The Red Seal Your Road to FSMA and





























- Slides: 33

The Red Seal – Your Road to FSMA and Quality Compliance Thomas Vogel – Director, Food Safety DFA of California Jeremiah Szabo – Director of Operations, DFA of California

Our Presenters Today – Thomas Vogel • Auditor, Trainer, Consultant – DFA of California • Driving force behind our food safety operations in the areas of Good Manufacturing Practice (GMP), Good Agricultural Practice (GAP) and supplier assurance programs, including HACCP, SQF and BRC • 35 years of experience in the food industry, while working for national and international companies in quality assurance, product development and auditing. • BSC in Chemistry and an accomplished food safety trainer for numerous food safety manager certification courses • In addition, he is a GMP/HACCP instructor, a Lead Auditor trainer, and is also certified to audit on the Global Food Safety Initiative for SQF and BRC. He is certified in numerous product categories

Our Presenters Today – Jeremiah Szabo • Operations Director for commodity inspection and laboratory programs, Auditor, Trainer, Consultant – DFA of California • 10 years of food industry experience working in Quality Control, Quality Assurance, Laboratory management, and food safety. • BSC in Biological sciences, certified HACCP auditor, certified to audit to Global Food Safety Initiative (GFSI) audits for BRC and SQF audits, GMP/HACCP/Internal Auditor trainer,

Purpose Of This Session • Overview of Food Safety Modernization Act • Provide an update on the implementation of FSMA • How the Red Seal can assist with FSMA compliance • How the Red Seal can give a market advantage • Q&A

Food Safety Modernization Act (FSMA) Congress passed FSMA on December 20 th 2010 President Obama signed it into law on January 4 th, 2011 Congress sets the laws, FDA develops the regulations to fit with the laws and then enforces the laws. Initial timetable had a 3 year implementation plan with parts being implemented immediately and other parts gradually but FDA has not been able to meet this timeframe and it is now likely to be a 5 -7 year plan.

Food Safety Modernization Act (FSMA) Federal Judge from the US District Court in Northern California has ruled that FDA has to meet the following timetable. Regulations all had to be published by November 30 th, 2013. All comment periods have to be closed out by March 31 st, 2014. Final regulations need to be put into effect by June 30 th, 2015. Followed by a 3 year implementation plan for Industry.

Food Safety Modernization Act 2010 Title 1 Designed to improve capacity to prevent food safety problems Title 2 Designed to improve capacity to detect and respond to food safety problems Title 3 Designed to improve the safety of imported food Title 4 Includes miscellaneous provisions

Food Safety Modernization Act 2010 Exempted Businesses • FSMA does not apply to facilities regulated by USDA (meat, poultry and eggs) • Also exempted are the following industries from any changes: • Juice manufacturers • Seafood processors • Alcohol-related facilities • Low acid canning (except to expand their Hazard Analysis) • Small Businesses < $500, 000 sales and 50% of their sales within 275 miles of their facility (Tester amendment) • FDA is considering modified requirements for warehouses and having Preventive Controls only if they are storing refrigerated products.

Title 1 Sections Designed to prevent food safety problems • Section 101 FDA access to your records • Section 102 Registration and possible suspension by FDA • Section 103 Preventive Controls (HACCP) • Section 104 FDA hazard information • Section 105 Produce food safety guidelines • Section 106 Regulations to prevent intentional adulteration • Section 107 Fee assessments by FDA • Section 108 National agriculture and food defense strategy

Title 1 Sections Continued • Section 109 Annual reporting by Secretary of Homeland Security • Section 110 Building domestic capacity • Section 111 Sanitary transportation regulations • Section 112 Allergen education • Section 113 New dietary ingredients

Sections Update We are for the purpose of this update confining our updates to the following Sections of FSMA: Section 103 Preventive Controls Rule proposal. Section 105 Section 106 Section 301 Section 306 Section 307 Produce Guidelines. Intentional Adulteration of Foods. Voluntary Importer Program. Risk Assessment on Imported Foods. Third Party Accredited Audits.

Section 103 c. GMP and Hazard Analysis and Risk-Based Preventive Controls for Human food. • Requires registered facilities unless exempt, to: • Have a written food safety plan • Perform a Hazard analysis • Implement risk based preventive control measures • Conduct Monitoring • Perform Corrective Actions • Verify the effectiveness of these preventive controls • Maintain records (FDA to provide list of what records will be required).

Section 103 c. GMP and Hazard Analysis and Risk-Based Preventive Controls for Human food. • Requires all FDA registered facilities to comply with the exception of Dietary Supplement manufacturers • Seafood companies. • Juice manufacturers. • Alcohol manufacturers. • Low acid canning. • Proposing to possibly exempt • grinding, milling, or crushing of grains. • In-farm packing of intact fruit and vegetables. • Facilities storing unexposed products.

PREVENTIVE CONTROLS • • • "Preventive Controls" vs. HACCP CCPs vs. CPs. Knowing your risks Managing your Risks Training

PREVENTIVE CONTROLS vs. HACCP FDA makes a point in this proposed rule that although Preventive Controls should be determined using Principle 1 (Hazard Analysis) as in developing a HACCP program the Preventive Controls should not be limited to just CCPs. Need to include • Pre-requisite programs • Recall program • Sanitation programs • Environmental Pathogens and controls • Allergens and controls • Equipment Calibration • Food Defense Program see Section 106.

PREVENTIVE CONTROLS vs. HACCP FDA's support information for requiring companies to develop and implement Preventive Controls programs vs. HACCP plans states that companies with HACCP plans have not put the due diligence into implementing and monitoring their CPs. Thoughts are here that industry has deferred to a CP instead of a CCP so as to avoid having to monitor, record and react to possible variances required of a CCP in a HACCP Plan and this has thus continued to cause contaminated products to be distributed and hence cause subsequent Recalls. Industry has also deferred to their Suppliers control of certain possible hazards to the extent that they trust their Suppliers but never verify their Suppliers can and do achieve this level of control of an "likely to occur" hazard.

Section 103 c. GMP and Hazard Analysis and Risk-based Preventive Controls for Human food. This proposed rule requires also: • That low acid canning facilities expand their hazard analysis to include chemical and physical hazards • Requires that each hazard analysis to include the possibility of deliberate or "terroristic" contamination of your products. (However preventive controls for this are to be covered under your Food Defense plan (See Section 106) not this plan). • Requires all companies to reanalyze their potential hazards every three years

Section 103 c. GMP and Hazard Analysis and Risk-based Preventive Controls for Human food. FSMA asks companies to assess chemical, radiological hazards, natural toxins, pesticides, drug residues in their hazard analysis, ( arsenic in chicken, aflatoxins in figs, or acrylamide in corn chips). (FDA recently lowered the allowable arsenic level in apple juice to match that allowed in drinking water).

c. GMP Working Group presented to the FDA 7 areas of opportunity for updating the c. GMPs. • Training • • Documented Allergen controls Documented environmental pathogen control program • Documented SSOPs • Maintenance of food safety records • Possibly removing the exemption given to companies who just handle raw agricultural products • Getting public comment for time and temperature as related to the safe storage of hot and cold foods

Red Seal Certification Program

Certification Program Requirements • Red Seal Certificate Available to DFA of California and Specialty Crop Trade Council members only! • DFA Red Seal Member companies must meet… Facility Requirements Quality Requirements

No other Product Certification in the industry gives the customer…. Facility Food Safety Certification Customer specific Product Quality Certification Customer specific Product Laboratory Certification

Red Seal Certified Commodities Tree Nuts…… Dried Fruit……

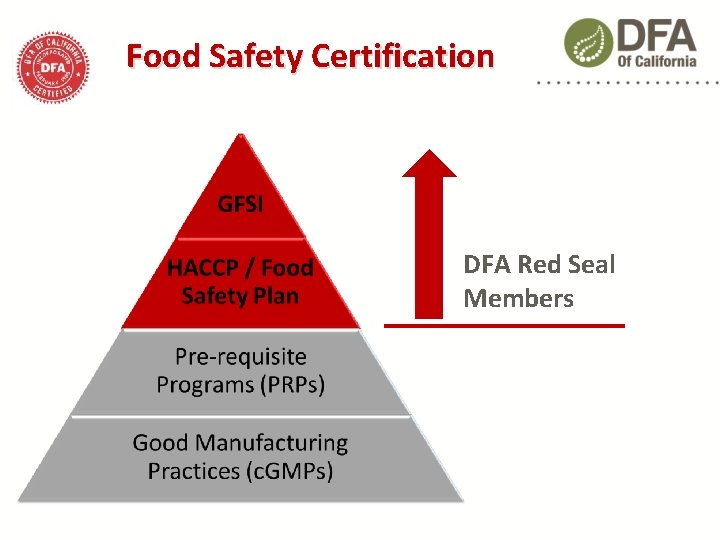
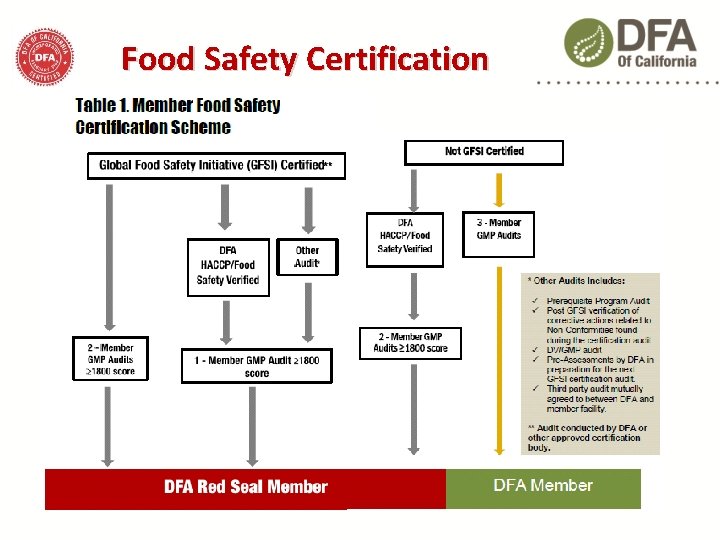
Food Safety Certification Red Seal Member facilities must…. Demonstrate that their products are handled, produced, packaged and stored at a facility operating according to… Codex Alimentarius (HACCP) and the National Advisory Committee on Microbiological Criteria of Foods (NACMCF) guidelines Certified to a Global Food Safety Initiative (GFSI) standard Verified by an authorized DFA food safety auditor

Food Safety Certification DFA Red Seal Members

Food Safety Certification

Product Quality Certification Customer Satisfaction is #1! Quality provisions for Red Seal Certified products: Lot averaging not allowed! Positive lot identification Inspection within 30 days of shipment No arbitration on quality No Rejected segments Grading to meet customer specifications Crop year certified Certify “Contract Requirements” (i. e. Laboratory testing)

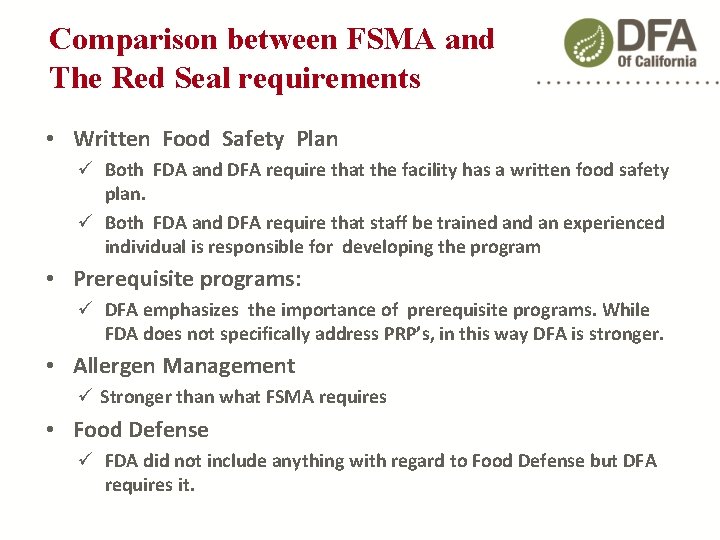
Comparison between FSMA and The Red Seal requirements • Written Food Safety Plan Both FDA and DFA require that the facility has a written food safety plan. Both FDA and DFA require that staff be trained an experienced individual is responsible for developing the program • Prerequisite programs: DFA emphasizes the importance of prerequisite programs. While FDA does not specifically address PRP’s, in this way DFA is stronger. • Allergen Management Stronger than what FSMA requires • Food Defense FDA did not include anything with regard to Food Defense but DFA requires it.

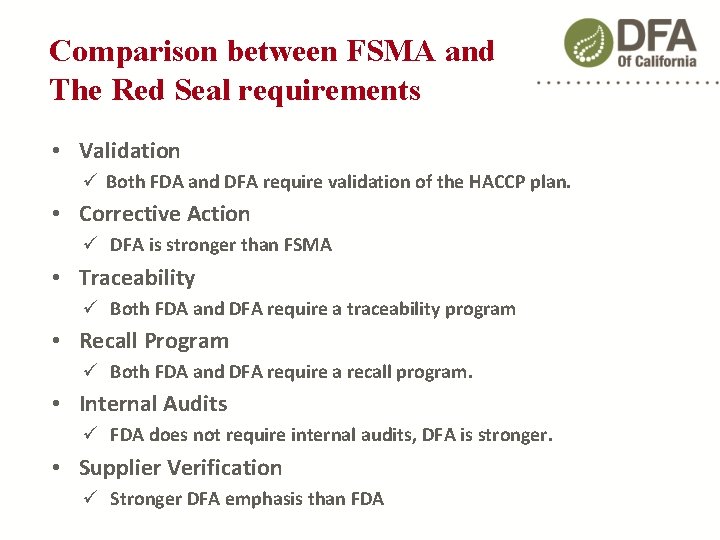
Comparison between FSMA and The Red Seal requirements • Validation Both FDA and DFA require validation of the HACCP plan. • Corrective Action DFA is stronger than FSMA • Traceability Both FDA and DFA require a traceability program • Recall Program Both FDA and DFA require a recall program. • Internal Audits FDA does not require internal audits, DFA is stronger. • Supplier Verification Stronger DFA emphasis than FDA

Product Quality Certification Safer Products Consistent Uniformity Superior Quality Ship Red Seal Certified! redseal@agfoodsafety. org/quality-inspection/red-seal-certificate

Auditing, Certification, Training & Improvement Solutions • • BRC Food Safety SQF HACCP Training In House Public Courses Consultation

Food Safety Services • Training Public and onsite training (SQF, BRC, HACCP, Advanced HACCP, Internal Auditor, Food Defense, GMP) • Consulting On-site GAP analysis HACCP program facilitation Program review & development Allergen program review and validation Product specification building • Audits Non-Accredited: GMP, Warehouse, HACCP Verification Accredited: GFSI benchmarked certification audits (DFA Global Certifications, LLC)

THANK YOU FOR ATTENDING v Q&A
 Fsma overview
Fsma overview Fsma pcp
Fsma pcp Paved road vs unpaved road
Paved road vs unpaved road Give us your hungry your tired your poor
Give us your hungry your tired your poor A red red rose poem questions and answers
A red red rose poem questions and answers Simile in the song stereo hearts
Simile in the song stereo hearts Down the road
Down the road And i was thinking on the drive down
And i was thinking on the drive down Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh