The Patient with Low HDL Cholesterol James A
















- Slides: 67

The Patient with Low HDL Cholesterol James A. Underberg, MD, MS, FACPM, FACP, FASH, FNLA Preventive Cardiovascular Medicine Clinical Assistant Professor of Medicine Division of General Internal Medicine, NYU Medical School & NYU Center for Prevention of Cardiovascular Disease Director Bellevue Hospital Primary Care Lipid Clinic ASH Hypertension Specialist Diplomate American Board of Clinical Lipidology Fellow Society of Vascular Medicine & Biology Specialist

Disclosures • Honoraria for Speakers Bureau (Pharma) : Astra. Zeneca, Abbott, Forest, Glaxo. Smith. Kline, Daiichi-Sankyo, Kowa, Novartis, Pfizer, Liposcience, dia. Dexus, Merck, Eli Lilly • Honoraria for CME Programs : American Heart Association, National Lipid Association, American College of Reproductive Medicine, Pri. Med, Primary Care Network • Consulting Income: Liposcience, News Corporation, Publicis Inc. , Summer Street Consulting Inc. Guidepoint Global • Advisory Boards: Kowa, Abbott, Merck • Clincial Research Funding: Genzyme, Glaxo. Smith. Kline, • Medical Education Committee Member : NAMS, ASH, NLA • Editoral Board Member: Journal of Clinical Lipidology

Case#1 • 52 year old male presents with one week of shaking chills, fever, and sweats • He has lost 12 lbs over the past 6 weeks without change in diet or exercise • He denies cough, urinary symptoms or change in bowel habits • He does note a decrease in his appetite • Who would like to give him aspirin for the fever and send him home?

Case #2 • 41 year old white male presents for evaluation of low HDL cholesterol ( HDL-C) • No significant past medical history • Physical exam unremarkable. Normal BP, BMI 23. 4 WC 32” • Father with MI age 51, died age 52 sudden death. Was a smoker, never told he had a lipid disorder. • Patient does not drink alcohol, and does not smoke, He exercises 4 times a week and eats mostly fish and vegetables, salad. Small amounts of lean meat and poultry.

Case #2 • • Lab Evaluation Total Cholesterol 140 mg/dl HDL-C 21 mg/dl LDL-C 94 TG 125 mg/dl FBS 90 mg/dl, Hb. A 1 c 5. 2% Bun/Cr. 15/0. 9 LFT and all other labs WNL

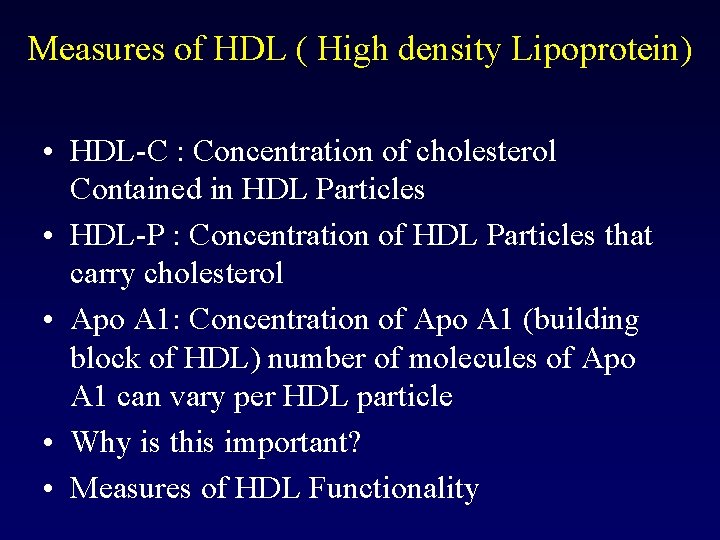
Measures of HDL ( High density Lipoprotein) • HDL-C : Concentration of cholesterol Contained in HDL Particles • HDL-P : Concentration of HDL Particles that carry cholesterol • Apo A 1: Concentration of Apo A 1 (building block of HDL) number of molecules of Apo A 1 can vary per HDL particle • Why is this important? • Measures of HDL Functionality

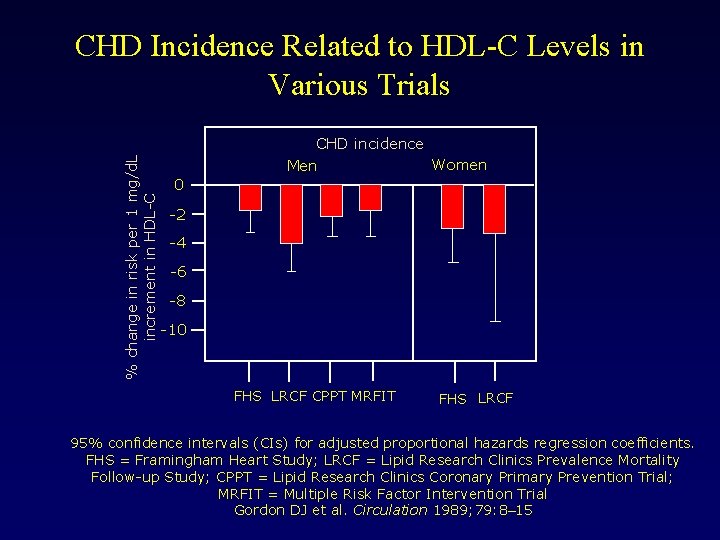
% change in risk per 1 mg/d. L increment in HDL-C CHD Incidence Related to HDL-C Levels in Various Trials CHD incidence Women Men 0 -2 -4 -6 -8 -10 FHS LRCF CPPT MRFIT FHS LRCF 95% confidence intervals (CIs) for adjusted proportional hazards regression coefficients. FHS = Framingham Heart Study; LRCF = Lipid Research Clinics Prevalence Mortality Follow-up Study; CPPT = Lipid Research Clinics Coronary Primary Prevention Trial; MRFIT = Multiple Risk Factor Intervention Trial Gordon DJ et al. Circulation 1989; 79: 8– 15

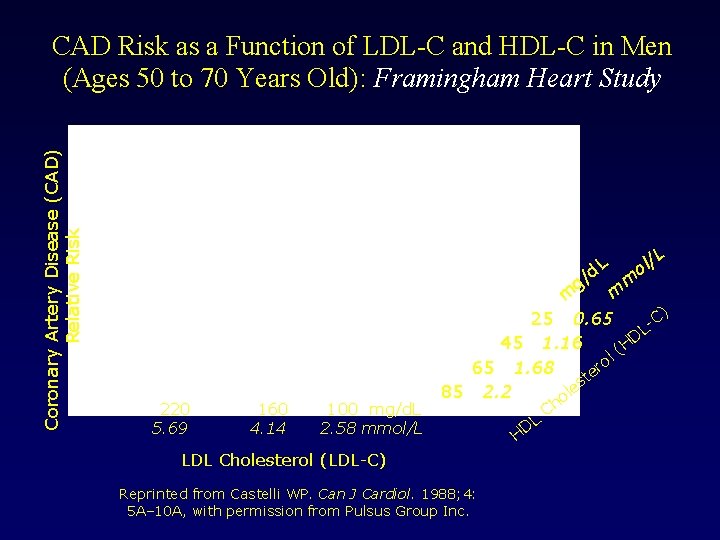
Coronary Artery Disease (CAD) Relative Risk CAD Risk as a Function of LDL-C and HDL-C in Men (Ages 50 to 70 Years Old): Framingham Heart Study d. L g/ 220 5. 69 160 4. 14 100 mg/d. L 2. 58 mmol/L /L l o m m m C) 25 0. 65 L D 45 1. 16 (H l o 65 1. 68 er t es 85 2. 2 ol LDL Cholesterol (LDL-C) Reprinted from Castelli WP. Can J Cardiol. 1988; 4: 5 A– 10 A, with permission from Pulsus Group Inc. L D H Ch

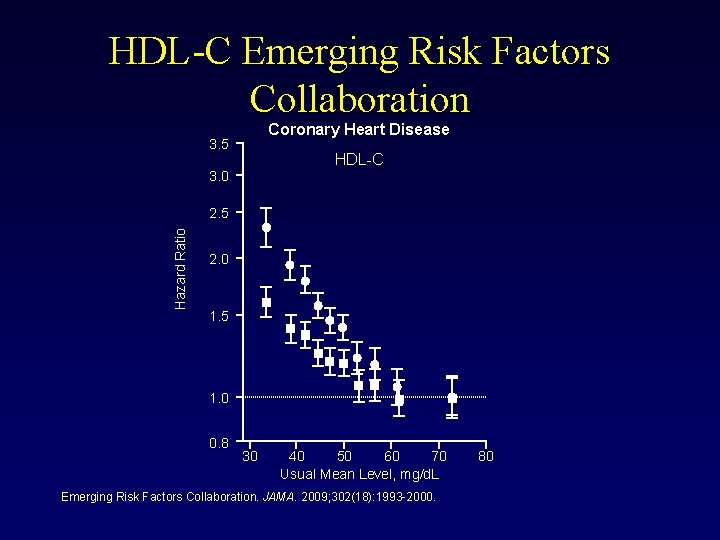
HDL-C Emerging Risk Factors Collaboration Coronary Heart Disease 3. 5 HDL-C 3. 0 Hazard Ratio 2. 5 2. 0 1. 5 1. 0 0. 8 30 40 50 60 70 Usual Mean Level, mg/d. L Emerging Risk Factors Collaboration. JAMA. 2009; 302(18): 1993 -2000. 80

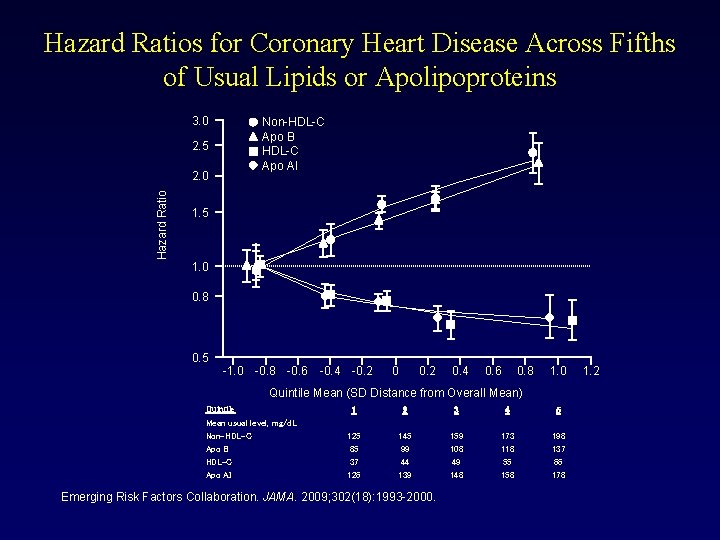
Hazard Ratios for Coronary Heart Disease Across Fifths of Usual Lipids or Apolipoproteins 3. 0 Non-HDL-C Apo B HDL-C Apo AI 2. 5 Hazard Ratio 2. 0 1. 5 1. 0 0. 8 0. 5 -1. 0 -0. 8 -0. 6 -0. 4 -0. 2 0. 4 0. 6 0. 8 1. 0 Quintile Mean (SD Distance from Overall Mean) Quintile Mean usual level, mg/d. L Non-HDL-C Apo B HDL-C Apo AI 1 2 3 4 5 125 85 37 126 145 99 44 139 159 108 49 148 173 118 55 158 198 137 66 178 Emerging Risk Factors Collaboration. JAMA. 2009; 302(18): 1993 -2000. 1. 2

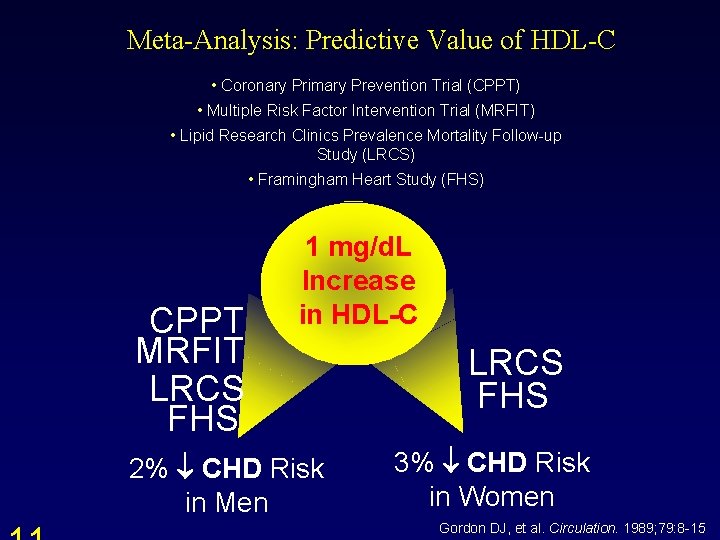
Meta-Analysis: Predictive Value of HDL-C • Coronary Primary Prevention Trial (CPPT) • Multiple Risk Factor Intervention Trial (MRFIT) • Lipid Research Clinics Prevalence Mortality Follow-up Study (LRCS) • Framingham Heart Study (FHS) CPPT MRFIT LRCS FHS 1 mg/d. L Increase in HDL-C 2% CHD Risk in Men LRCS FHS 3% CHD Risk in Women Gordon DJ, et al. Circulation. 1989; 79: 8 -15.

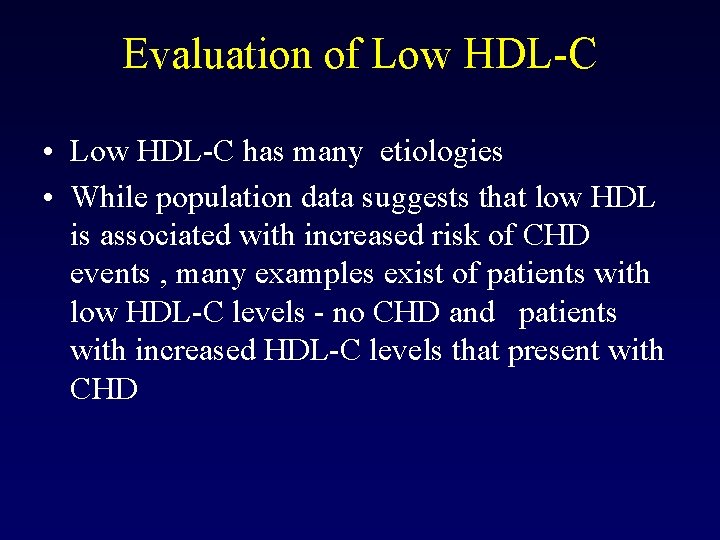
Evaluation of Low HDL-C • Low HDL-C has many etiologies • While population data suggests that low HDL is associated with increased risk of CHD events , many examples exist of patients with low HDL-C levels - no CHD and patients with increased HDL-C levels that present with CHD

HDL-C Levels Often Do Not Predict ASHD • • • Torcetrapib Apo. A-1 Milano WHI Estrogen SRB 1 rodent data LCAT deficiency Tangier Disease

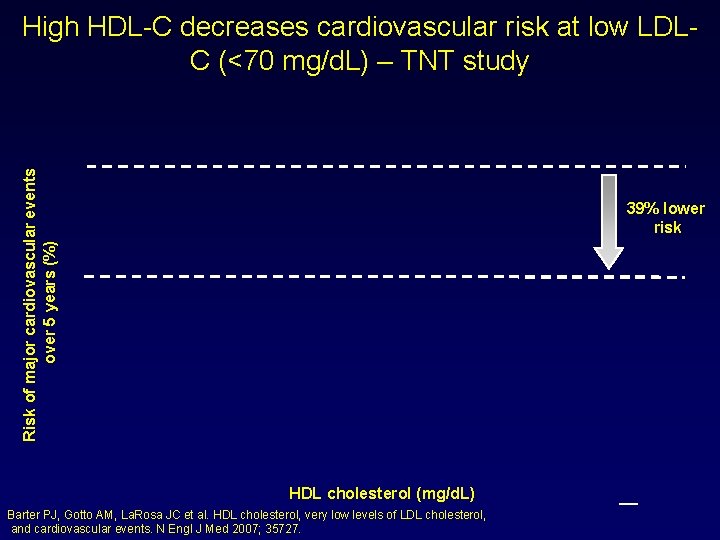
Risk of major cardiovascular events over 5 years (%) High HDL-C decreases cardiovascular risk at low LDLC (<70 mg/d. L) – TNT study 39% lower risk HDL cholesterol (mg/d. L) Barter PJ, Gotto AM, La. Rosa JC et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007; 35727. _


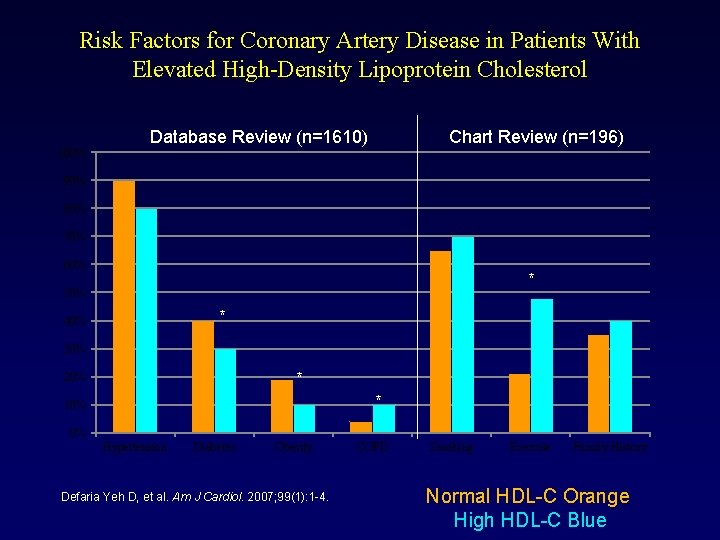
Risk Factors for Coronary Artery Disease in Patients With Elevated High-Density Lipoprotein Cholesterol 100% Database Review (n=1610) Chart Review (n=196) 90% 80% 70% 60% * 50% * 40% 30% * 20% * 10% 0% Hypertension Diabetes Obesity Defaria Yeh D, et al. Am J Cardiol. 2007; 99(1): 1 -4. COPD Smoking Exercise Family History Normal HDL-C Orange High HDL-C Blue

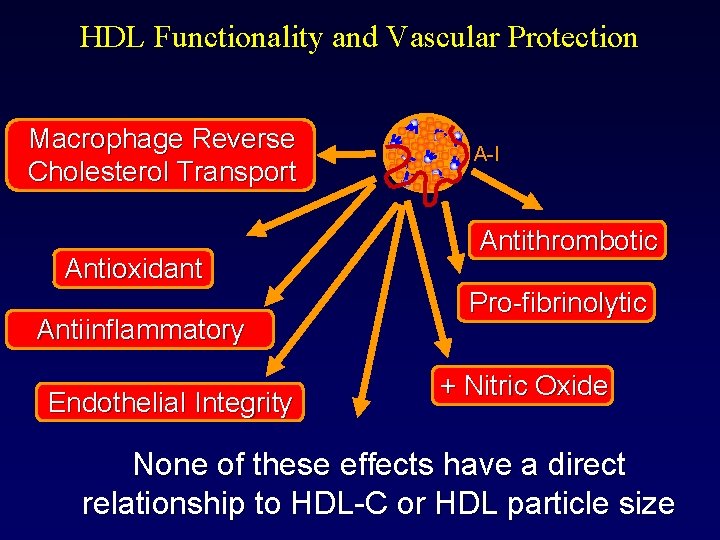
HDL Functionality and Vascular Protection Macrophage Reverse Cholesterol Transport Antioxidant Antiinflammatory Endothelial Integrity A-I Antithrombotic Pro-fibrinolytic + Nitric Oxide None of these effects have a direct relationship to HDL-C or HDL particle size

Dysfunctional HDL-C Factors that can make the “good” cholesterol better – or worse • Proven to promote the anti-inflammatory effect of high-density lipoprotein (HDL) – Apolipoprotein (apo) A-I mimeticsa – Exercise, low-fat diet – Polyunsaturated fat diet – Statins • May promote HDL’s anti-inflammatory effect – Antirheumatic biologicals – Apo A-IMilanoa – Delipidated HDLa • Promote proinflammatory HDL – Coronary atherosclerosis – Diabetes mellitus – Hemodialysis – Diet high in saturated fat – Infection – Rheumatoid arthritis – Surgery – Systemic lupus erythematosus Currently in development Ansell BJ. Cleve Clin J Med. 2007; 74(10): 697 -700, 703 -705. a

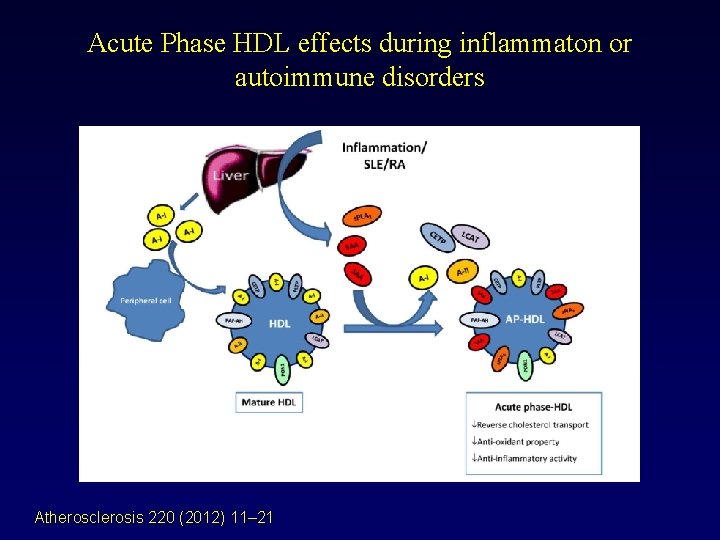
Acute Phase HDL effects during inflammaton or autoimmune disorders Atherosclerosis 220 (2012) 11– 21

Cholesterol Efflux Capacity N Engl J Med 2011; 364: 127 -35 .

HDL-C Levels vs. Functionality • At no time can a man say “It is, ” only “ I believe it was, " and “I hope it shall be. " Transformation is the only constant. From earth we come and to earth we shall return, but while above it we shall be shapeless, unsettled, as water. -Tandou Armah Cisse, So Far from Home

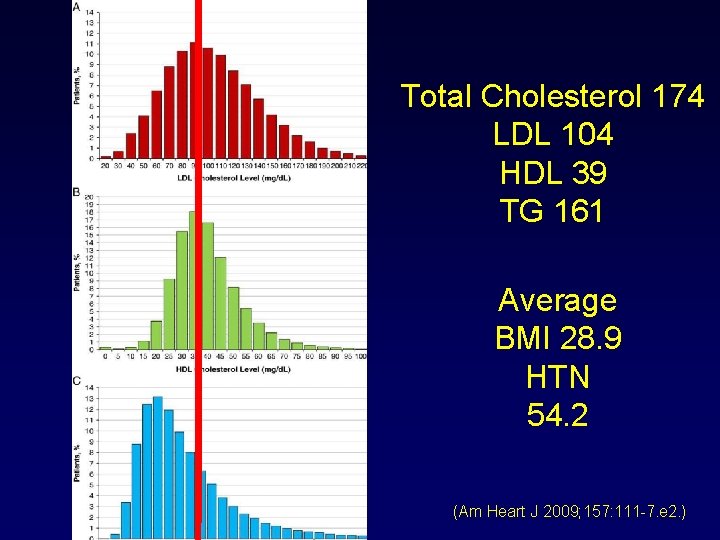
Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136, 905 hospitalizations in Get With The Guidelines Amit Sachdeva, MD, a Christopher P. Cannon, MD, b Prakash C. Deedwania, MD, c Kenneth A. La. Bresh, MD, d Sidney C. Smith, Jr, MD, e David Dai, MS, f Adrian Hernandez, MD, f and Gregg C. Fonarow, MD a on behalf of the GWTG Steering Committee and Hospitals Los Angeles and San Francisco, CA; Boston and Waltham, MA; and Chapel Hill and Durham, NC (Am Heart J 2009; 157: 111 -7. e 2. )

Total Cholesterol 174 LDL 104 HDL 39 TG 161 Average BMI 28. 9 HTN 54. 2 (Am Heart J 2009; 157: 111 -7. e 2. )

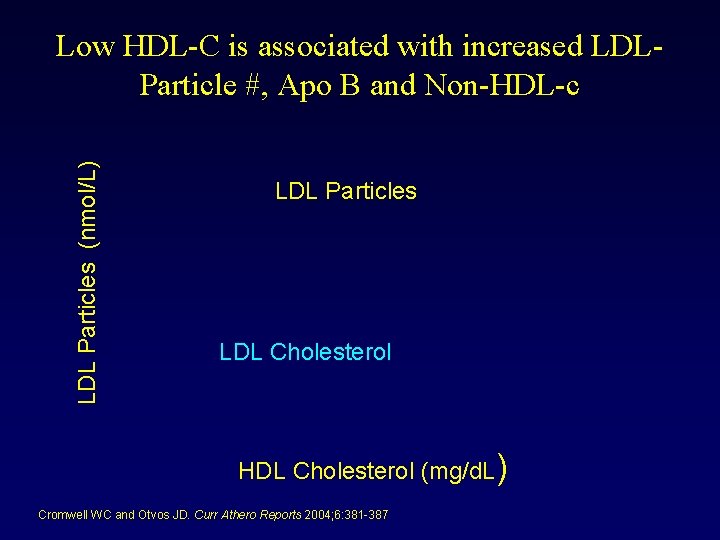
LDL Particles (nmol/L) Low HDL-C is associated with increased LDLParticle #, Apo B and Non-HDL-c LDL Particles LDL Cholesterol HDL Cholesterol (mg/d. L) Cromwell WC and Otvos JD. Curr Athero Reports 2004; 6: 381 -387

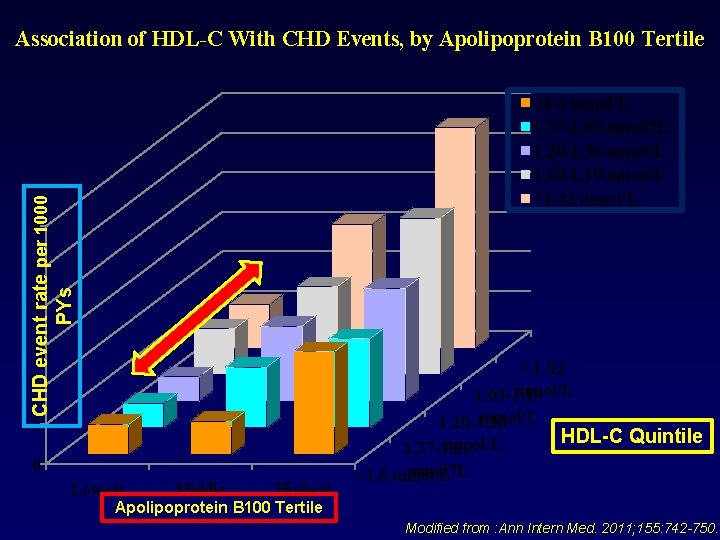
Association of HDL-C With CHD Events, by Apolipoprotein B 100 Tertile CHD event rate per 1000 PYs >1. 6 mmol/L 1. 37 -1. 60 mmol? L 1. 20 -1. 36 mmol/L 1. 03 -1. 19 mmol/L <1. 02 mmol/L 6 5 4 3 2 1 0 Lowest Middle Highest <1. 02 mmol/L 1. 03 -1. 19 mmol/L 1. 20 -1. 36 HDL-C Quintile mmol/L 1. 37 -1. 60 mmol? L >1. 6 mmol/L Apolipoprotein B 100 Tertile Modified from : Ann Intern Med. 2011; 155: 742 -750.

Differential Dx of Low HDLc • Diagnosis often imparts risk or lack thereof • Diagnosis help to understand need for treatment • Diagnosis helps to understand how aggressive treatment should be • Diagnosis helps guide the management other secondary causes of low HDL-C

Causes of Low HDL-C • • • Lifestyle Diet Smoking Medications Secondary Disease States Genetic disorders

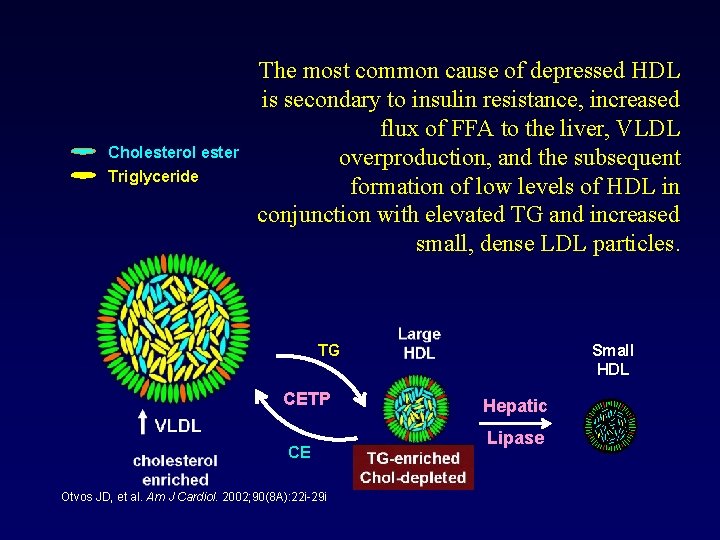
Cholesterol ester Triglyceride The most common cause of depressed HDL is secondary to insulin resistance, increased flux of FFA to the liver, VLDL overproduction, and the subsequent formation of low levels of HDL in conjunction with elevated TG and increased small, dense LDL particles. Small HDL TG CETP CE Otvos JD, et al. Am J Cardiol. 2002; 90(8 A): 22 i-29 i Hepatic Lipase

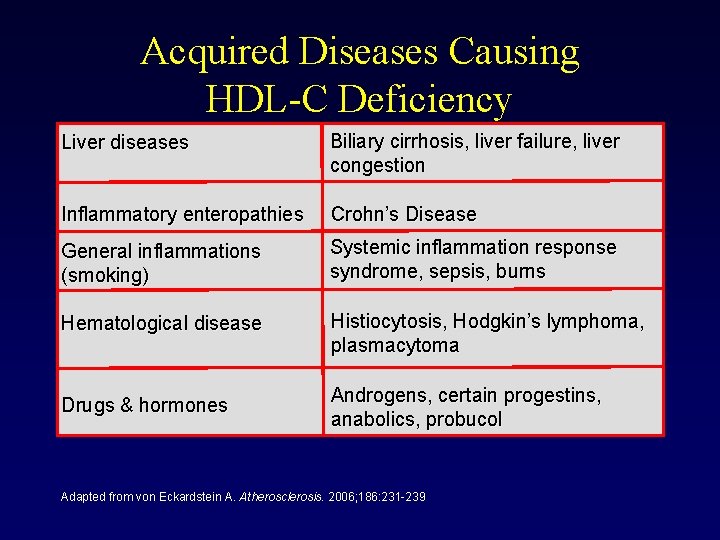
Acquired Diseases Causing HDL-C Deficiency Liver diseases Biliary cirrhosis, liver failure, liver congestion Inflammatory enteropathies Crohn’s Disease General inflammations (smoking) Systemic inflammation response syndrome, sepsis, burns Hematological disease Histiocytosis, Hodgkin’s lymphoma, plasmacytoma Drugs & hormones Androgens, certain progestins, anabolics, probucol Adapted from von Eckardstein A. Atherosclerosis. 2006; 186: 231 -239

HDL-C Deficiency Table 1: Common Causes of High-density Lipoprotein Deficiency • Obesity, especially visceral obesity • Insulin resistance • Hypertriglyceridemia • Metabolic syndrome • Type 2 diabetes • HIV disease • Low fat intake or diets enriched with polyunsaturated fat • Cigarette smoking • Severe stress states, e. g. , sepsis, burns • Liver disease • Renal insufficiency • Drugs – – – – Isotretinoin Sirolimus (rapamycin) Protease inhibitors Androgenic steroids Non-selective β-blockers Probucol Recombinant interleukin-2 Brown BG, et al. J Clin Lipidol. 2007; 1(1): 88 -94.

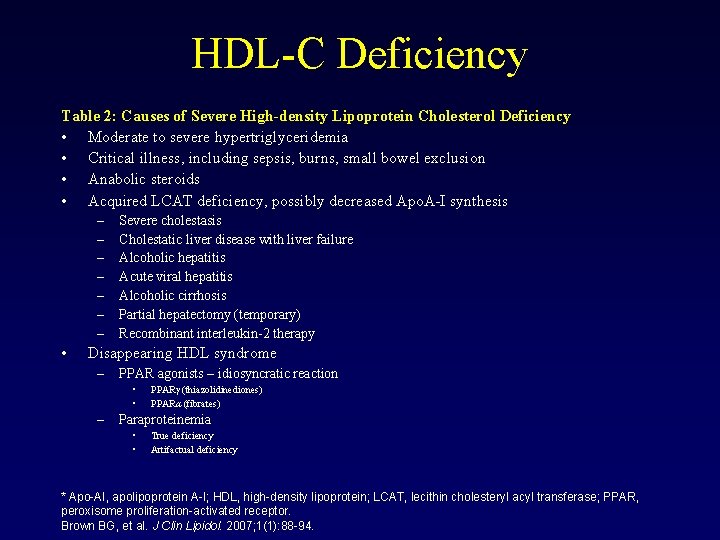
HDL-C Deficiency Table 2: Causes of Severe High-density Lipoprotein Cholesterol Deficiency • Moderate to severe hypertriglyceridemia • Critical illness, including sepsis, burns, small bowel exclusion • Anabolic steroids • Acquired LCAT deficiency, possibly decreased Apo. A-I synthesis – – – – • Severe cholestasis Cholestatic liver disease with liver failure Alcoholic hepatitis Acute viral hepatitis Alcoholic cirrhosis Partial hepatectomy (temporary) Recombinant interleukin-2 therapy Disappearing HDL syndrome – PPAR agonists – idiosyncratic reaction • • – PPARγ (thiazolidinediones) PPARα (fibrates) Paraproteinemia • • True deficiency Artifactual deficiency * Apo-AI, apolipoprotein A-I; HDL, high-density lipoprotein; LCAT, lecithin cholesteryl acyl transferase; PPAR, peroxisome proliferation-activated receptor. Brown BG, et al. J Clin Lipidol. 2007; 1(1): 88 -94.

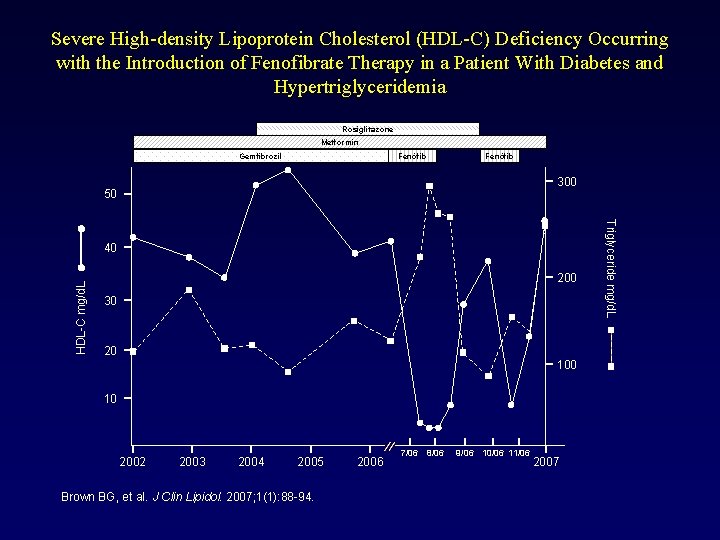
Severe High-density Lipoprotein Cholesterol (HDL-C) Deficiency Occurring with the Introduction of Fenofibrate Therapy in a Patient With Diabetes and Hypertriglyceridemia Rosiglitazone Metformin Fenòfib Gemfibrozil Fenòfib 300 50 HDL-C mg/d. L 200 30 20 10 2002 2003 2004 2005 Brown BG, et al. J Clin Lipidol. 2007; 1(1): 88 -94. 2006 7/06 8/06 9/06 10/06 11/06 2007 Triglyceride mg/d. L 40

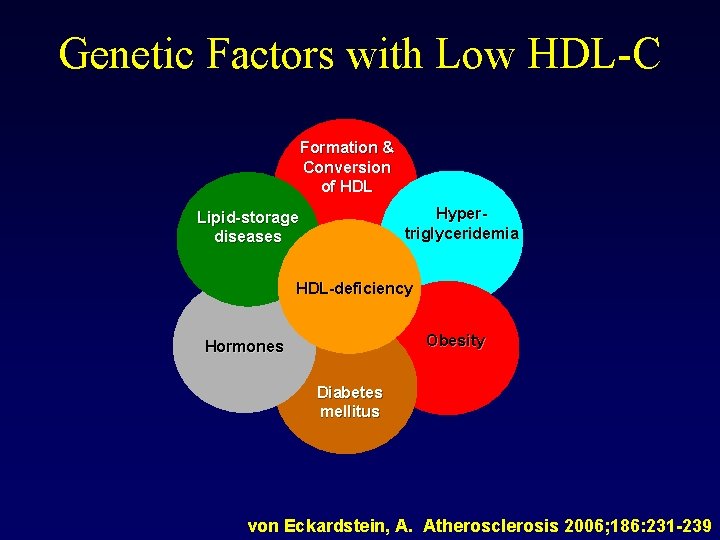
Genetic Factors with Low HDL-C Formation & Conversion of HDL Hypertriglyceridemia Lipid-storage diseases HDL-deficiency Obesity Hormones Diabetes mellitus von Eckardstein, A. Atherosclerosis 2006; 186: 231 -239

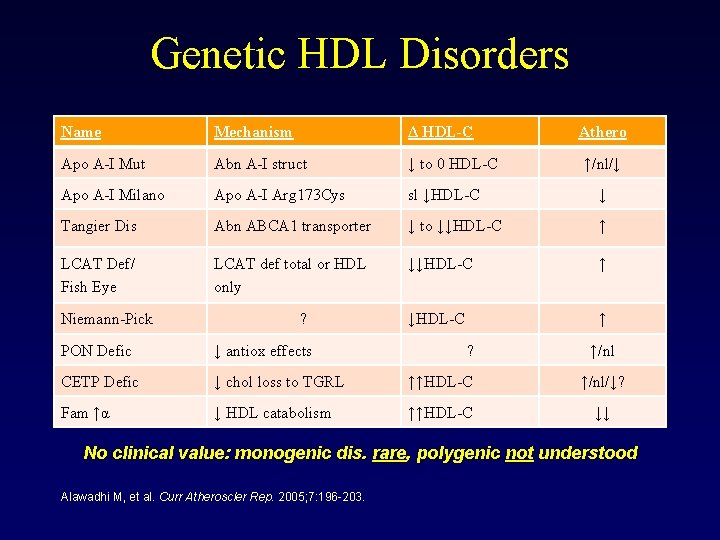
Genetic HDL Disorders Name Mechanism Δ HDL-C Apo A-I Mut Abn A-I struct ↓ to 0 HDL-C Apo A-I Milano Apo A-I Arg 173 Cys sl ↓HDL-C ↓ Tangier Dis Abn ABCA 1 transporter ↓ to ↓↓HDL-C ↑ LCAT Def/ Fish Eye LCAT def total or HDL only ↓↓HDL-C ↑ Niemann-Pick ? ? Athero ↑/nl/↓ PON Defic ↓ antiox effects ↑/nl CETP Defic ↓ chol loss to TGRL ↑↑HDL-C ↑/nl/↓? Fam ↑α ↓ HDL catabolism ↑↑HDL-C ↓↓ No clinical value: monogenic dis. rare, polygenic not understood Alawadhi M, et al. Curr Atheroscler Rep. 2005; 7: 196 -203.

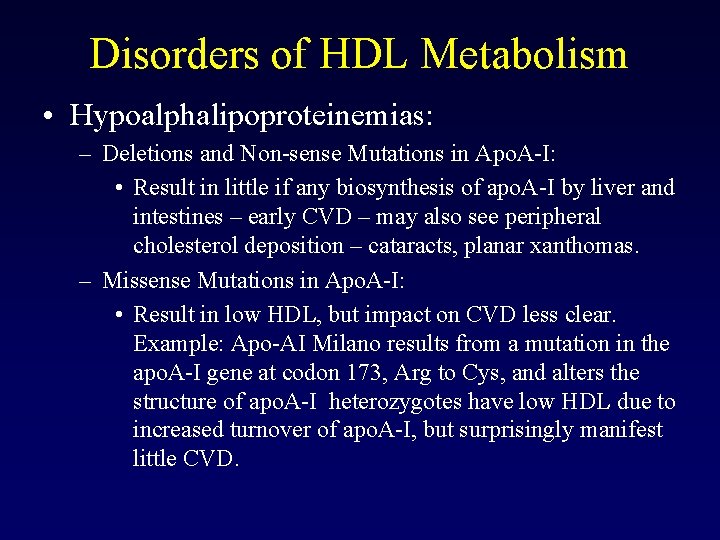
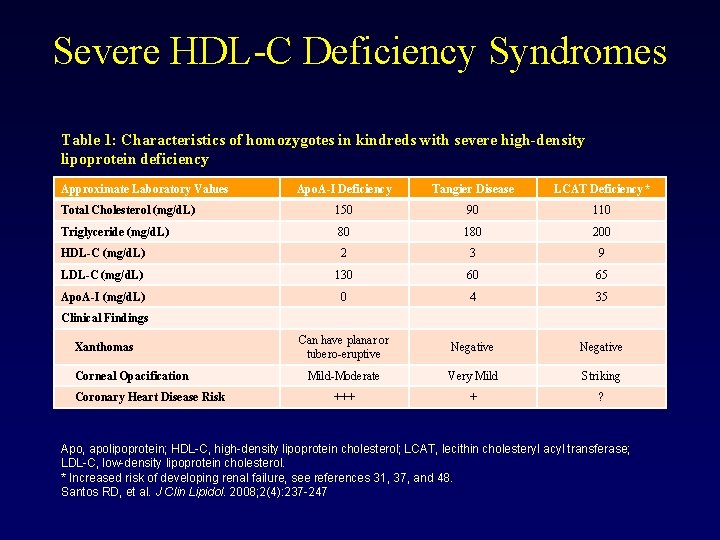
Disorders of HDL Metabolism • Hypoalphalipoproteinemias: – Deletions and Non-sense Mutations in Apo. A-I: • Result in little if any biosynthesis of apo. A-I by liver and intestines – early CVD – may also see peripheral cholesterol deposition – cataracts, planar xanthomas. – Missense Mutations in Apo. A-I: • Result in low HDL, but impact on CVD less clear. Example: Apo-AI Milano results from a mutation in the apo. A-I gene at codon 173, Arg to Cys, and alters the structure of apo. A-I heterozygotes have low HDL due to increased turnover of apo. A-I, but surprisingly manifest little CVD.

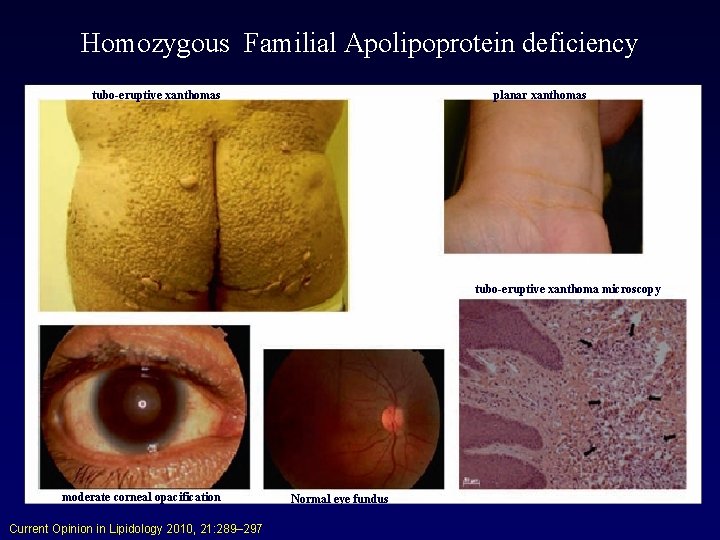
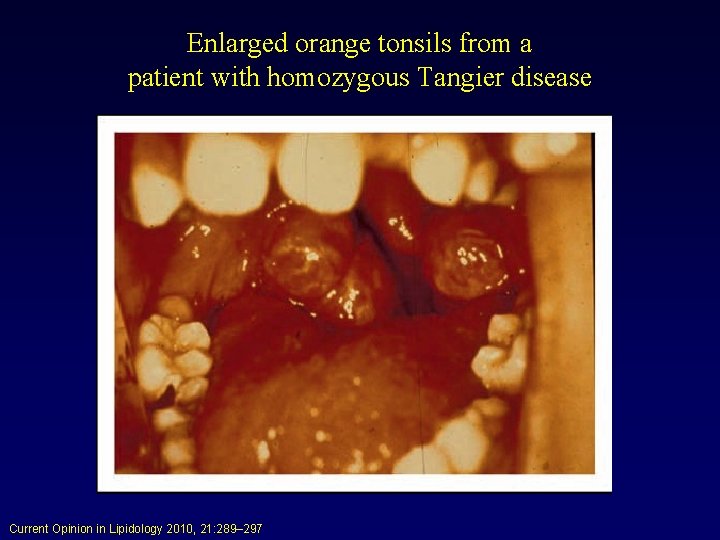
Disorders of HDL Metabolism • Hypoalphalipoproteinemias: – Tangier Disease: Results from a defect in cholesterol and PL efflux from cells to apo. A-I. Poorly lipidated apo. A-I is then rapidly removed by the kidney. Basic defect is in double dose mutation in ABCA 1 gene. HDL markedly abnormal. Chylomicron-like particles are sequestered by the reticular endothelial cells (yellow tonsils). Early atherosclerosis is not a major feature.

Disorders of HDL Metabolism • Hypoalphalipoproteinemias: – LCAT Deficiency and Fish Eye Disease: Free cholesterol must be esterified to produce a spherical HDL; esterification is achieved via LCAT. Patients with LCAT deficiency have markedly reduced HDL. (Other lipoproteins are also abnormal). Clinical findings include glomerulosclerosis, normochromic anemia, and corneal opacities. Although CAD is not prominent, it has been reported. Classic LCAT deficiency – both α and β LCAT deficient – all lipoproteins abnl. If only α LCAT is abnormal – fish eye disease – a major finding is corneal opacities.

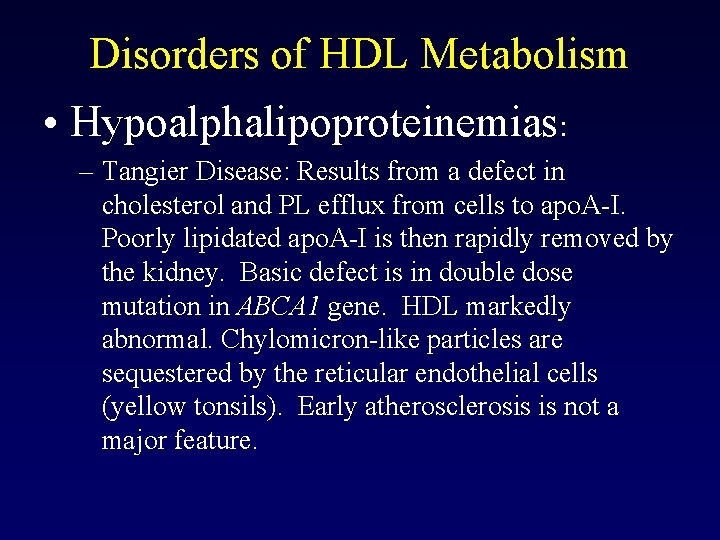
Severe HDL-C Deficiency Syndromes Table 1: Characteristics of homozygotes in kindreds with severe high-density lipoprotein deficiency Approximate Laboratory Values Apo. A-I Deficiency Tangier Disease LCAT Deficiency* Total Cholesterol (mg/d. L) 150 90 110 Triglyceride (mg/d. L) 80 180 200 HDL-C (mg/d. L) 2 3 9 LDL-C (mg/d. L) 130 60 65 Apo. A-I (mg/d. L) 0 4 35 Can have planar or tubero-eruptive Negative Mild-Moderate Very Mild Striking +++ + ? Clinical Findings Xanthomas Corneal Opacification Coronary Heart Disease Risk Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; LCAT, lecithin cholesteryl acyl transferase; LDL-C, low-density lipoprotein cholesterol. * Increased risk of developing renal failure, see references 31, 37, and 48. Santos RD, et al. J Clin Lipidol. 2008; 2(4): 237 -247

Homozygous Familial Apolipoprotein deficiency tubo-eruptive xanthomas planar xanthomas tubo-eruptive xanthoma microscopy moderate corneal opacification Current Opinion in Lipidology 2010, 21: 289– 297 Normal eye fundus

Enlarged orange tonsils from a patient with homozygous Tangier disease Current Opinion in Lipidology 2010, 21: 289– 297

LCAT remains a potential target JACC Vol. 58, No. 24, 2011 December 6, 2011: 2488– 90

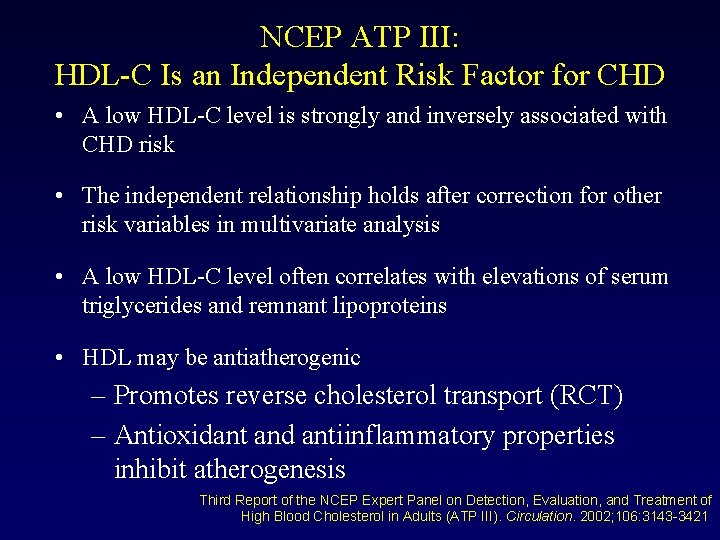
NCEP ATP III: HDL-C Is an Independent Risk Factor for CHD • A low HDL-C level is strongly and inversely associated with CHD risk • The independent relationship holds after correction for other risk variables in multivariate analysis • A low HDL-C level often correlates with elevations of serum triglycerides and remnant lipoproteins • HDL may be antiatherogenic – Promotes reverse cholesterol transport (RCT) – Antioxidant and antiinflammatory properties inhibit atherogenesis Third Report of the NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III). Circulation. 2002; 106: 3143 -3421.

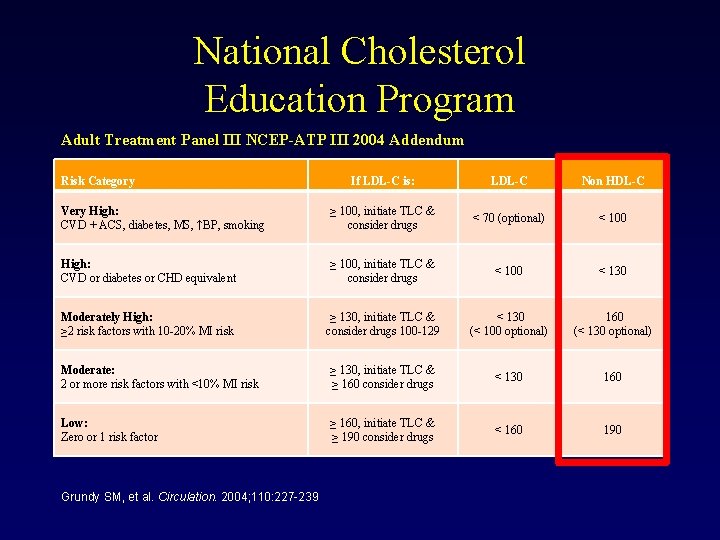
National Cholesterol Education Program Adult Treatment Panel III NCEP-ATP III 2004 Addendum Risk Category If LDL-C is: LDL-C Non HDL-C Very High: CVD + ACS, diabetes, MS, ↑BP, smoking ≥ 100, initiate TLC & consider drugs < 70 (optional) < 100 High: CVD or diabetes or CHD equivalent ≥ 100, initiate TLC & consider drugs < 100 < 130 Moderately High: ≥ 2 risk factors with 10 -20% MI risk ≥ 130, initiate TLC & consider drugs 100 -129 < 130 (< 100 optional) 160 (< 130 optional) Moderate: 2 or more risk factors with <10% MI risk ≥ 130, initiate TLC & ≥ 160 consider drugs < 130 160 Low: Zero or 1 risk factor ≥ 160, initiate TLC & ≥ 190 consider drugs < 160 190 Grundy SM, et al. Circulation. 2004; 110: 227 -239

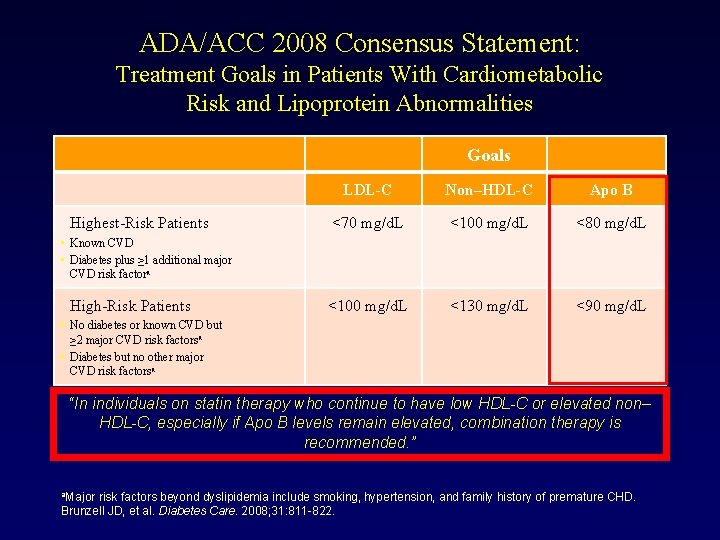
ADA/ACC 2008 Consensus Statement: Treatment Goals in Patients With Cardiometabolic Risk and Lipoprotein Abnormalities Goals Highest-Risk Patients LDL-C Non–HDL-C Apo B <70 mg/d. L <100 mg/d. L <80 mg/d. L <100 mg/d. L <130 mg/d. L <90 mg/d. L • Known CVD • Diabetes plus ≥ 1 additional major CVD risk factora High-Risk Patients • No diabetes or known CVD but ≥ 2 major CVD risk factorsa • Diabetes but no other major CVD risk factorsa “In individuals on statin therapy who continue to have low HDL-C or elevated non– HDL-C, especially if Apo B levels remain elevated, combination therapy is recommended. ” a. Major risk factors beyond dyslipidemia include smoking, hypertension, and family history of premature CHD. Brunzell JD, et al. Diabetes Care. 2008; 31: 811 -822.

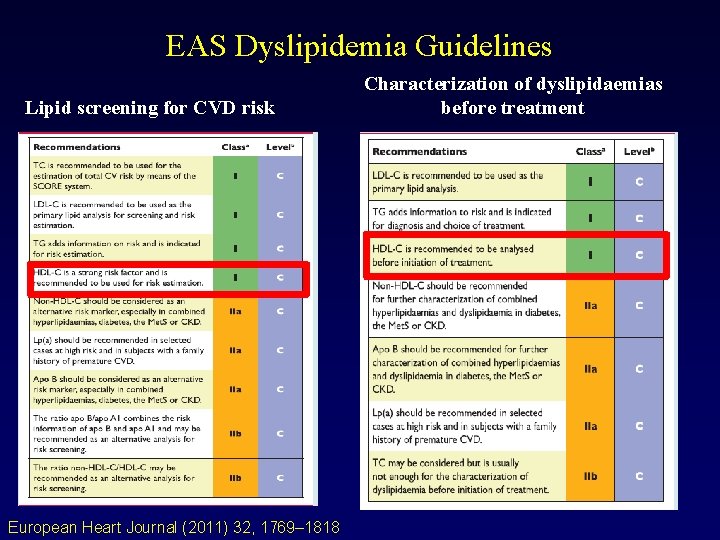
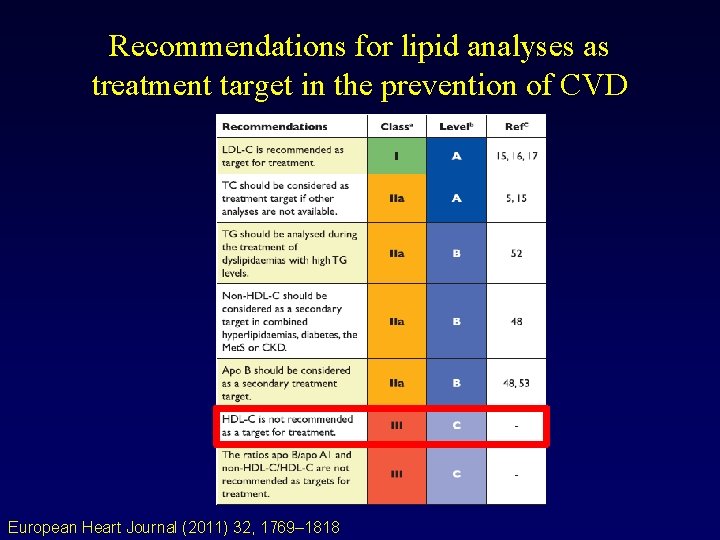
EAS Dyslipidemia Guidelines Lipid screening for CVD risk European Heart Journal (2011) 32, 1769– 1818 Characterization of dyslipidaemias before treatment

Recommendations for lipid analyses as treatment target in the prevention of CVD European Heart Journal (2011) 32, 1769– 1818

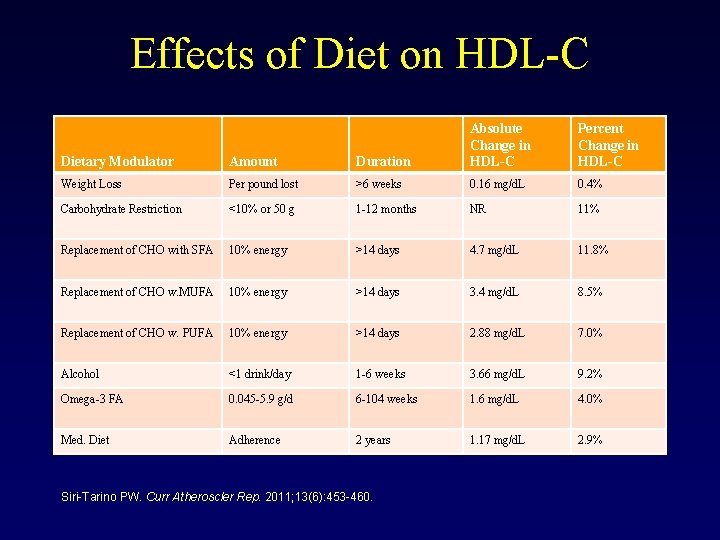
Effects of Diet on HDL-C Dietary Modulator Amount Duration Absolute Change in HDL-C Weight Loss Per pound lost >6 weeks 0. 16 mg/d. L 0. 4% Carbohydrate Restriction <10% or 50 g 1 -12 months NR 11% Replacement of CHO with SFA 10% energy >14 days 4. 7 mg/d. L 11. 8% Replacement of CHO w. MUFA 10% energy >14 days 3. 4 mg/d. L 8. 5% Replacement of CHO w. PUFA 10% energy >14 days 2. 88 mg/d. L 7. 0% Alcohol <1 drink/day 1 -6 weeks 3. 66 mg/d. L 9. 2% Omega-3 FA 0. 045 -5. 9 g/d 6 -104 weeks 1. 6 mg/d. L 4. 0% Med. Diet Adherence 2 years 1. 17 mg/d. L 2. 9% Siri-Tarino PW. Curr Atheroscler Rep. 2011; 13(6): 453 -460. Percent Change in HDL-C

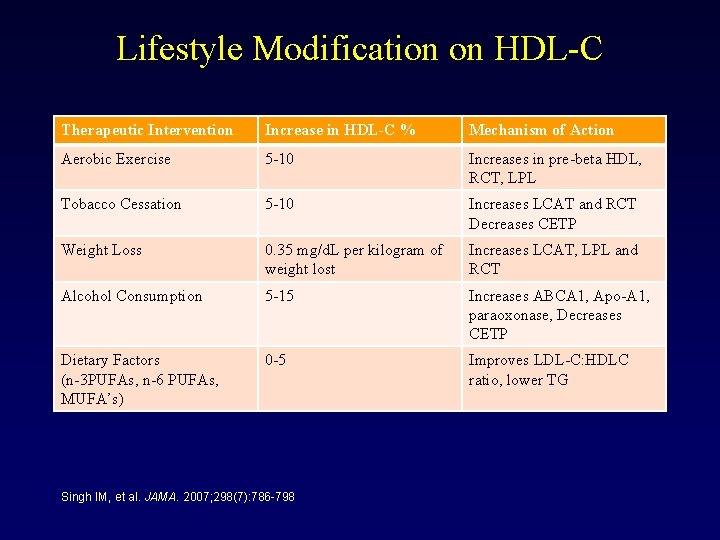
Lifestyle Modification on HDL-C Therapeutic Intervention Increase in HDL-C % Mechanism of Action Aerobic Exercise 5 -10 Increases in pre-beta HDL, RCT, LPL Tobacco Cessation 5 -10 Increases LCAT and RCT Decreases CETP Weight Loss 0. 35 mg/d. L per kilogram of weight lost Increases LCAT, LPL and RCT Alcohol Consumption 5 -15 Increases ABCA 1, Apo-A 1, paraoxonase, Decreases CETP Dietary Factors (n-3 PUFAs, n-6 PUFAs, MUFA’s) 0 -5 Improves LDL-C: HDLC ratio, lower TG Singh IM, et al. JAMA. 2007; 298(7): 786 -798

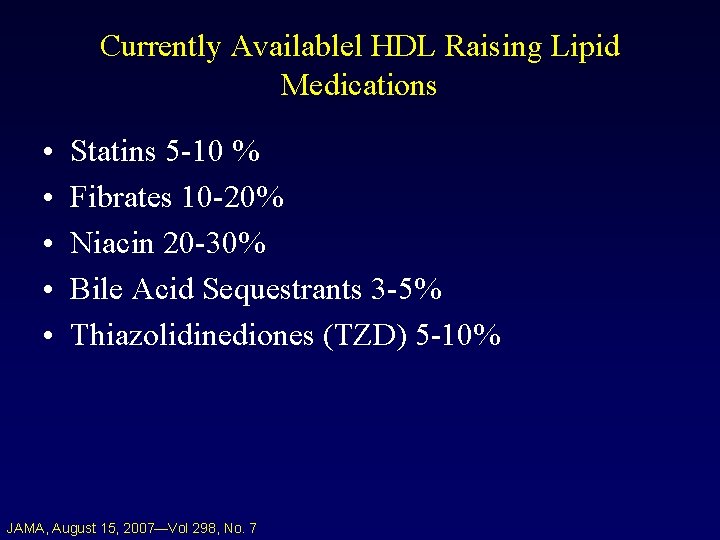
Currently Availablel HDL Raising Lipid Medications • • • Statins 5 -10 % Fibrates 10 -20% Niacin 20 -30% Bile Acid Sequestrants 3 -5% Thiazolidinediones (TZD) 5 -10% JAMA, August 15, 2007—Vol 298, No. 7

Developmental treatments for raising HDL cholesterol • Cholesteryl ester transfer protein (CETP) inhibitors • Recombinant apolipoprotein A-I • Reconstituted HDL • Synthetic HDL particles • LXR Agonists

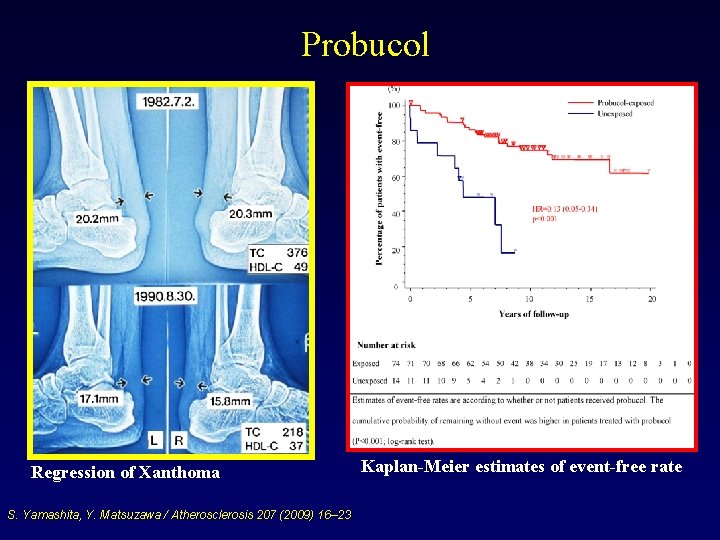
Probucol Regression of Xanthoma S. Yamashita, Y. Matsuzawa / Atherosclerosis 207 (2009) 16– 23 Kaplan-Meier estimates of event-free rate

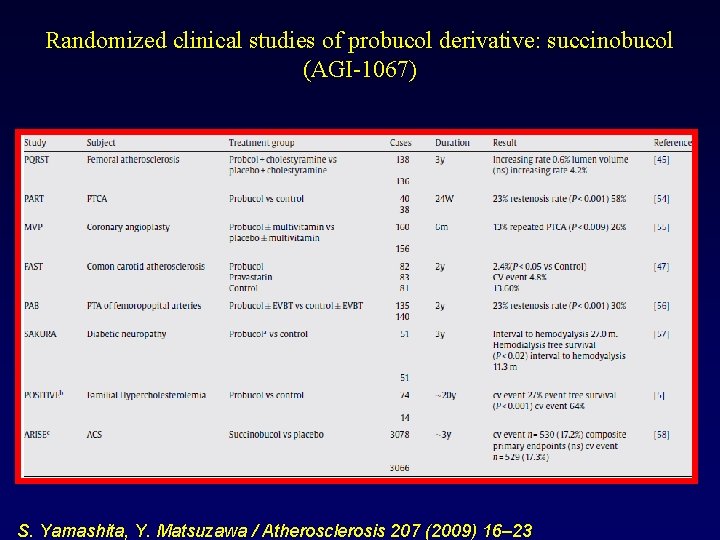
Randomized clinical studies of probucol derivative: succinobucol (AGI-1067) S. Yamashita, Y. Matsuzawa / Atherosclerosis 207 (2009) 16– 23

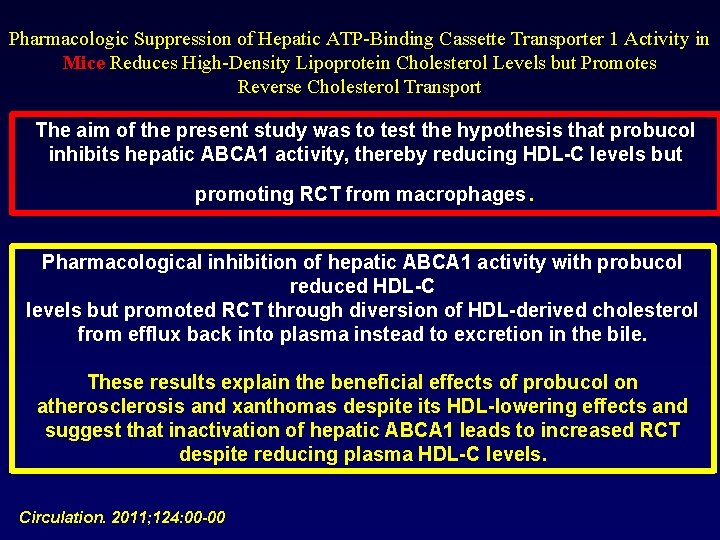
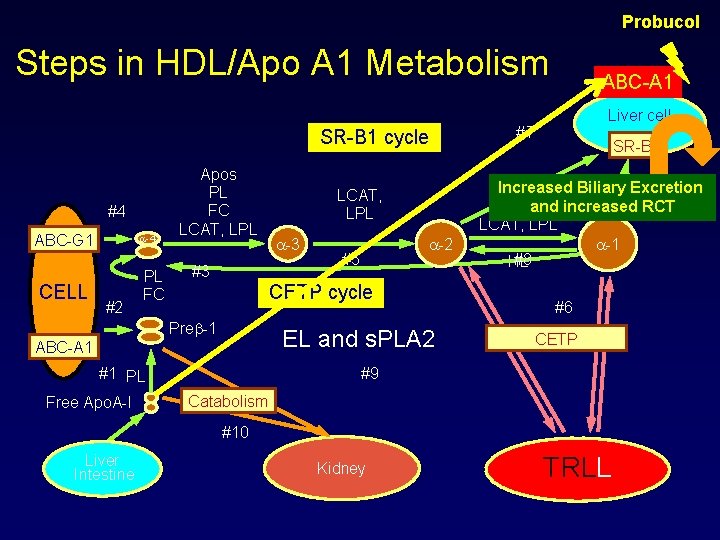
Pharmacologic Suppression of Hepatic ATP-Binding Cassette Transporter 1 Activity in Mice Reduces High-Density Lipoprotein Cholesterol Levels but Promotes Reverse Cholesterol Transport The aim of the present study was to test the hypothesis that probucol inhibits hepatic ABCA 1 activity, thereby reducing HDL-C levels but . promoting RCT from macrophages Pharmacological inhibition of hepatic ABCA 1 activity with probucol reduced HDL-C levels but promoted RCT through diversion of HDL-derived cholesterol from efflux back into plasma instead to excretion in the bile. These results explain the beneficial effects of probucol on atherosclerosis and xanthomas despite its HDL-lowering effects and suggest that inactivation of hepatic ABCA 1 leads to increased RCT despite reducing plasma HDL-C levels. Circulation. 2011; 124: 00 -00

Probucol Steps in HDL/Apo A 1 Metabolism SR-B 1 cycle #4 ABC-G 1 CELL -4 #2 PL FC Apos PL FC LCAT, LPL #3 -2 #5 CETP cycle Pre -1 EL and s. PLA 2 ABC-A 1 #1 PL Free Apo. A-I LCAT, LPL ABC-A 1 Liver cell #7 SR-B 1 Increased Biliary Excretion and increased RCT LCAT, LPL -1 #8 HL #6 CETP #9 Catabolism #10 Liver Intestine Kidney TRLL

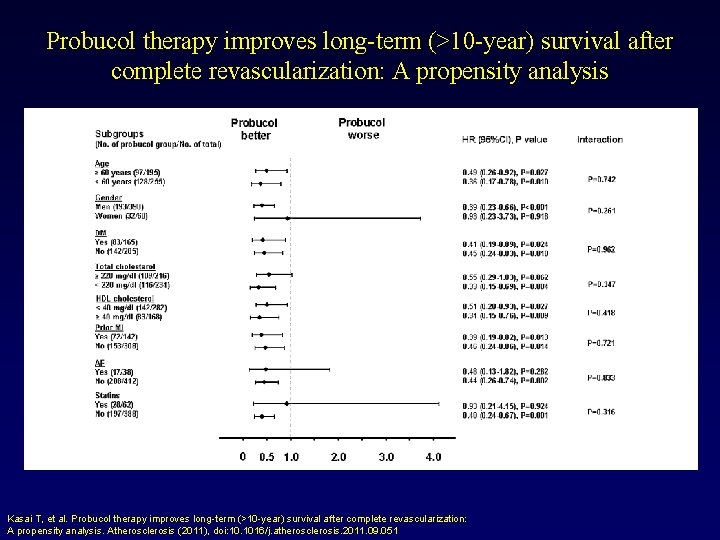
Probucol therapy improves long-term (>10 -year) survival after complete revascularization: A propensity analysis Kasai T, et al. Probucol therapy improves long-term (>10 -year) survival after complete revascularization: A propensity analysis. Atherosclerosis (2011), doi: 10. 1016/j. atherosclerosis. 2011. 09. 051

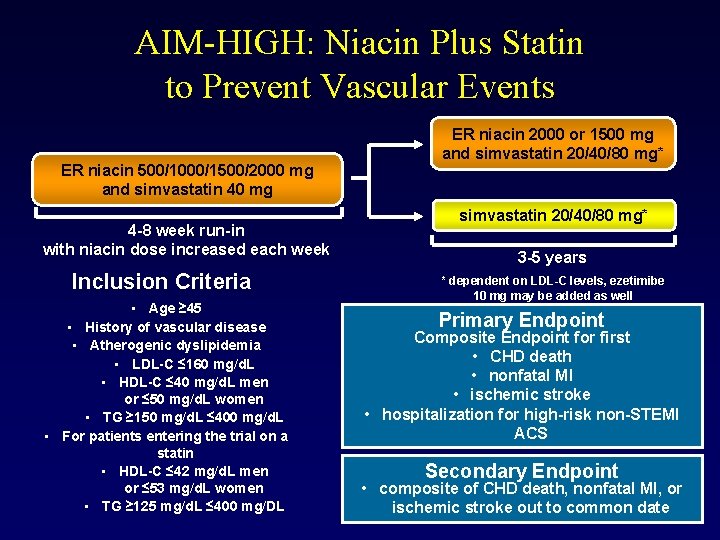
AIM-HIGH: Niacin Plus Statin to Prevent Vascular Events ER niacin 500/1000/1500/2000 mg and simvastatin 40 mg 4 -8 week run-in with niacin dose increased each week Inclusion Criteria • Age ≥ 45 • History of vascular disease • Atherogenic dyslipidemia • LDL-C ≤ 160 mg/d. L • HDL-C ≤ 40 mg/d. L men or ≤ 50 mg/d. L women • TG ≥ 150 mg/d. L ≤ 400 mg/d. L • For patients entering the trial on a statin • HDL-C ≤ 42 mg/d. L men or ≤ 53 mg/d. L women • TG ≥ 125 mg/d. L ≤ 400 mg/DL ER niacin 2000 or 1500 mg and simvastatin 20/40/80 mg* 3 -5 years * dependent on LDL-C levels, ezetimibe 10 mg may be added as well Primary Endpoint Composite Endpoint for first • CHD death • nonfatal MI • ischemic stroke • hospitalization for high-risk non-STEMI ACS Secondary Endpoint • composite of CHD death, nonfatal MI, or ischemic stroke out to common date

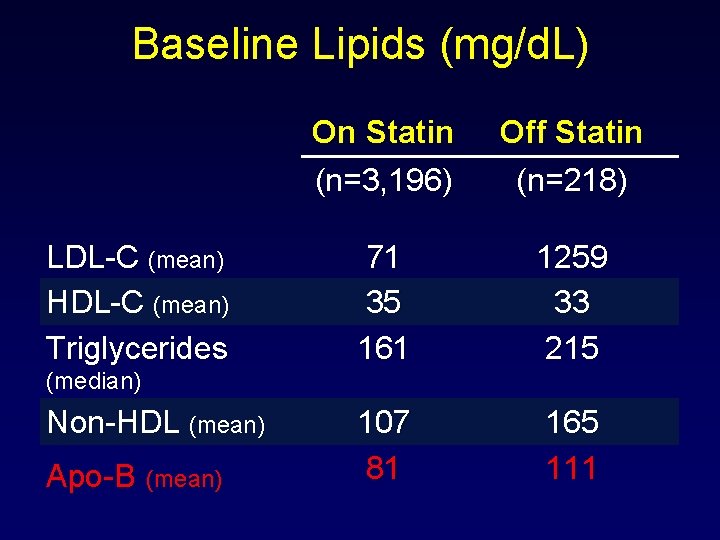
Baseline Lipids (mg/d. L) LDL-C (mean) HDL-C (mean) Triglycerides On Statin (n=3, 196) Off Statin (n=218) 71 35 161 1259 33 215 107 81 165 111 (median) Non-HDL (mean) Apo-B (mean)

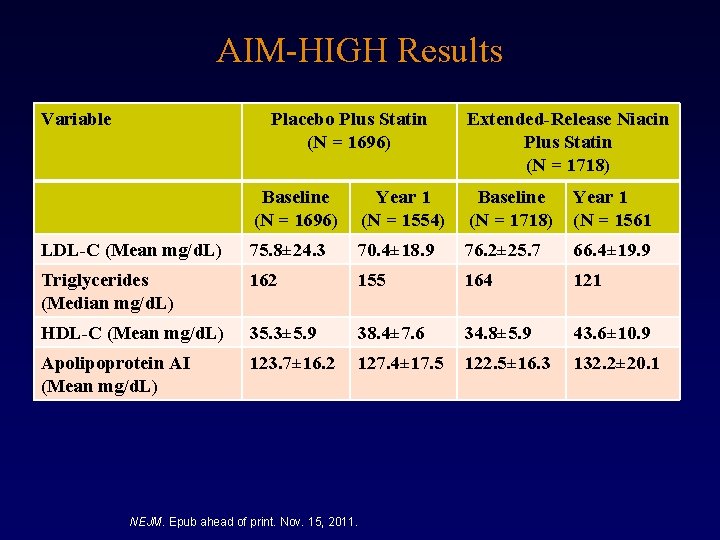
AIM-HIGH Results Variable Placebo Plus Statin (N = 1696) Extended-Release Niacin Plus Statin (N = 1718) Baseline (N = 1696) Year 1 (N = 1554) Baseline (N = 1718) Year 1 (N = 1561 LDL-C (Mean mg/d. L) 75. 8± 24. 3 70. 4± 18. 9 76. 2± 25. 7 66. 4± 19. 9 Triglycerides (Median mg/d. L) 162 155 164 121 HDL-C (Mean mg/d. L) 35. 3± 5. 9 38. 4± 7. 6 34. 8± 5. 9 43. 6± 10. 9 Apolipoprotein AI (Mean mg/d. L) 123. 7± 16. 2 127. 4± 17. 5 122. 5± 16. 3 132. 2± 20. 1 NEJM. Epub ahead of print. Nov. 15, 2011.

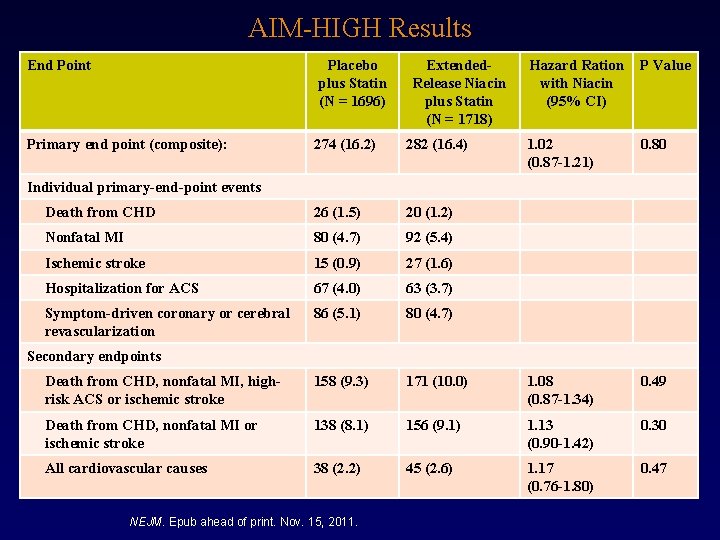
AIM-HIGH Results End Point Placebo plus Statin (N = 1696) Primary end point (composite): Extended. Release Niacin plus Statin (N = 1718) Hazard Ration with Niacin (95% CI) P Value 1. 02 (0. 87 -1. 21) 0. 80 274 (16. 2) 282 (16. 4) Death from CHD 26 (1. 5) 20 (1. 2) Nonfatal MI 80 (4. 7) 92 (5. 4) Ischemic stroke 15 (0. 9) 27 (1. 6) Hospitalization for ACS 67 (4. 0) 63 (3. 7) Symptom-driven coronary or cerebral revascularization 86 (5. 1) 80 (4. 7) Death from CHD, nonfatal MI, highrisk ACS or ischemic stroke 158 (9. 3) 171 (10. 0) 1. 08 (0. 87 -1. 34) 0. 49 Death from CHD, nonfatal MI or ischemic stroke 138 (8. 1) 156 (9. 1) 1. 13 (0. 90 -1. 42) 0. 30 All cardiovascular causes 38 (2. 2) 45 (2. 6) 1. 17 (0. 76 -1. 80) 0. 47 Individual primary-end-point events Secondary endpoints NEJM. Epub ahead of print. Nov. 15, 2011.

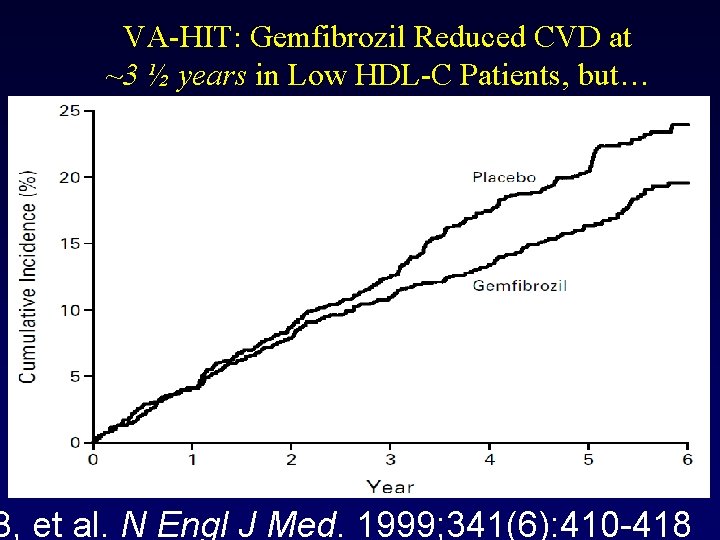
VA-HIT: Gemfibrozil Reduced CVD at ~3 ½ years in Low HDL-C Patients, but… B, et al. N Engl J Med. 1999; 341(6): 410 -418

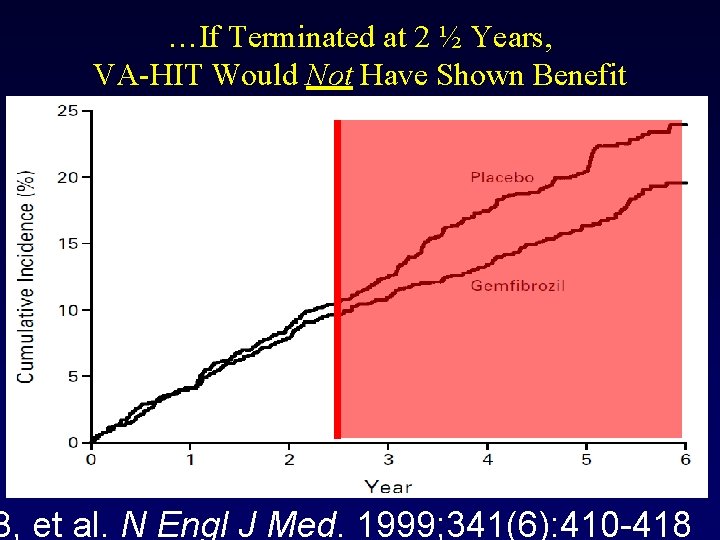
…If Terminated at 2 ½ Years, VA-HIT Would Not Have Shown Benefit B, et al. N Engl J Med. 1999; 341(6): 410 -418

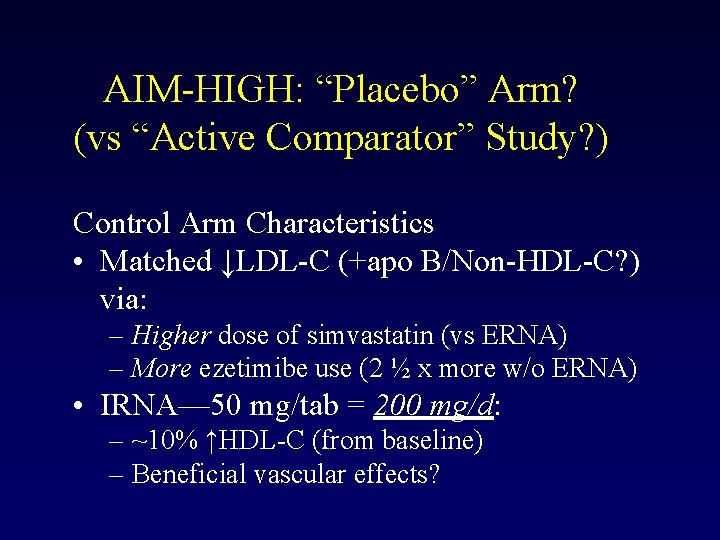
AIM-HIGH: “Placebo” Arm? (vs “Active Comparator” Study? ) Control Arm Characteristics • Matched ↓LDL-C (+apo B/Non-HDL-C? ) via: – Higher dose of simvastatin (vs ERNA) – More ezetimibe use (2 ½ x more w/o ERNA) • IRNA— 50 mg/tab = 200 mg/d: – ~10% ↑HDL-C (from baseline) – Beneficial vascular effects?

So What Happened? • In this aggressively managed group of patients was there no residual risk, hence no need for niacin? • Prior studies done with Niacin IR, perhaps Niacin ER ineffective? • Placebo effect ? • Niacin did not lower Apo B or LDLp? • HDL raising not effective if LDLp or Apo B at goal? • Perhaps raising HDLc with no impact on HDLp with low Apo. B/LDLp levels is not effective? • Diabetes risk? ? /CVD • Stroke? No prior signals. ? AFIB? • “The Surprising AIM-HIGH Results Are Not Surprising When Viewed Through a Particle Lens” (Otvos JCL 2011)

CETP Inhibitors and Modulators Evacetrapib CETP Barter P, et al. N Engl J Med. 2007; 357: 2109 -2122; http: //www. amaassn. org/ama 1/pub/upload/mm/365/dalcetrapib. doc; http: //www. amaassn. org/ama 1/pub/upload/mm/365/torcetrapib. doc; Qiu X, et al. Nat Struct Mol Biol. 2007; 14(2): 106 -13. http: //www. amaassn. org/ama 1/pub/upload/mm/365/anacetrapib. pdf.

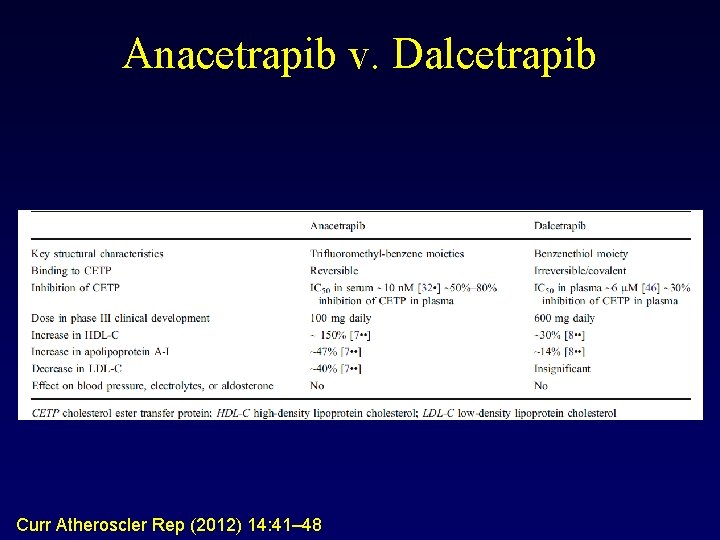
Anacetrapib v. Dalcetrapib Curr Atheroscler Rep (2012) 14: 41– 48

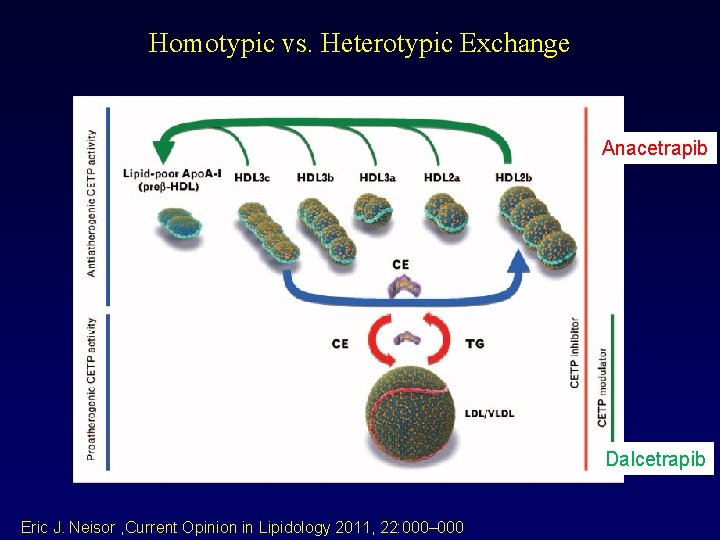
Homotypic vs. Heterotypic Exchange Anacetrapib Dalcetrapib Eric J. Neisor , Current Opinion in Lipidology 2011, 22: 000– 000

Plan for our patient • • • Additional Lipoprotein testing Apo B 133 mg/dl (High) LDLp 2200 nmol/L (High) Size 19. 2 nm (small) Patient has elevated Apo. B/LDLp in setting of small dense LDL particles • Plan statin therapy followed by additional LDLp/Apo. B lowering Rx to achieve target Apo. B/LDLp levels
 Low hdl
Low hdl Patient 2 patient
Patient 2 patient Sociability continuum
Sociability continuum Significant figures
Significant figures Low voltage = low hazard
Low voltage = low hazard Middle = low + (high - low) / 2
Middle = low + (high - low) / 2 Testing the difference between two means dependent samples
Testing the difference between two means dependent samples Cholesterol precursor
Cholesterol precursor Cholesterol in urine
Cholesterol in urine Visual puns quiz
Visual puns quiz Carrier vs channel proteins
Carrier vs channel proteins Isolation of cholesterol from egg yolk
Isolation of cholesterol from egg yolk Structure of cholesterol
Structure of cholesterol Xanthome des plis palmaires
Xanthome des plis palmaires Lipid digestion
Lipid digestion Symcard cholesterol
Symcard cholesterol Function of cholesterol
Function of cholesterol Cppp nucleus
Cppp nucleus Sex and cholesterol
Sex and cholesterol How to determine total cholesterol
How to determine total cholesterol Steroids and cholesterol
Steroids and cholesterol Hugh cholesterol
Hugh cholesterol Emily lundstrom
Emily lundstrom Cholesterol cislovani
Cholesterol cislovani Cholesterol uptake
Cholesterol uptake Regulation of cholesterol biosynthesis
Regulation of cholesterol biosynthesis Hugh cholesterol
Hugh cholesterol Subunits of fat
Subunits of fat Good and bad cholesterol
Good and bad cholesterol How is cholesterol made
How is cholesterol made Cholesterol homeostasis
Cholesterol homeostasis Is cholesterol a lipid
Is cholesterol a lipid Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol Cholesterol treatment trialists collaboration
Cholesterol treatment trialists collaboration Lipid profile calculation chart
Lipid profile calculation chart A dietitian wishes to see if a person's cholesterol
A dietitian wishes to see if a person's cholesterol What do these images have in common?
What do these images have in common? Biochem
Biochem Good and bad cholesterol
Good and bad cholesterol Biological significance of cholesterol
Biological significance of cholesterol Cholesterol homeostasis
Cholesterol homeostasis Cholesterol esterification
Cholesterol esterification High cholesterol
High cholesterol Stephanie seneff cholesterol
Stephanie seneff cholesterol Steroid
Steroid Clay lawson and russell odom
Clay lawson and russell odom James russell odom
James russell odom Hdl buspro setup tool 2
Hdl buspro setup tool 2 Hdl formation
Hdl formation Region definition
Region definition Hdl genesys
Hdl genesys Panichelli luisa pediatra bergamo
Panichelli luisa pediatra bergamo Structural vs dataflow verilog
Structural vs dataflow verilog Knx presentation
Knx presentation A stepper motor hdl application must include
A stepper motor hdl application must include Hdl-mcip-rf02.10
Hdl-mcip-rf02.10 Ram16 hdl
Ram16 hdl Alu nand to tetris
Alu nand to tetris Hdl buspro setup tool 2 download
Hdl buspro setup tool 2 download Hdl designer tutorial
Hdl designer tutorial Fungsi hdl
Fungsi hdl Abel hdl
Abel hdl Advanced digital design with the verilog hdl
Advanced digital design with the verilog hdl Colestasi
Colestasi Hardware description language (hdl) can be used as a
Hardware description language (hdl) can be used as a Hdl code
Hdl code Verilog
Verilog Port connection rules in verilog
Port connection rules in verilog