Zumdahl De Coste World of CHEMISTRY Chapter 4









- Slides: 18

Zumdahl • De. Coste World of CHEMISTRY

Chapter 4 Nomenclature

Goals of Chapter 4 1. 2. 3. 4. 5. Name binary compounds of metal and nonmetal Name binary compounds containing only non-metals Learn names of polyatomic ions and how to use them in naming Learn common acids and how to name them Write the formula of a compound when name is given Copyright © Houghton Mifflin Company 3

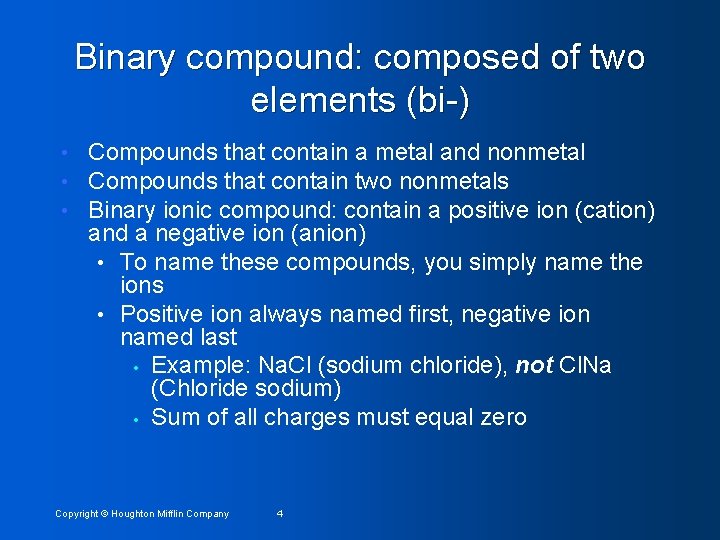
Binary compound: composed of two elements (bi-) • • • Compounds that contain a metal and nonmetal Compounds that contain two nonmetals Binary ionic compound: contain a positive ion (cation) and a negative ion (anion) • To name these compounds, you simply name the ions • Positive ion always named first, negative ion named last • Example: Na. Cl (sodium chloride), not Cl. Na (Chloride sodium) • Sum of all charges must equal zero Copyright © Houghton Mifflin Company 4

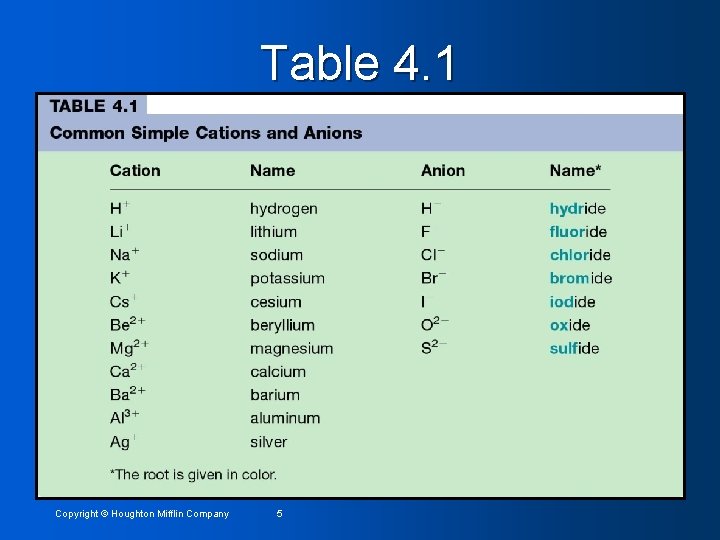
Table 4. 1 Copyright © Houghton Mifflin Company 5

Type I Binary Compounds: The metal present only forms one type of cation. Group 1 and 2 metals (sometimes Group 3) • See Table 4. 1 • Rules for naming • • The cation is always named first and the anion named second • A simple cation (obtained from a single atom) takes its name from the name of the element • A simple anion (obtained from a single atom) is named by taking the first part of the element name (the root) and adding –ide. Copyright © Houghton Mifflin Company 6

Examples of Type I Binary Compounds • Na. Cl • Sodium Chloride • Na+1 combines with Cl-1, sum is zero • KI • Potassium Iodide • K+1 combines with I-1, sum is zero • Mg. Cl 2 • Magnesium Chloride • Mg+2 combines with 2 Cl-1, sum is zero Copyright © Houghton Mifflin Company 7

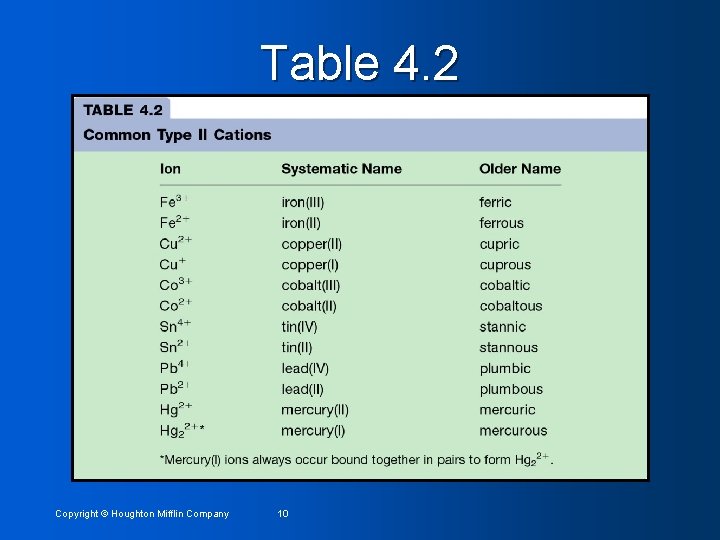
Type II Binary Compounds: The metal present can form two (or more) cations that have different charges. Many transition metals (some Group 3) • See Table 4. 2 • Rules for naming • • Use basically same procedure as Type I, except • Use Roman Numerals to designate charge on cations (i. e. Fe 2+ = Iron (II)) • Old system (sometimes still used): Ion with the higher charge has a name ending in –ic and ion with the lower charge has a name ending in –ous. For example Fe 2+ = ferrous ion and Fe 3+ = ferric ion. • Do not use Roman numerals for Type I compounds! Copyright © Houghton Mifflin Company 8

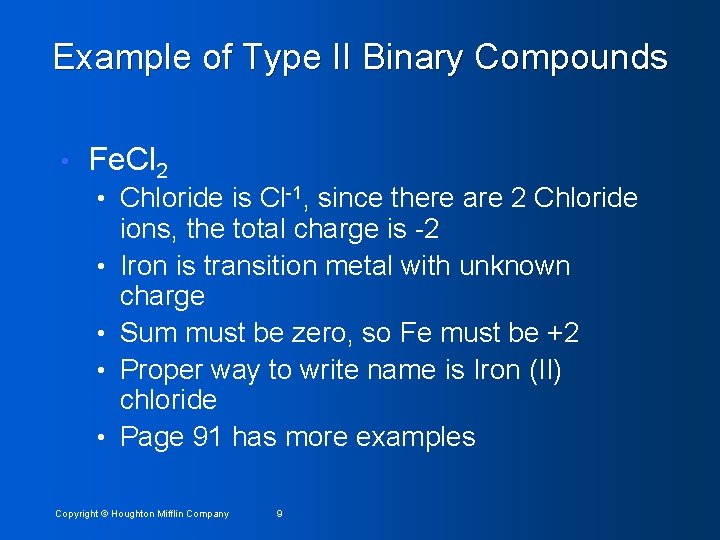
Example of Type II Binary Compounds • Fe. Cl 2 • Chloride is Cl-1, since there are 2 Chloride • • ions, the total charge is -2 Iron is transition metal with unknown charge Sum must be zero, so Fe must be +2 Proper way to write name is Iron (II) chloride Page 91 has more examples Copyright © Houghton Mifflin Company 9

Table 4. 2 Copyright © Houghton Mifflin Company 10

Type III Binary Compounds: compounds containing only nonmetals See prefixes in Table 4. 3 • Rules for naming • • The first element in the formula is named first • The second element named as though it were an anion (oxygen → oxide) • Prefixes are used to denote the numbers of atoms present (O 2 = dioxide) • The prefix mono- is never used when naming the first element. (CO is carbon monoxide not monocarbon monoxide) • To avoid awkward pronunciation, drop final o or a of prefix when second element is oxygen Copyright © Houghton Mifflin Company 11

Some compounds are always referred to by the common names: • H 2 O = water • NH 3 = ammonia Copyright © Houghton Mifflin Company 12

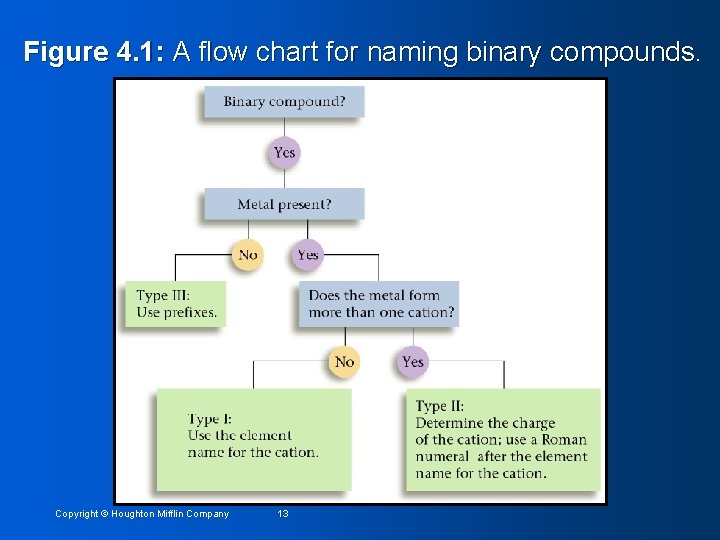
Figure 4. 1: A flow chart for naming binary compounds. Copyright © Houghton Mifflin Company 13

Polyatomic Ions: charged entities composed of several atoms bound together • • Entire group has a positive or negative charge Oxyanions: contain atom of a given element and different numbers of oxygen atoms (i. e. nitrate, nitrite) • Name of one with smaller number of oxygen atoms ends in –ite (nitrite, sulfite) • Name of one with larger number of oxygen atoms ends in –ate (nitrate, sulfate) • When more than two oxyanions in series, use hypo- (less than – for fewest) and per- (more than – for most) (hypochlorite, chlorate, perchlorate) • Rules for naming • Must recognize the polyatomic ion (break into two parts) • Use rules similar to naming binary ionic compounds • Treat polyatomic same as individual element, determine whether Type I, II, or III • See flow chart on page 102 Copyright © Houghton Mifflin Company 14

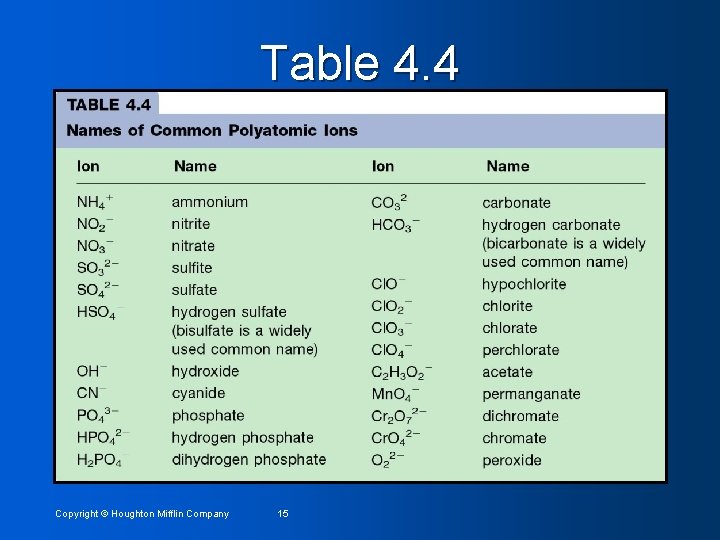
Table 4. 4 Copyright © Houghton Mifflin Company 15

Acids: molecules that produce H+ ions when dissolved in water • • First recognized by sour taste Molecule with one or more H+ ions attached to an anion Naming depends on whether or not oxygen present in anion Rules for naming • If anion does not contain oxygen: acid named with the prefix hydro- and the suffix –ic attached to the root of the name of the element (i. e. hydrochloric acid = HCl, hydrocyanic acid = HCN) • When anion contains oxygen: the acid name is formed from the root name of the central element of the anion or the anion name with a suffix of –ic or –ous. When the anion name ends in –ate, the suffix –ic is used (i. e. H 2 SO 4 = sulfuric acid). When anion name ends in -ite, the suffix – ous is used (i. e. H 2 SO 3 = sulfurous acid) • See flow chart on page 105 Copyright © Houghton Mifflin Company 16

Writing names from formulas Use naming process and work backwards

Figure 4. 2: Overall strategy for naming chemical compounds. Copyright © Houghton Mifflin Company 18
 Ap chemistry notes zumdahl
Ap chemistry notes zumdahl C/ lambda
C/ lambda Azimuthal quantum number
Azimuthal quantum number Zumdahl chemistry
Zumdahl chemistry Zumdahl chemistry
Zumdahl chemistry Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Algoritmo de costo uniforme
Algoritmo de costo uniforme Coste explícito
Coste explícito Coste marginal
Coste marginal Mural uv
Mural uv Il mare di baffin lambisce le coste
Il mare di baffin lambisce le coste Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Ap world history chapter 25 africa and the atlantic world
Ap world history chapter 25 africa and the atlantic world Primate opposable thumb
Primate opposable thumb New world to old world columbian exchange
New world to old world columbian exchange Real world vs digital world
Real world vs digital world What is world of forms
What is world of forms