily identified (Group # on Periodic Table) Ion Oxidation State Na+ +1 Cl- -1 Mg 2+ +2 O 2 - -2

Covalent Compounds: Oxidation States n n n No ions present in covalent compounds (electrons are shared) Useful to assign imaginary charges to elements in covalent compounds which = the oxidation states Assume shared electrons are transferred to more electronegative atom

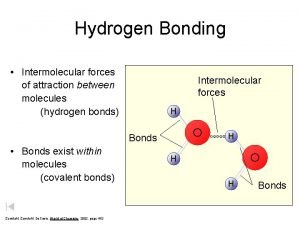
Example: Water (H 2 O) Oxygen is more electronegative than Hydrogen n Each hydrogen loses electron to oxygen n Hydrogen = oxidation state of +1 n Oxygen = oxidation state of -2 (1 electron from each hydrogen) n n Most electronegative: F>O>N>Cl n NO 2 n Oxygen = -2 Nitrogen = +4

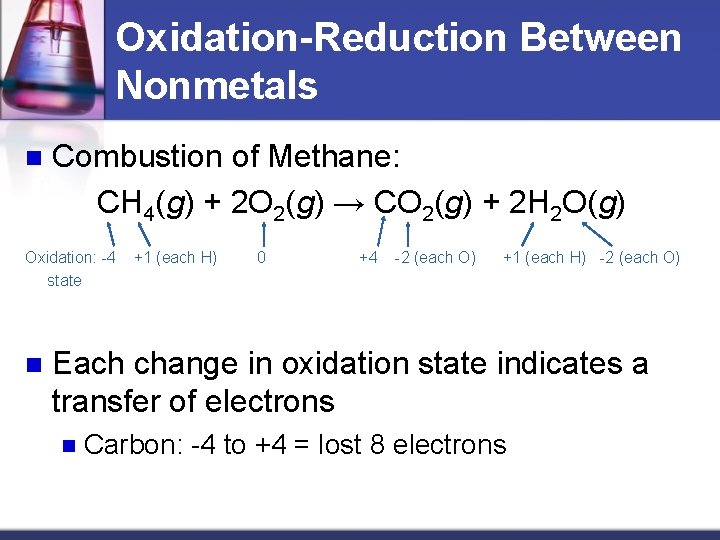
Oxidation-Reduction Between Nonmetals n Combustion of Methane: CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) Oxidation: -4 state n +1 (each H) 0 +4 -2 (each O) +1 (each H) -2 (each O) Each change in oxidation state indicates a transfer of electrons n Carbon: -4 to +4 = lost 8 electrons

Oxidation/Reduction Redefined Oxidation: an increase in oxidation state (loss of electrons) n Reduction: a decrease in oxidation state (gain of electrons) n Oxidizing agent = electron acceptor, reactant containing element that is reduced n Reducing agent = electron donor, reactant containing element that is oxidized n

Combustion of Methane: Another Look CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) Oxidation: -4 state +1 (each H) 0 +4 -2 (each O) +1 (each H) -2 (each O) Carbon = oxidized (increase in oxidation state) n CH 4 = reducing agent (contains oxidized carbon & furnishes electrons) n Oxygen = reduced (decrease in oxidation state) n Oxygen = oxidizing agent n

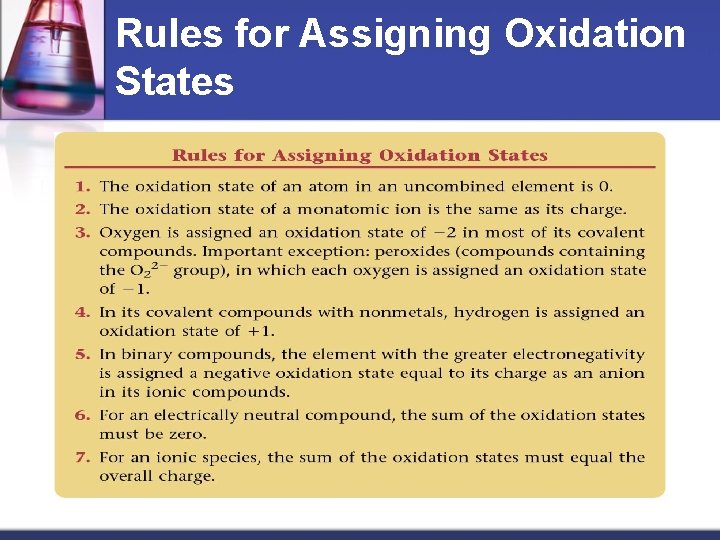
Rules for Assigning Oxidation States

Electrochemistry The study of the interchange of chemical and electrical energy n Battery uses energy from oxidation-reduction reaction to produce energy n Involves two types of processes n Production of an electric current from a chemical reaction n Use of electric current to produce a chemical change n

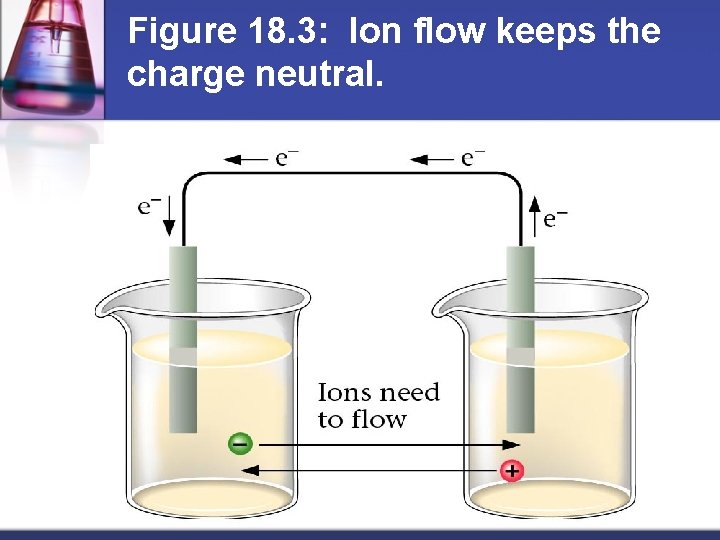
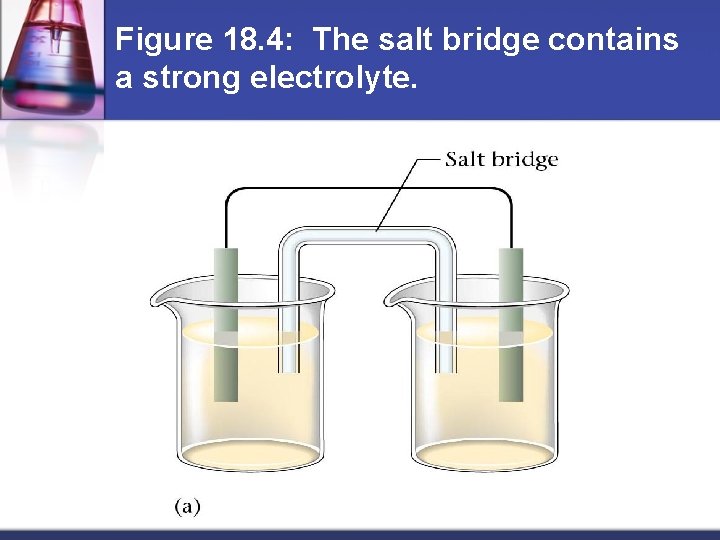
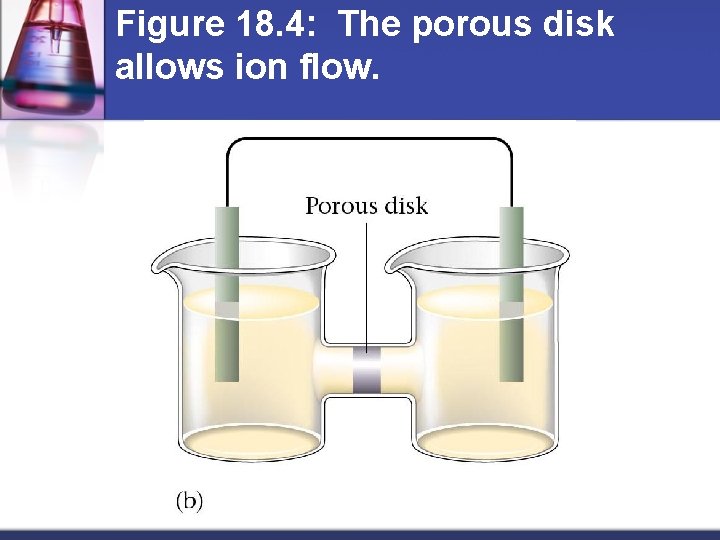
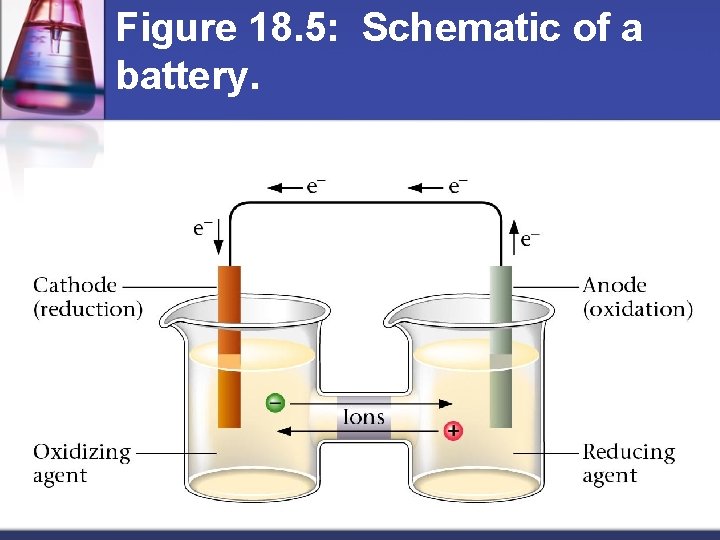
How do batteries work? Separate oxidizing agent (electron acceptor) from reducing agent (electron donor) n Electron transfer must occur through a wire n Current produced in wire by electron flow can be directed through a device to produce useful work n Anode = electrode where oxidation occurs n Cathode = electrode where reduction occurs n

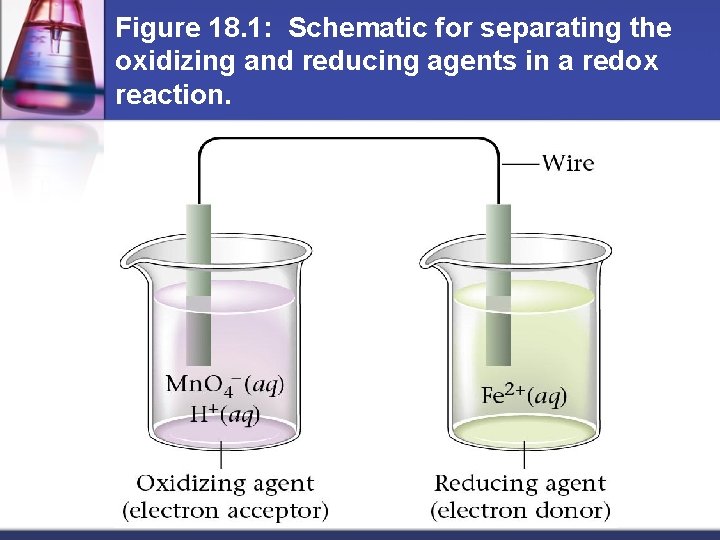
Figure 18. 1: Schematic for separating the oxidizing and reducing agents in a redox reaction.

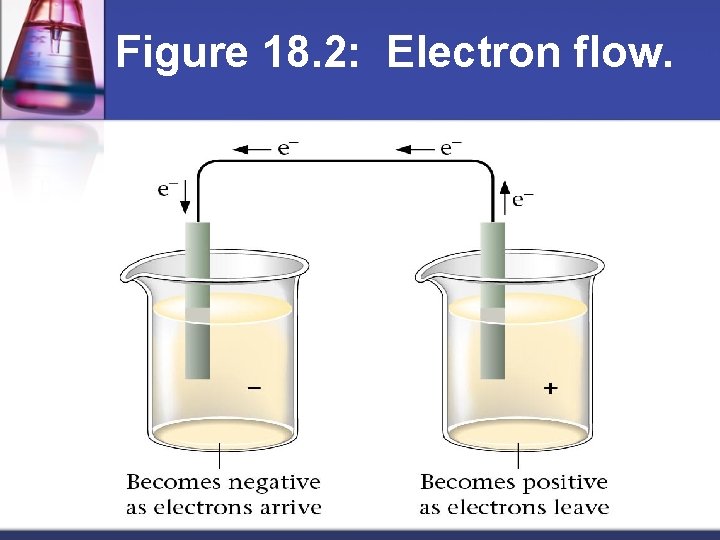
Figure 18. 2: Electron flow.

Figure 18. 3: Ion flow keeps the charge neutral.

Figure 18. 4: The salt bridge contains a strong electrolyte.

Figure 18. 4: The porous disk allows ion flow.

Figure 18. 5: Schematic of a battery.

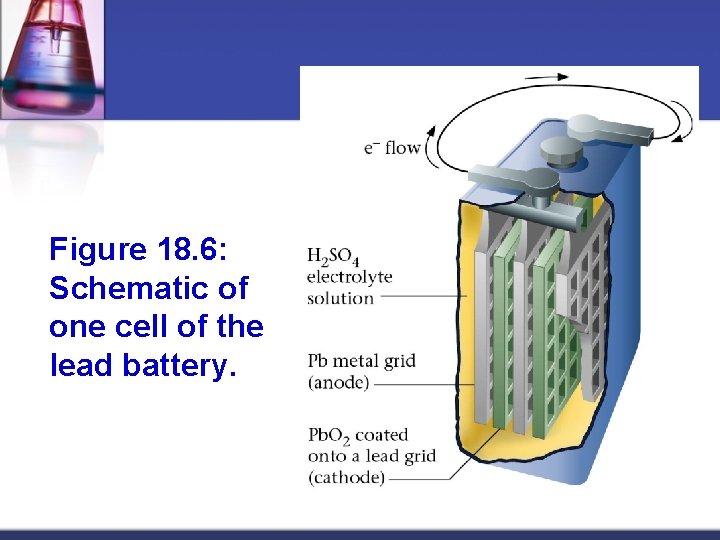
Figure 18. 6: Schematic of one cell of the lead battery.

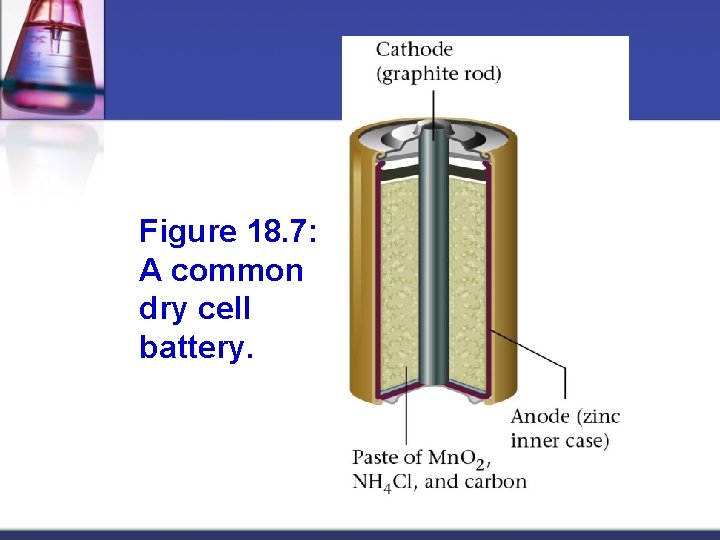
Figure 18. 7: A common dry cell battery.

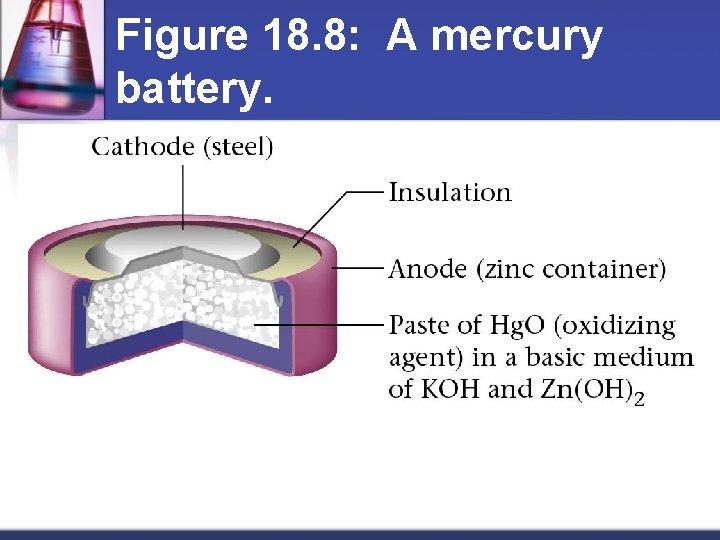
Figure 18. 8: A mercury battery.

Electrolysis Electrical energy is used to produce a chemical change n Force current through a cell to produce a chemical change that would not otherwise occur n
 Ap chemistry notes zumdahl
Ap chemistry notes zumdahl C/lamba
C/lamba Zumdahl chemistry
Zumdahl chemistry Example of ion dipole
Example of ion dipole Electron cloud model
Electron cloud model Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Busqueda de coste uniforme
Busqueda de coste uniforme Coste explícito
Coste explícito Coste marginal
Coste marginal Activos financieros a coste
Activos financieros a coste Il mare di baffin lambisce le coste
Il mare di baffin lambisce le coste Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Ap world history chapter 25 africa and the atlantic world
Ap world history chapter 25 africa and the atlantic world Old vs new world monkeys
Old vs new world monkeys Coffee new world or old world
Coffee new world or old world Real world vs digital world
Real world vs digital world Theory of forms plato
Theory of forms plato The changing world output and world trade picture
The changing world output and world trade picture Bad world tour
Bad world tour Sour cream walls is an example of pun
Sour cream walls is an example of pun Open handed map
Open handed map English world 1 unit 9
English world 1 unit 9 The changing world output and world trade picture
The changing world output and world trade picture Approach chemistry chalk chapter
Approach chemistry chalk chapter Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Pericyclic
Pericyclic