Theoretical Methods for Surface Science part II Johan

![Thermodynamics for adsorption a ma Host Definition of adsorbate energy: Eads=DG=G[host+ads]-{G[host]+Na ma} where G(T, Thermodynamics for adsorption a ma Host Definition of adsorbate energy: Eads=DG=G[host+ads]-{G[host]+Na ma} where G(T,](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-16.jpg)
![DF[e. V] Adsorbate induced work function change Tang et al. , Surf. Sci. Lett. DF[e. V] Adsorbate induced work function change Tang et al. , Surf. Sci. Lett.](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-34.jpg)
![The polar Zn. O{0001}-surface Zn-terminated [0001]-surface [0001] O-terminated [0001]-surface International Max-Planck Research School Theoretical The polar Zn. O{0001}-surface Zn-terminated [0001]-surface [0001] O-terminated [0001]-surface International Max-Planck Research School Theoretical](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-42.jpg)
![STM of Zn. O[0001]-surface Dulub et al. , PRL 90, 016102 (2003) Triangular islands STM of Zn. O[0001]-surface Dulub et al. , PRL 90, 016102 (2003) Triangular islands](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-45.jpg)
![Surface Phase diagram of Zn. O[0001] Kresse et al. , PRB 68, 245409 (2003) Surface Phase diagram of Zn. O[0001] Kresse et al. , PRB 68, 245409 (2003)](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-46.jpg)

- Slides: 48

Theoretical Methods for Surface Science part II Johan M. Carlsson Theory Department Fritz-Haber-Institut der Max-Planck-Gesellschaft Faradayweg 4 -6, 14195 Berlin International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 1

Summary Last lecture: The foundations of the DFT How to calculate bulk properties and electronic structure How to model surfaces Surface structures This lecture: Electronic structure at surfaces Adsorption International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 2

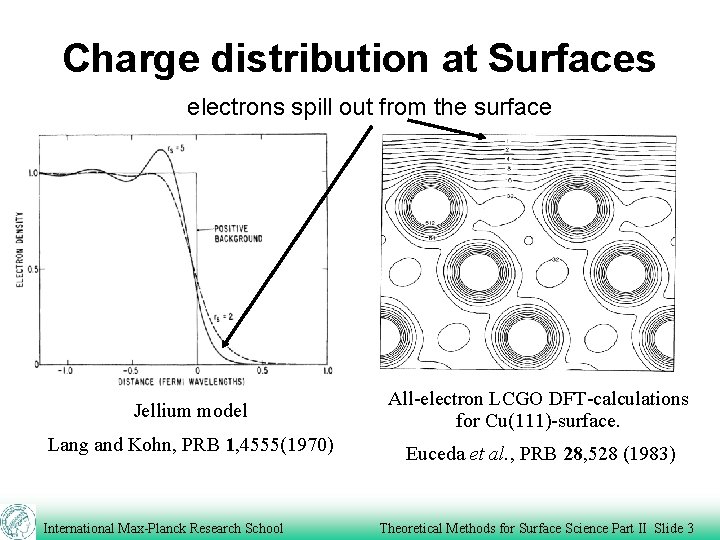
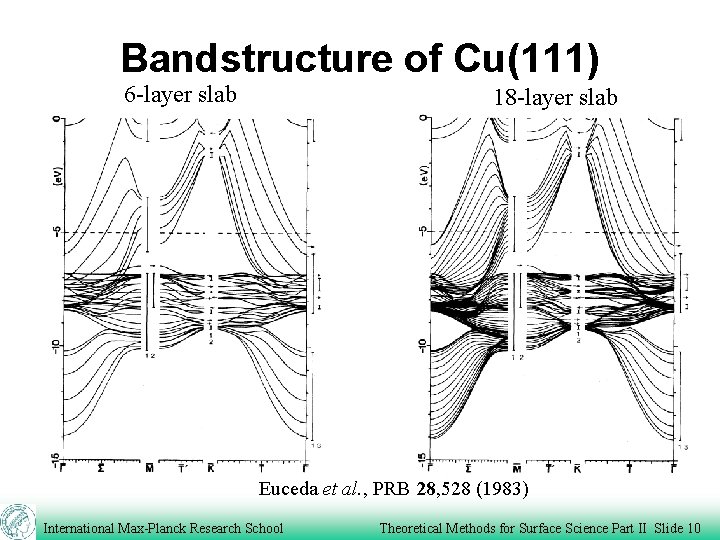
Charge distribution at Surfaces electrons spill out from the surface Jellium model All-electron LCGO DFT-calculations for Cu(111)-surface. Lang and Kohn, PRB 1, 4555(1970) Euceda et al. , PRB 28, 528 (1983) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 3

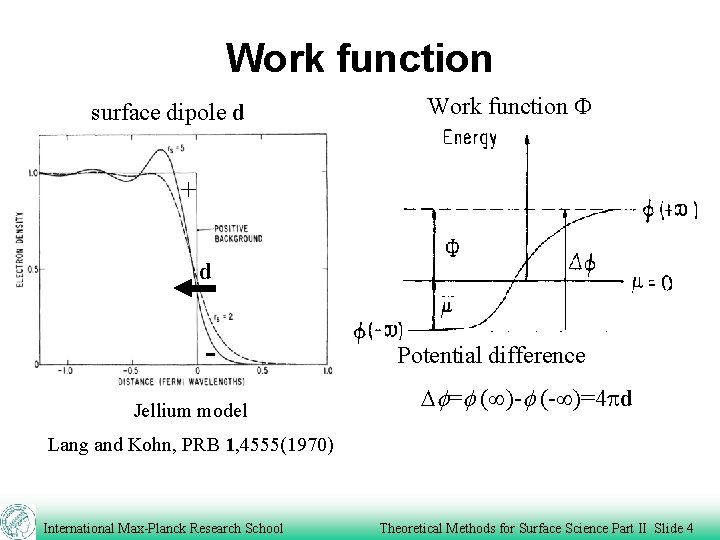
Work function surface dipole d Work function F + d Jellium model Potential difference Df=f ( )-f (- )=4 pd Lang and Kohn, PRB 1, 4555(1970) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 4

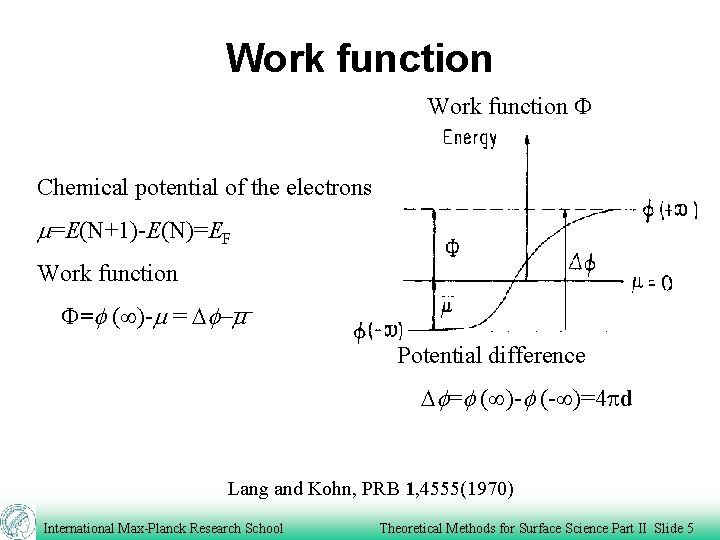
Work function F Chemical potential of the electrons m=E(N+1)-E(N)=EF Work function F=f ( )-m = Df-m Potential difference Df=f ( )-f (- )=4 pd Lang and Kohn, PRB 1, 4555(1970) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 5

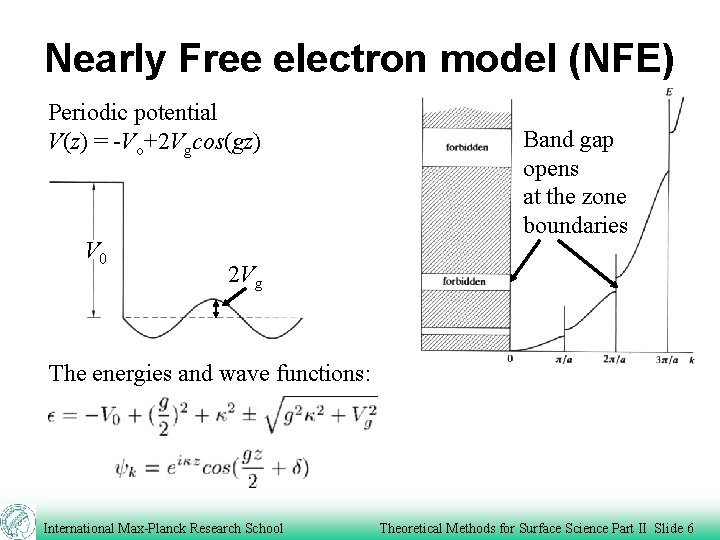
Nearly Free electron model (NFE) Periodic potential V(z) = -Vo+2 Vgcos(gz) V 0 Band gap opens at the zone boundaries 2 Vg The energies and wave functions: International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 6

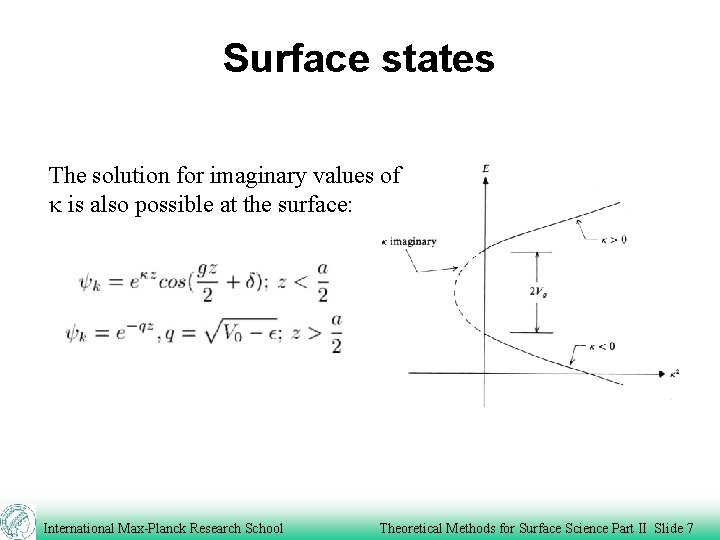
Surface states The solution for imaginary values of k is also possible at the surface: International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 7

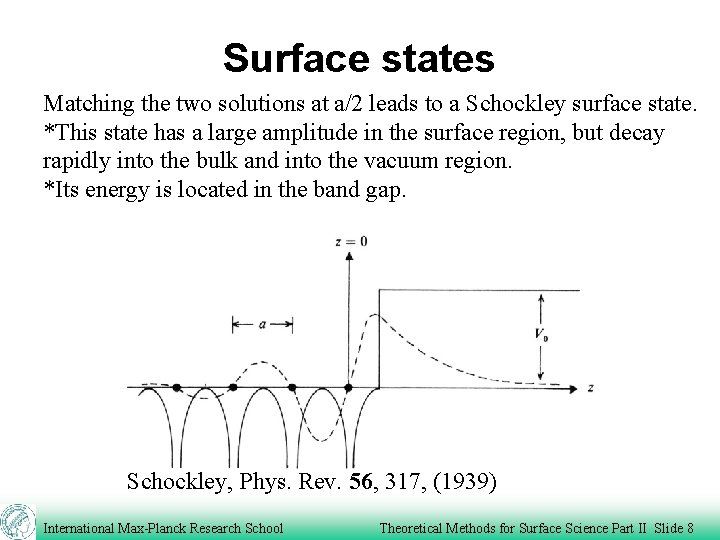
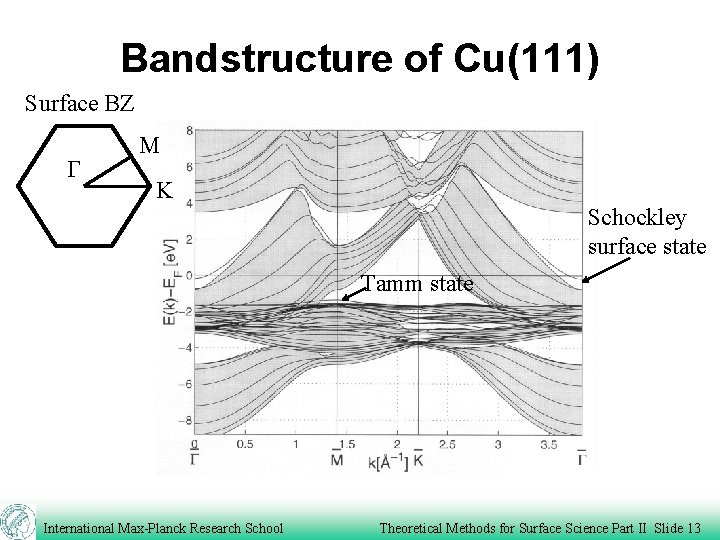
Surface states Matching the two solutions at a/2 leads to a Schockley surface state. *This state has a large amplitude in the surface region, but decay rapidly into the bulk and into the vacuum region. *Its energy is located in the band gap. Schockley, Phys. Rev. 56, 317, (1939) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 8

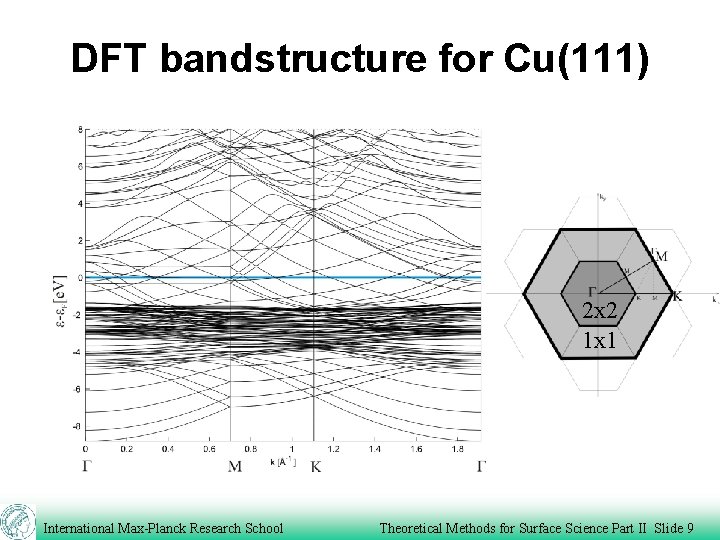
DFT bandstructure for Cu(111) 2 x 2 1 x 1 International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 9

Bandstructure of Cu(111) 6 -layer slab 18 -layer slab Euceda et al. , PRB 28, 528 (1983) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 10

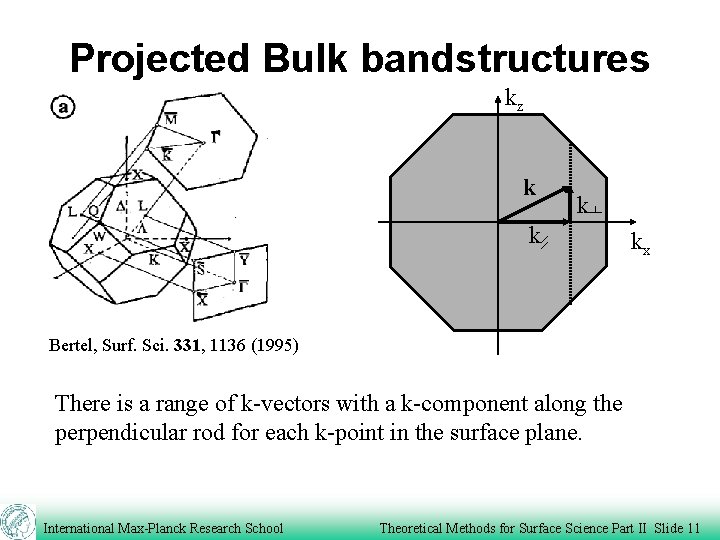
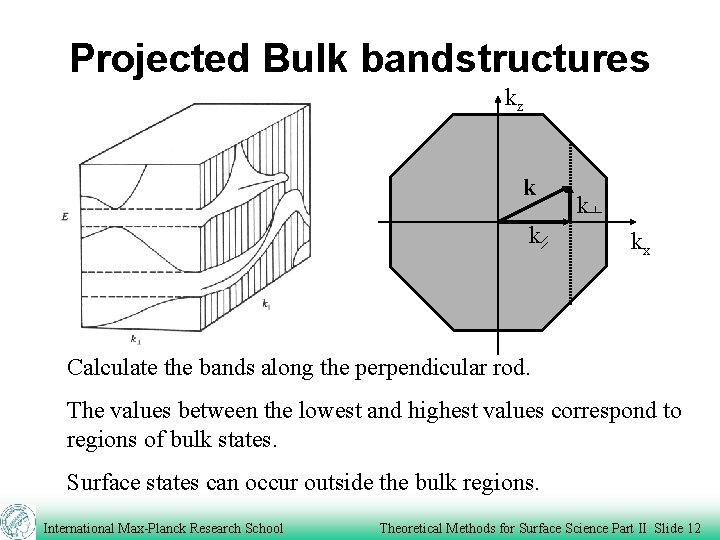
Projected Bulk bandstructures kz k kx Bertel, Surf. Sci. 331, 1136 (1995) There is a range of k-vectors with a k-component along the perpendicular rod for each k-point in the surface plane. International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 11

Projected Bulk bandstructures kz k kx Calculate the bands along the perpendicular rod. The values between the lowest and highest values correspond to regions of bulk states. Surface states can occur outside the bulk regions. International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 12

Bandstructure of Cu(111) Surface BZ G M K Schockley surface state Tamm state International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 13

Adsorption International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 14

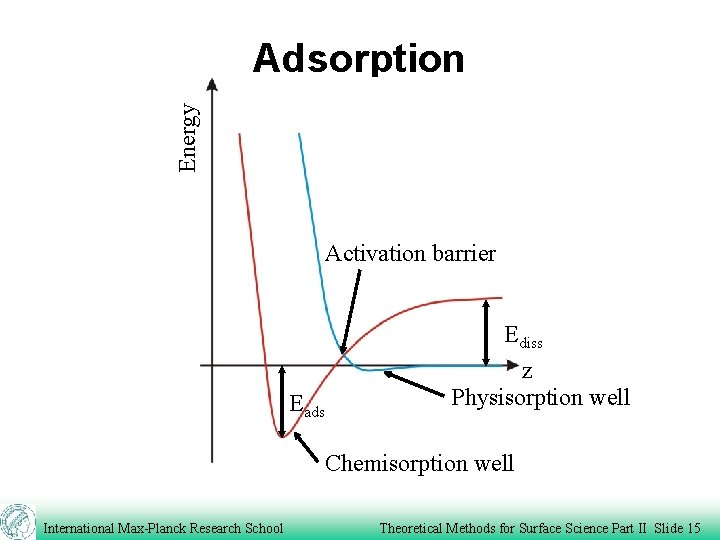
Energy Adsorption Activation barrier Eads Ediss z Physisorption well Chemisorption well International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 15
![Thermodynamics for adsorption a ma Host Definition of adsorbate energy EadsDGGhostadsGhostNa ma where GT Thermodynamics for adsorption a ma Host Definition of adsorbate energy: Eads=DG=G[host+ads]-{G[host]+Na ma} where G(T,](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-16.jpg)
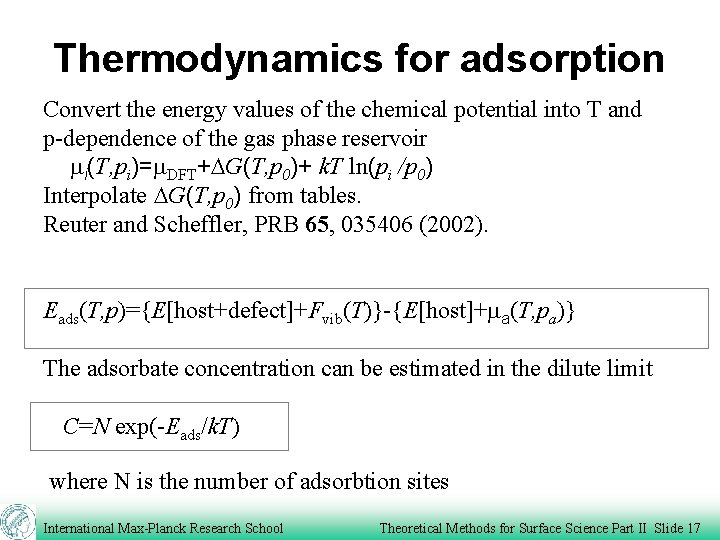
Thermodynamics for adsorption a ma Host Definition of adsorbate energy: Eads=DG=G[host+ads]-{G[host]+Na ma} where G(T, p)= E-TS + p. V=F+p. V Ftrans, Frot, p. V negligible for solids, but not in the gas phase The adsorbates vibrate at the surface: Fvib(T, w)=Evib (T, w)-TSvib (T, w) This gives the adsorption energy Eads={E[host+defect]+Fvib(T, w)}-{E[host]+Na ma} International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 16

Thermodynamics for adsorption Convert the energy values of the chemical potential into T and p-dependence of the gas phase reservoir mi(T, pi)=m. DFT+DG(T, p 0)+ k. T ln(pi /p 0) Interpolate DG(T, p 0) from tables. Reuter and Scheffler, PRB 65, 035406 (2002). Eads(T, p)={E[host+defect]+Fvib(T)}-{E[host]+ma(T, pa)} The adsorbate concentration can be estimated in the dilute limit C=N exp(-Eads/k. T) where N is the number of adsorbtion sites International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 17

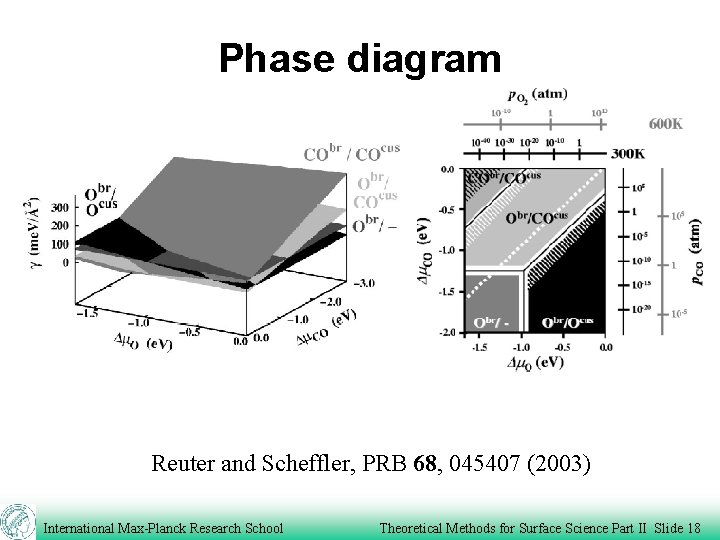
Phase diagram Reuter and Scheffler, PRB 68, 045407 (2003) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 18

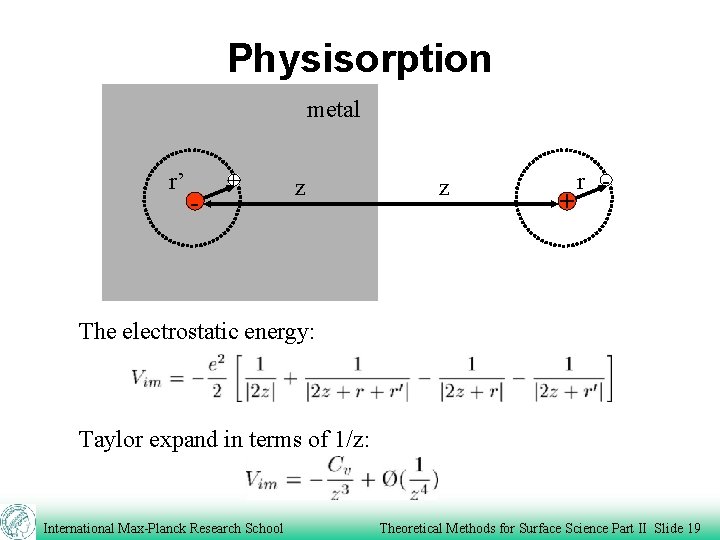
Physisorption metal r’ - + z z + r - The electrostatic energy: Taylor expand in terms of 1/z: International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 19

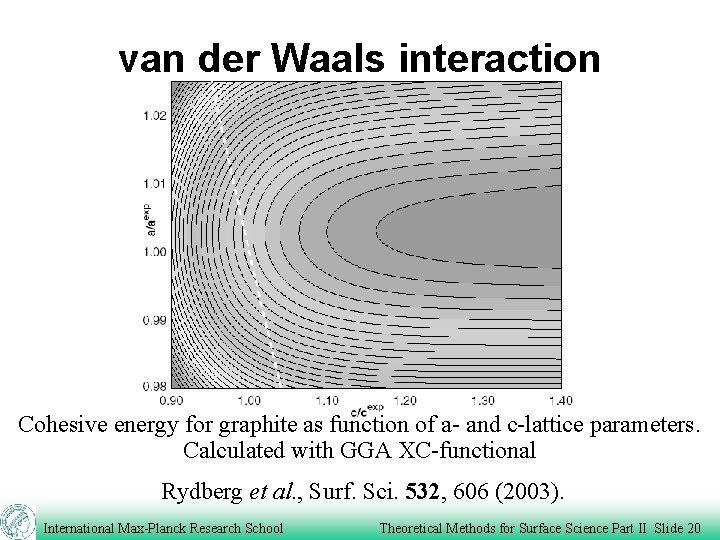
van der Waals interaction Cohesive energy for graphite as function of a- and c-lattice parameters. Calculated with GGA XC-functional Rydberg et al. , Surf. Sci. 532, 606 (2003). International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 20

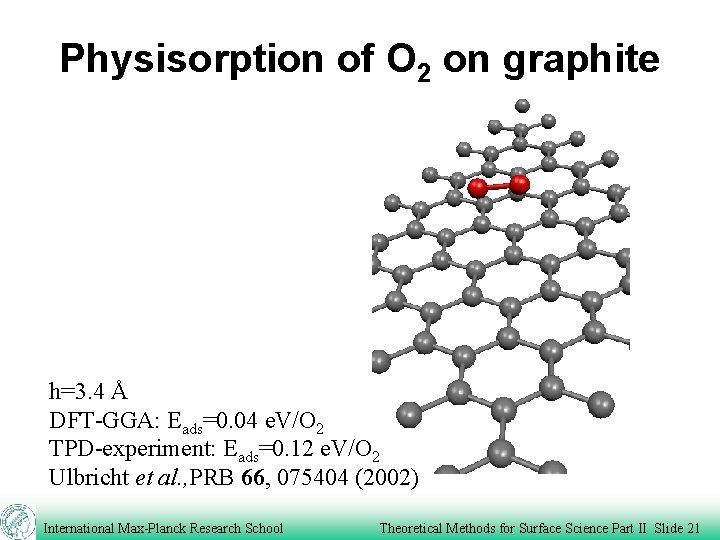
Physisorption of O 2 on graphite h=3. 4 Å DFT-GGA: Eads=0. 04 e. V/O 2 TPD-experiment: Eads=0. 12 e. V/O 2 Ulbricht et al. , PRB 66, 075404 (2002) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 21

Chemisorption International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 22

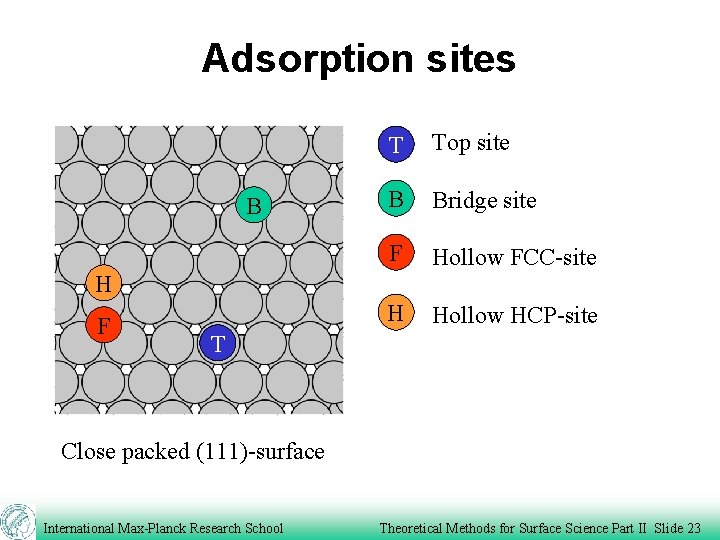
Adsorption sites B T Top site B Bridge site F Hollow FCC-site H Hollow HCP-site H F T Close packed (111)-surface International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 23

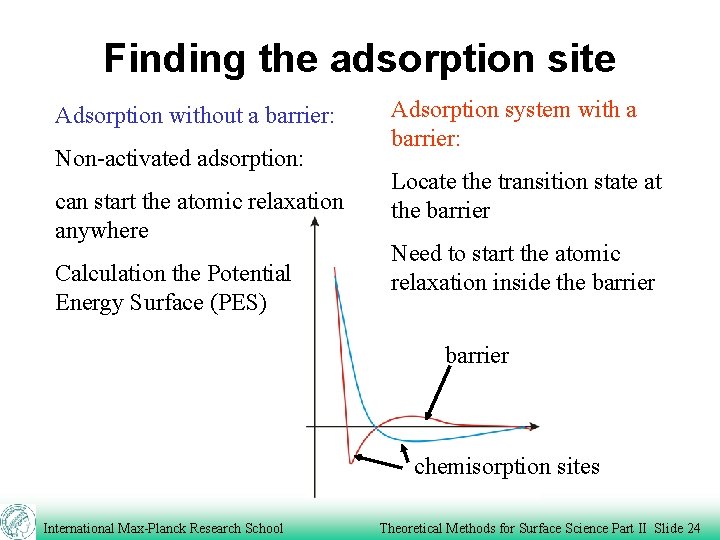
Finding the adsorption site Adsorption without a barrier: Non-activated adsorption: can start the atomic relaxation anywhere Calculation the Potential Energy Surface (PES) Adsorption system with a barrier: Locate the transition state at the barrier Need to start the atomic relaxation inside the barrier chemisorption sites International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 24

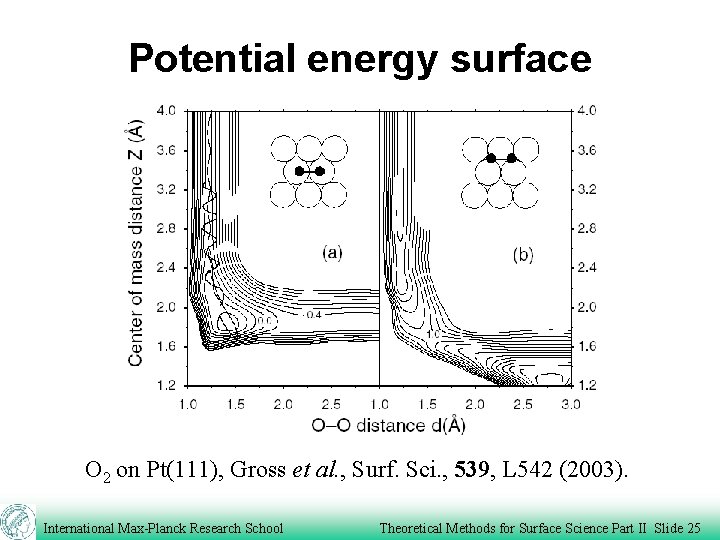
Potential energy surface O 2 on Pt(111), Gross et al. , Surf. Sci. , 539, L 542 (2003). International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 25

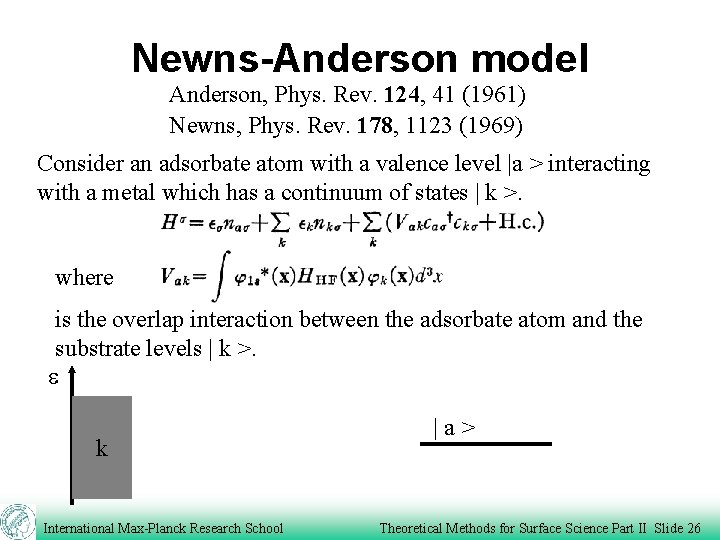
Newns-Anderson model Anderson, Phys. Rev. 124, 41 (1961) Newns, Phys. Rev. 178, 1123 (1969) Consider an adsorbate atom with a valence level |a > interacting with a metal which has a continuum of states | k >. where is the overlap interaction between the adsorbate atom and the substrate levels | k >. e k International Max-Planck Research School |a> Theoretical Methods for Surface Science Part II Slide 26

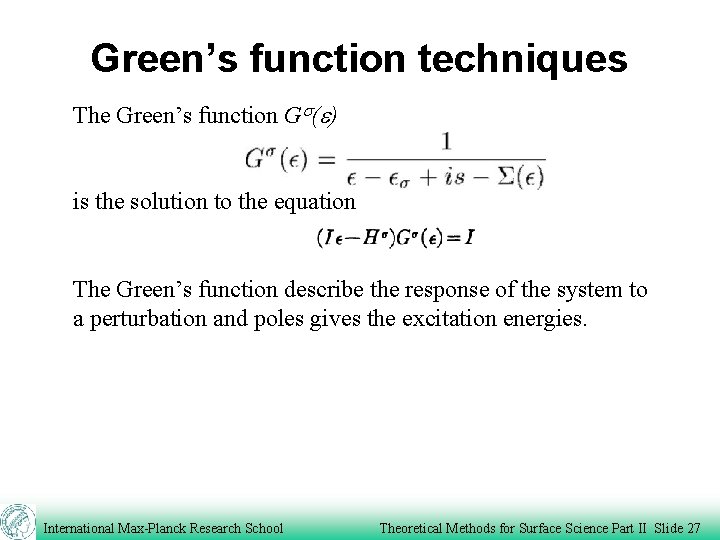
Green’s function techniques The Green’s function Gs(e) is the solution to the equation The Green’s function describe the response of the system to a perturbation and poles gives the excitation energies. International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 27

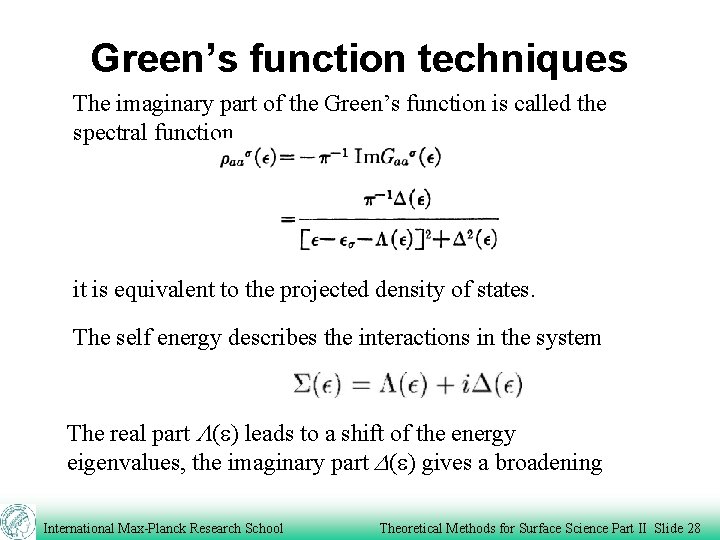
Green’s function techniques The imaginary part of the Green’s function is called the spectral function it is equivalent to the projected density of states. The self energy describes the interactions in the system The real part L(e) leads to a shift of the energy eigenvalues, the imaginary part D(e) gives a broadening International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 28

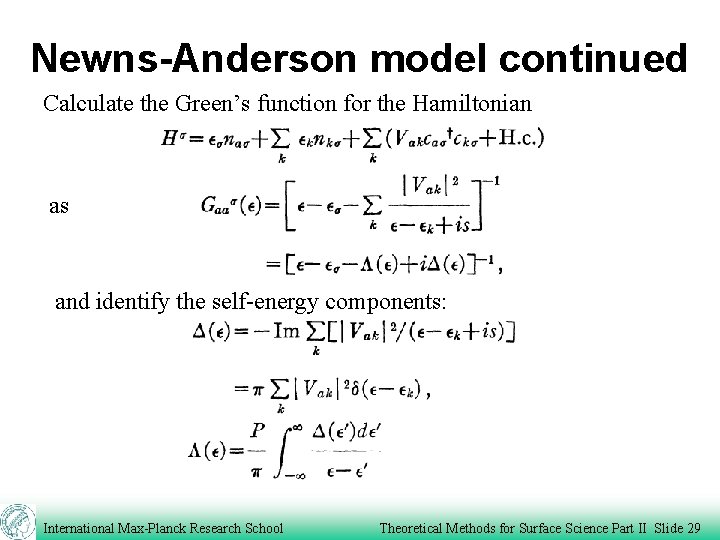
Newns-Anderson model continued Calculate the Green’s function for the Hamiltonian as and identify the self-energy components: International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 29

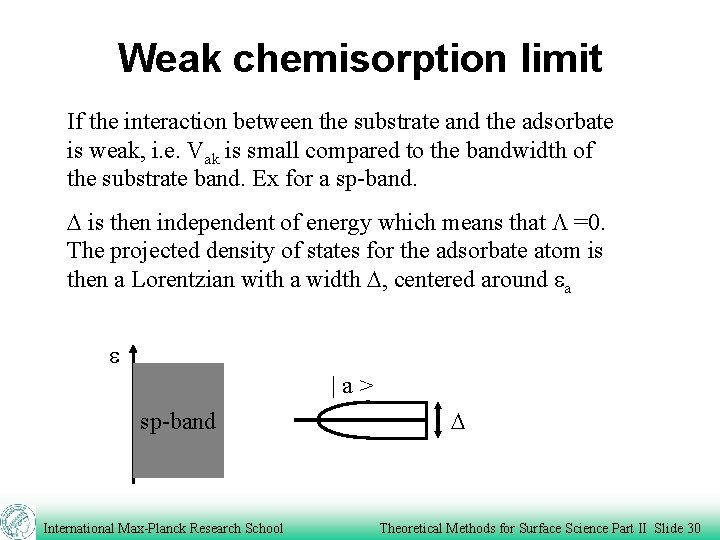
Weak chemisorption limit If the interaction between the substrate and the adsorbate is weak, i. e. Vak is small compared to the bandwidth of the substrate band. Ex for a sp-band. D is then independent of energy which means that L =0. The projected density of states for the adsorbate atom is then a Lorentzian with a width D, centered around ea e |a> sp-band International Max-Planck Research School D Theoretical Methods for Surface Science Part II Slide 30

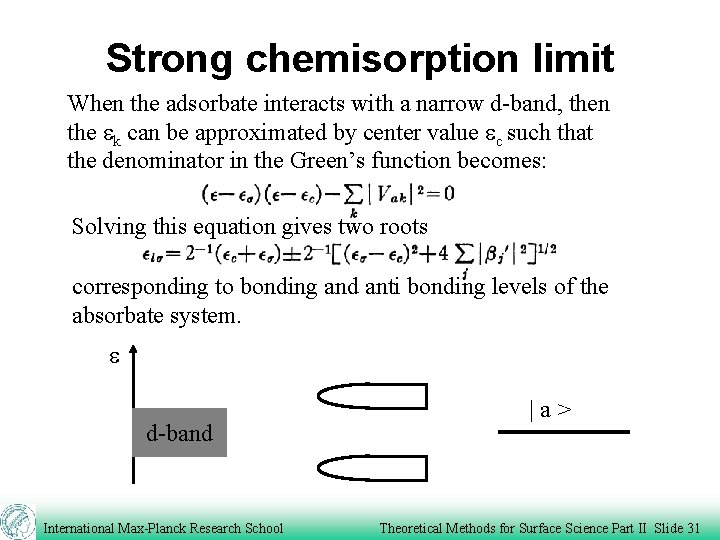
Strong chemisorption limit When the adsorbate interacts with a narrow d-band, then the ek can be approximated by center value ec such that the denominator in the Green’s function becomes: Solving this equation gives two roots corresponding to bonding and anti bonding levels of the absorbate system. e d-band International Max-Planck Research School |a> Theoretical Methods for Surface Science Part II Slide 31

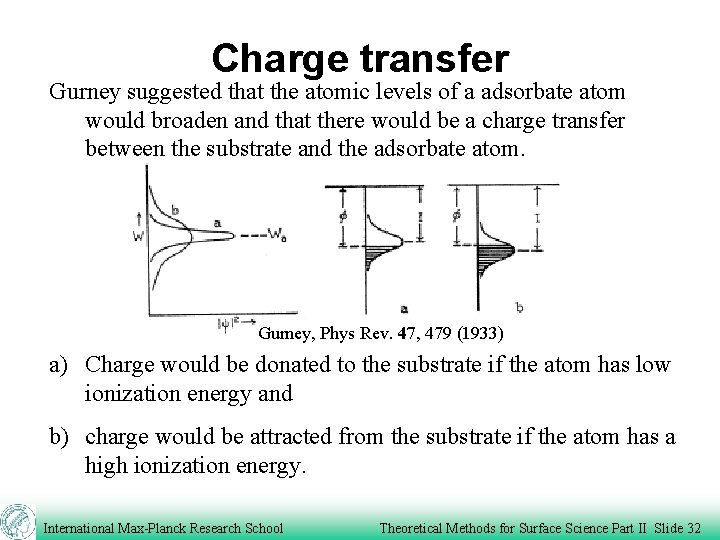
Charge transfer Gurney suggested that the atomic levels of a adsorbate atom would broaden and that there would be a charge transfer between the substrate and the adsorbate atom. Gurney, Phys Rev. 47, 479 (1933) a) Charge would be donated to the substrate if the atom has low ionization energy and b) charge would be attracted from the substrate if the atom has a high ionization energy. International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 32

Chemisorption on a metal surface Na/Cu(111) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 33
![DFe V Adsorbate induced work function change Tang et al Surf Sci Lett DF[e. V] Adsorbate induced work function change Tang et al. , Surf. Sci. Lett.](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-34.jpg)
DF[e. V] Adsorbate induced work function change Tang et al. , Surf. Sci. Lett. 255, L 497 (1991). International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 34

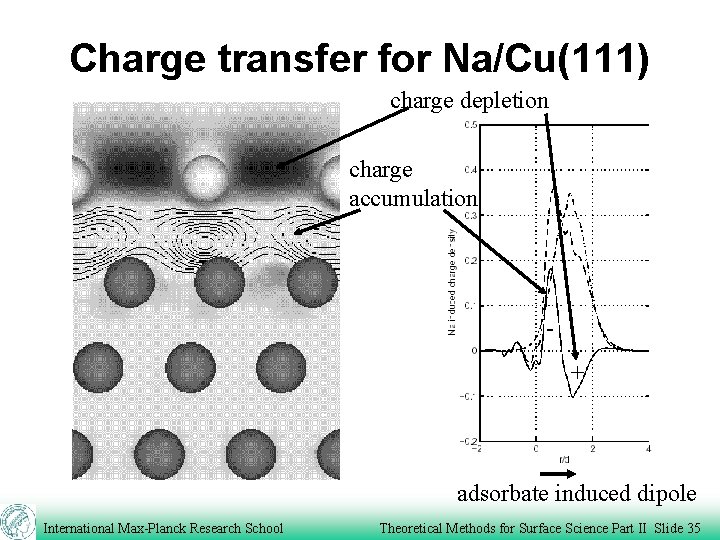
Charge transfer for Na/Cu(111) charge depletion charge accumulation + adsorbate induced dipole International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 35

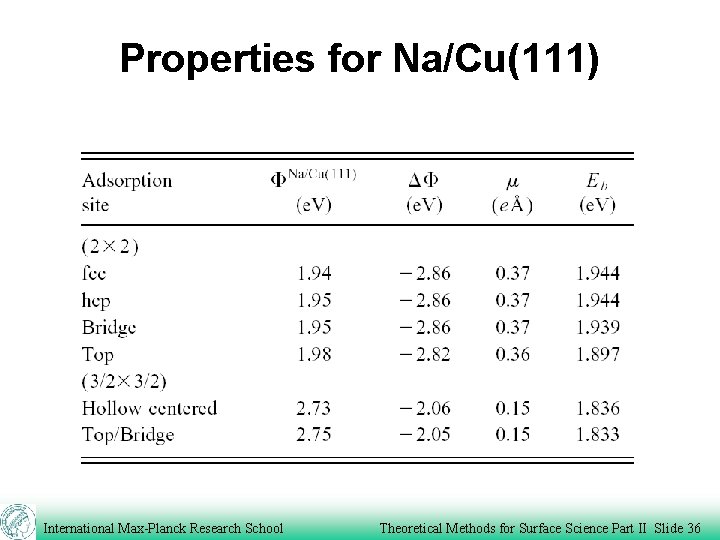
Properties for Na/Cu(111) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 36

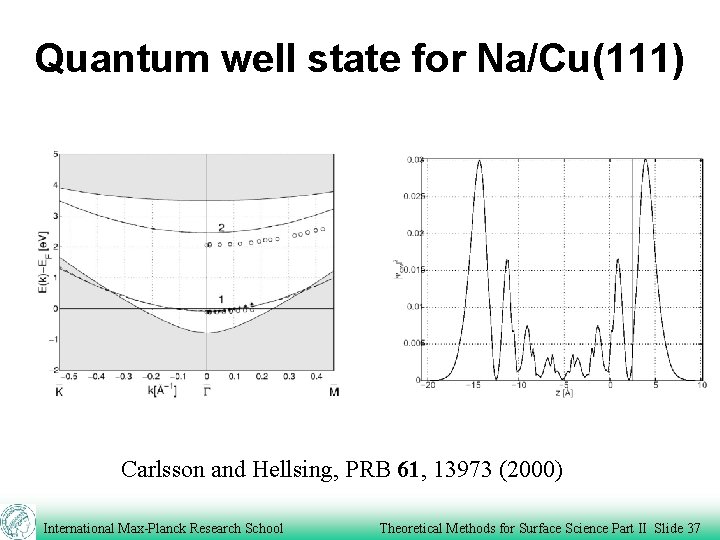
Quantum well state for Na/Cu(111) Carlsson and Hellsing, PRB 61, 13973 (2000) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 37

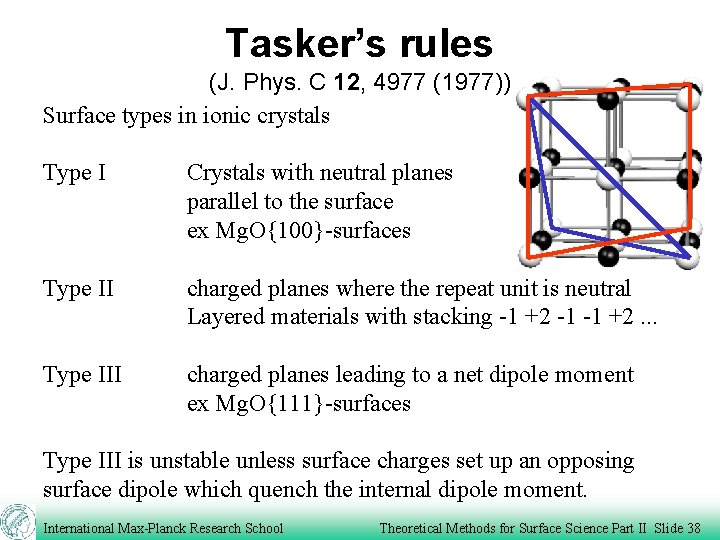
Tasker’s rules (J. Phys. C 12, 4977 (1977)) Surface types in ionic crystals Type I Crystals with neutral planes parallel to the surface ex Mg. O{100}-surfaces Type II charged planes where the repeat unit is neutral Layered materials with stacking -1 +2 -1 -1 +2. . . Type III charged planes leading to a net dipole moment ex Mg. O{111}-surfaces Type III is unstable unless surface charges set up an opposing surface dipole which quench the internal dipole moment. International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 38

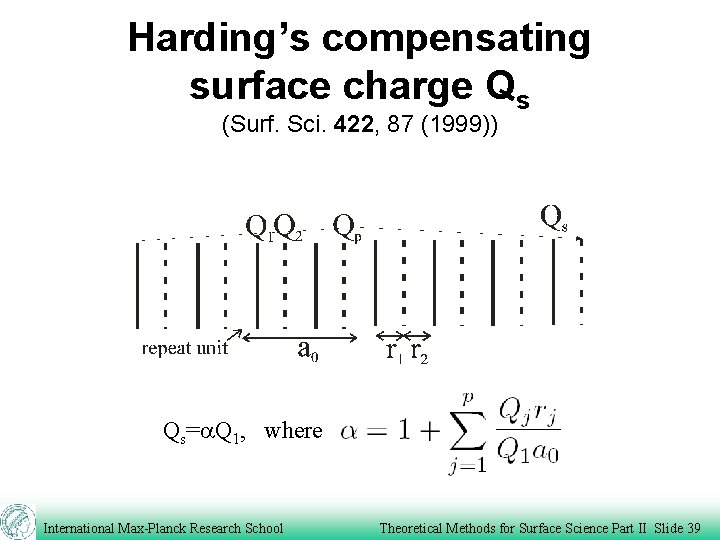
Harding’s compensating surface charge Qs (Surf. Sci. 422, 87 (1999)) Qs=a. Q 1, where International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 39

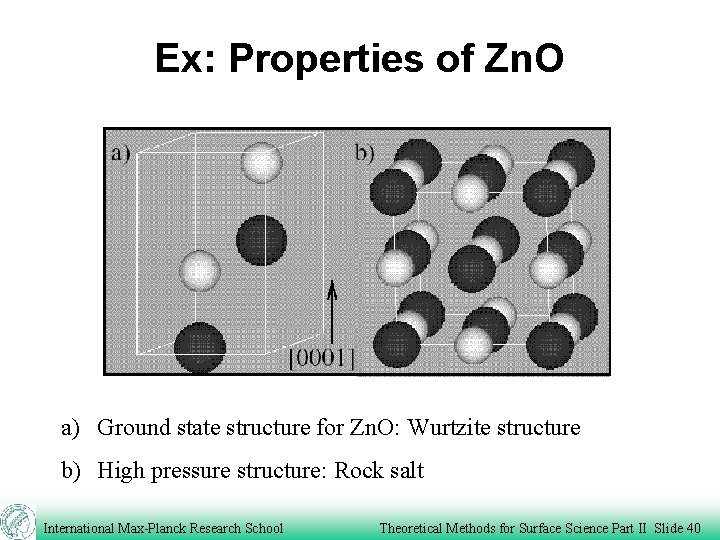
Ex: Properties of Zn. O a) Ground state structure for Zn. O: Wurtzite structure b) High pressure structure: Rock salt International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 40

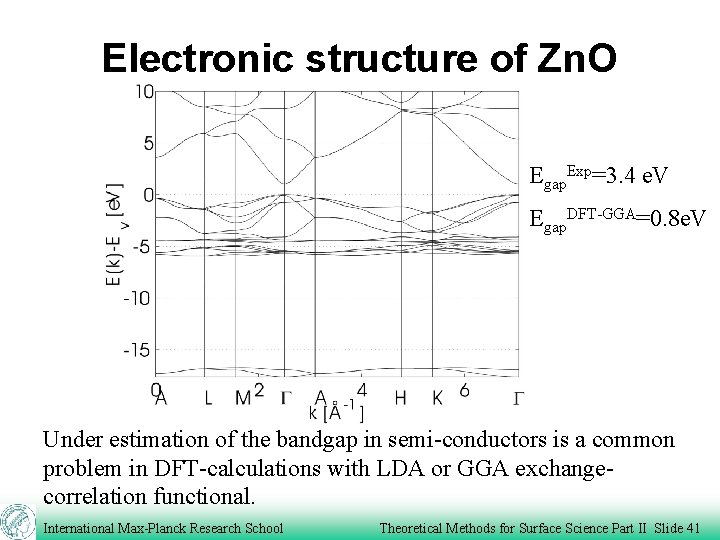
Electronic structure of Zn. O Egap. Exp=3. 4 e. V Egap. DFT-GGA=0. 8 e. V Under estimation of the bandgap in semi-conductors is a common problem in DFT-calculations with LDA or GGA exchangecorrelation functional. International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 41
![The polar Zn O0001surface Znterminated 0001surface 0001 Oterminated 0001surface International MaxPlanck Research School Theoretical The polar Zn. O{0001}-surface Zn-terminated [0001]-surface [0001] O-terminated [0001]-surface International Max-Planck Research School Theoretical](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-42.jpg)
The polar Zn. O{0001}-surface Zn-terminated [0001]-surface [0001] O-terminated [0001]-surface International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 42

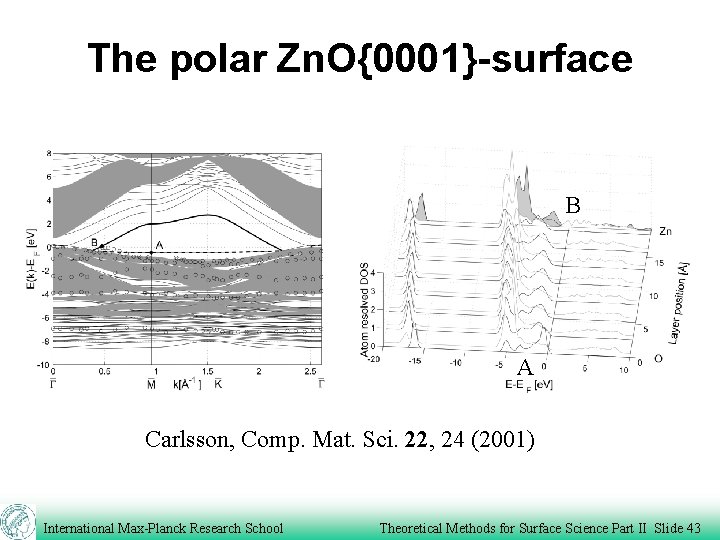
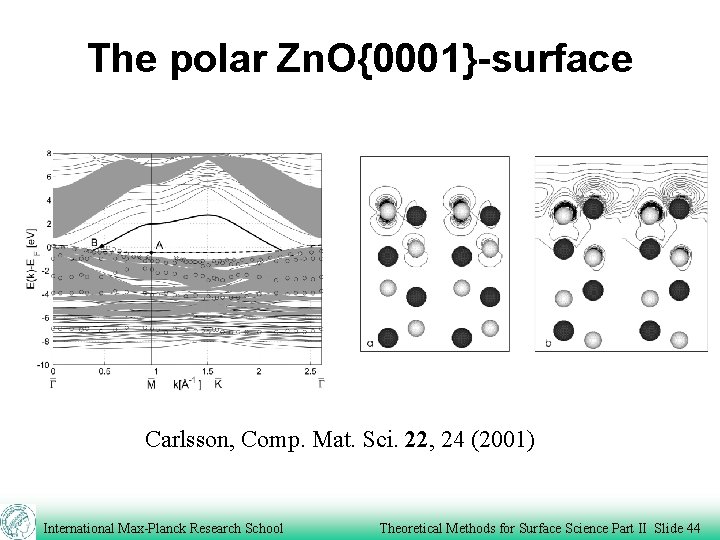
The polar Zn. O{0001}-surface B A Carlsson, Comp. Mat. Sci. 22, 24 (2001) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 43

The polar Zn. O{0001}-surface Carlsson, Comp. Mat. Sci. 22, 24 (2001) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 44
![STM of Zn O0001surface Dulub et al PRL 90 016102 2003 Triangular islands STM of Zn. O[0001]-surface Dulub et al. , PRL 90, 016102 (2003) Triangular islands](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-45.jpg)
STM of Zn. O[0001]-surface Dulub et al. , PRL 90, 016102 (2003) Triangular islands Step height=2. 7 Å=c/2 n=O-edge atoms International Max-Planck Research School b) Triangle # of O-atoms = n(n+1)/2 # of Zn-atoms =n(n-1)/2 Q=#Zn / #O =3/4 => n=7 L = (n-2)*a = 16. 25 Å c) Triangle with internal triangle # of O-atoms = 3 n(n+1)/2 -3 # of Zn-atoms = 3 n(n-1)/2 Q=#Zn / #O =3/4 => n=6 L = (2(n-1)-1)*a = 29. 25 Å Theoretical Methods for Surface Science Part II Slide 45
![Surface Phase diagram of Zn O0001 Kresse et al PRB 68 245409 2003 Surface Phase diagram of Zn. O[0001] Kresse et al. , PRB 68, 245409 (2003)](https://slidetodoc.com/presentation_image/3675673d96b4dfc05e90d8b25cfcc1da/image-46.jpg)
Surface Phase diagram of Zn. O[0001] Kresse et al. , PRB 68, 245409 (2003) International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 46

Summary • Surface energy • Atomic structure relaxation • Charge redistribution • Work function • Surface states • Adsorption International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 47

Literature Review article about DFT implementations: Payne et al. , Rev. Mod. Phys. 64, 1045 (1992). A. Zangwill, Physics at Surfaces, Cambridge University Press 1. A. Gross, Theoretical Surface Science A microscopic perspective, Springer Verlag 2. F. Bechstedt, Principles of Surface Physics, Springer Verlag International Max-Planck Research School Theoretical Methods for Surface Science Part II Slide 48