CHOLESTEROL SYNTHESIS CHOLESTEROL Cholesterol is the most highly


![[1] Mevalonate is converted to 5 pyrophosphomevalonate in two steps, each of which transfers [1] Mevalonate is converted to 5 pyrophosphomevalonate in two steps, each of which transfers](https://slidetodoc.com/presentation_image_h/8327bcd897d1c7ce0e022b8a688ee0db/image-13.jpg)
![[4] IPP and DPP condense to form ten-carbon geranyl pyro phosphate (GPP). [5] A [4] IPP and DPP condense to form ten-carbon geranyl pyro phosphate (GPP). [5] A](https://slidetodoc.com/presentation_image_h/8327bcd897d1c7ce0e022b8a688ee0db/image-14.jpg)
![[7] Squalene is converted to the sterol lanosterol by a sequence of reactions catalyzed [7] Squalene is converted to the sterol lanosterol by a sequence of reactions catalyzed](https://slidetodoc.com/presentation_image_h/8327bcd897d1c7ce0e022b8a688ee0db/image-15.jpg)
- Slides: 21

CHOLESTEROL SYNTHESIS

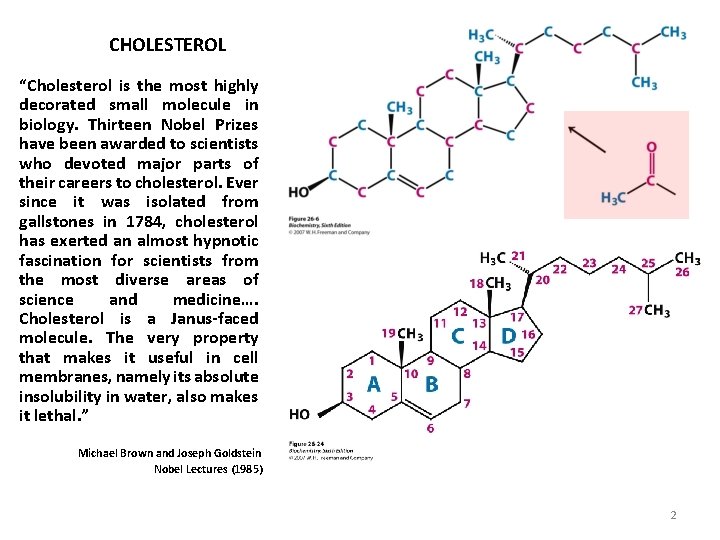
CHOLESTEROL “Cholesterol is the most highly decorated small molecule in biology. Thirteen Nobel Prizes have been awarded to scientists who devoted major parts of their careers to cholesterol. Ever since it was isolated from gallstones in 1784, cholesterol has exerted an almost hypnotic fascination for scientists from the most diverse areas of science and medicine…. Cholesterol is a Janus-faced molecule. The very property that makes it useful in cell membranes, namely its absolute insolubility in water, also makes it lethal. ” Michael Brown and Joseph Goldstein Nobel Lectures (1985) 2

CHOLESTEROL 3

CHOLESTEROL 4

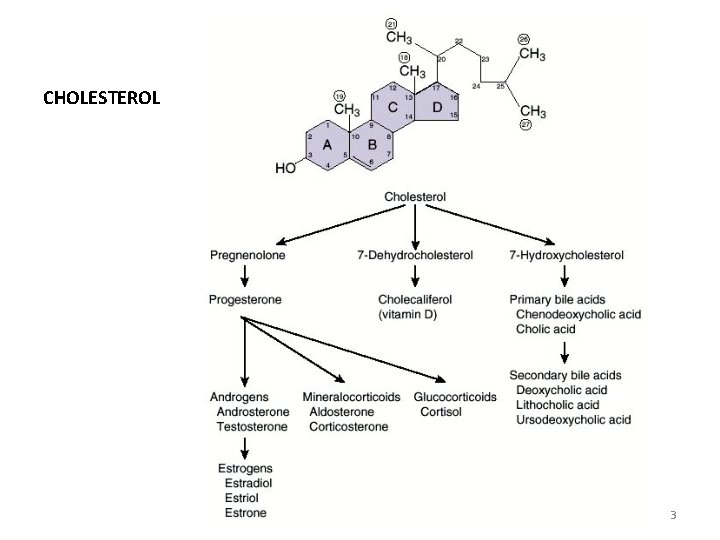
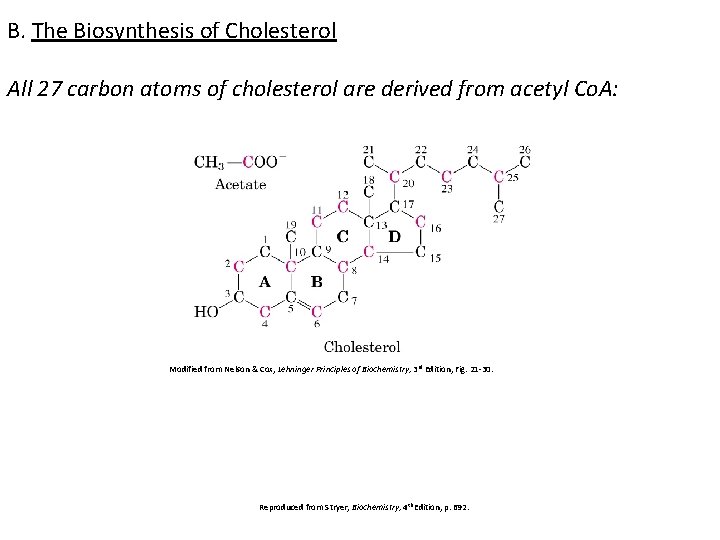
Cholesterol is synthesized by virtually all tissues in humans, although liver, intestine, adrenal cortex, and reproductive tissues, including ovaries, testes, and placenta, make the largest contributions to the body’s cholesterol pool. As with fatty acids, ALL THE CARBON ATOMS IN CHOLESTEROL ARE PROVIDED BY ACETATE, and NADPH provides the reducing equivalents. Biosynthesis of membrane lipids and steroids 1 5

Synthesis requires enzymes in both the cytosol and the membrane of the smooth endoplasmic reticulum (ER). The pathway is responsive to changes in cholesterol concentration, and regulatory mechanisms exist to balance the rate of cholesterol synthesis within the body against the rate of cholesterol excretion. An imbalance in this regulation can lead to an elevation in circulating levels of plasma cholesterol, with the potential for vascular disease. Biosynthesis of membrane lipids and steroids 1 6

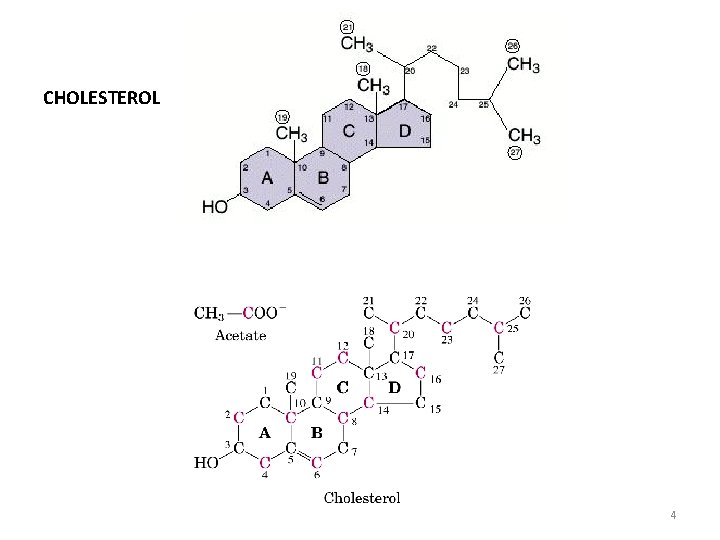
B. The Biosynthesis of Cholesterol All 27 carbon atoms of cholesterol are derived from acetyl Co. A: Modified from Nelson & Cox, Lehninger Principles of Biochemistry, 3 rd Edition, Fig. 21 -30. Reproduced from Stryer, Biochemistry, 4 th Edition, p. 692.

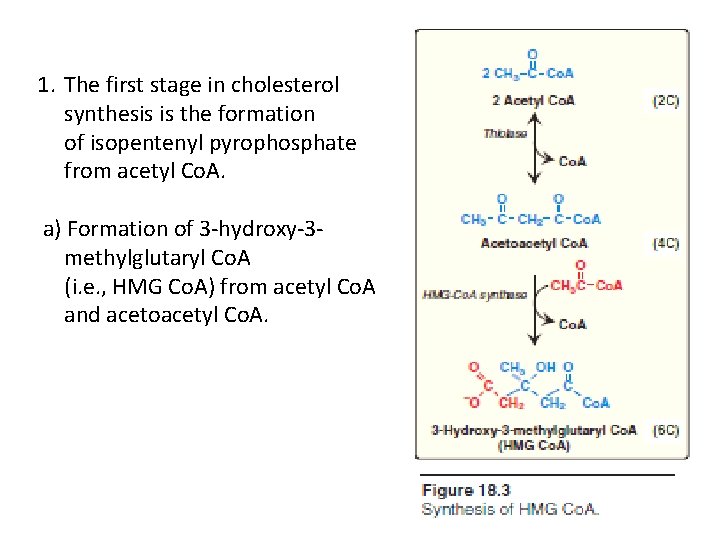
The first two reactions in the cholesterol synthetic pathway are similar to those in the pathway that produces ketone bodies (see Figure 16. 22, p. 196). They result in the production of HMG Co. A (Figure 18. 3). First, two acetyl Co. A molecules condense to form acetoacetyl Co. A. Next, a third molecule of acetyl Co. A is added, producing HMG Co. A, a six-carbon compound. [Note: Liver parenchymal cells contain two isoenzymes of HMG Co. A synthase. The cytosolic enzyme participates in cholesterol synthesis, whereas the mitochondrial enzyme functions in the pathway for ketone body synthesis. ] Biosynthesis of membrane lipids and steroids 1 8

• SYNTHESIS OF CHOLESTEROL Acetyl-Co. A à 3 -hydroxy-3 -methyl-glutaryl-Co. A (HMG-Co. A) HMG-Co. A àmevalonate Mevalonate àisopentenyl-PP 6 isopentenyl-pyrophosphate àsqualene Cyclisation of squalene àcholesterol Biosynthesis of membrane lipids and steroids 1 9

1. The first stage in cholesterol synthesis is the formation of isopentenyl pyrophosphate from acetyl Co. A. a) Formation of 3 -hydroxy-3 methylglutaryl Co. A (i. e. , HMG Co. A) from acetyl Co. A and acetoacetyl Co. A.

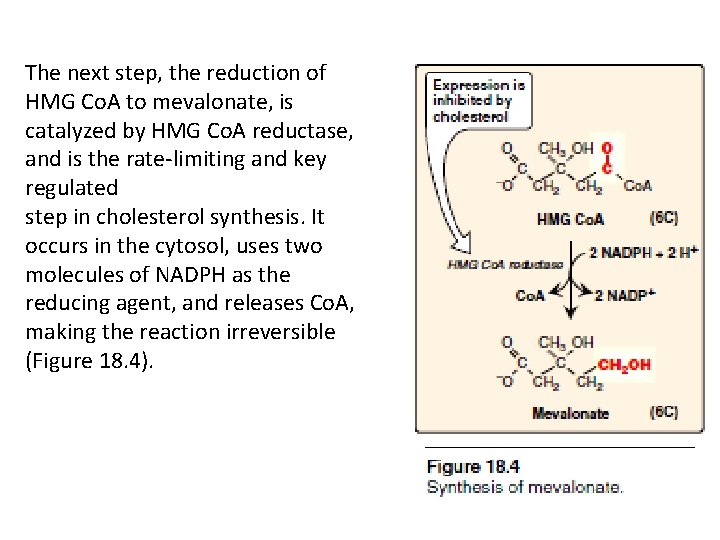
The next step, the reduction of HMG Co. A to mevalonate, is catalyzed by HMG Co. A reductase, and is the rate-limiting and key regulated step in cholesterol synthesis. It occurs in the cytosol, uses two molecules of NADPH as the reducing agent, and releases Co. A, making the reaction irreversible (Figure 18. 4).

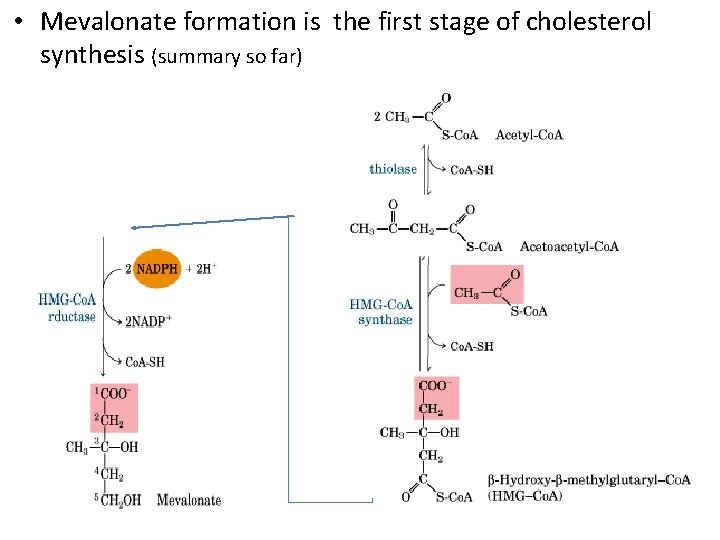
• Mevalonate formation is the first stage of cholesterol synthesis (summary so far)
![1 Mevalonate is converted to 5 pyrophosphomevalonate in two steps each of which transfers [1] Mevalonate is converted to 5 pyrophosphomevalonate in two steps, each of which transfers](https://slidetodoc.com/presentation_image_h/8327bcd897d1c7ce0e022b8a688ee0db/image-13.jpg)
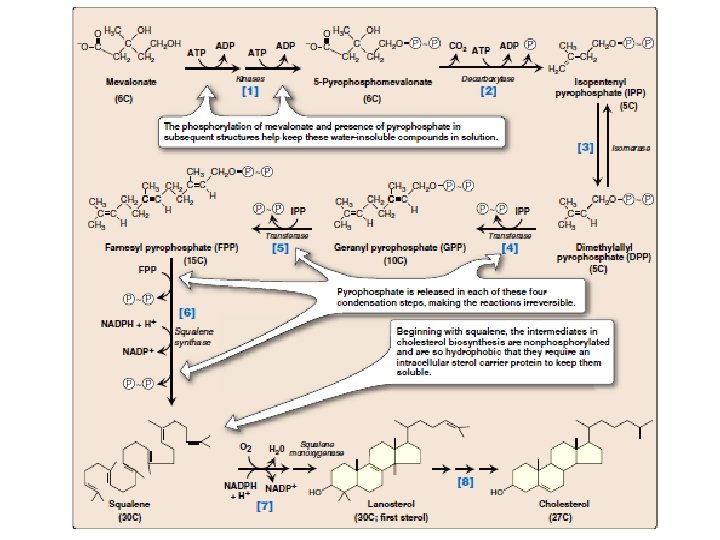
[1] Mevalonate is converted to 5 pyrophosphomevalonate in two steps, each of which transfers a phosphate group from ATP. [2] A five-carbon isoprene unit—isopentenyl pyrophosphate (IPP)— is formed by the decarboxylation of 5 -pyrophosphomevalonate. The reaction requires ATP. [3] IPP is isomerized to 3, 3 -dimethylallyl pyrophosphate (DPP).
![4 IPP and DPP condense to form tencarbon geranyl pyro phosphate GPP 5 A [4] IPP and DPP condense to form ten-carbon geranyl pyro phosphate (GPP). [5] A](https://slidetodoc.com/presentation_image_h/8327bcd897d1c7ce0e022b8a688ee0db/image-14.jpg)
[4] IPP and DPP condense to form ten-carbon geranyl pyro phosphate (GPP). [5] A second molecule of IPP then condenses with GPP to form 15 carbon farnesyl pyrophosphate (FPP). [Note: Covalent attachment of farnesyl to proteins, a process known as “prenylation, ” is one mechanism for anchoring proteins to plasma membranes. ] [6] Two molecules of FPP combine, releasing pyrophosphate, and are reduced, forming the 30 -carbon compound squalene. [Note: Squalene is formed from six isoprenoid units. Because three ATP are hydrolyzed per mevalonate residue converted to IPP, a total of 18 ATP are required to make the polyisoprenoid squalene. ]
![7 Squalene is converted to the sterol lanosterol by a sequence of reactions catalyzed [7] Squalene is converted to the sterol lanosterol by a sequence of reactions catalyzed](https://slidetodoc.com/presentation_image_h/8327bcd897d1c7ce0e022b8a688ee0db/image-15.jpg)
[7] Squalene is converted to the sterol lanosterol by a sequence of reactions catalyzed by ER-associated enzymes that use molecular oxygen and NADPH. The hydroxylation of squalene triggers the cyclization of the structure to lanosterol. [8] The conversion of lanosterol to cholesterol is a multistep process, resulting in the shortening of the carbon chain from 30 to 27 carbons, removal of the two methyl groups at carbon 4, migration of the double bond from carbon 8 to carbon 5, and reduction of the double bond between carbon 24 and carbon 25. [Note: This ER-associated pathway includes several different enzymatic reactions. Smith-Lemli-Opitz syndrome (SLOS), a relatively common autosomal recessive disorder of cholesterol biosynthesis, is caused by a partial deficiency in 7 -dehy dro cholesterol-7 -reductase— an enzyme involved in the migration of the double bond. SLOS is one of several multisystem, embryonic malformation syndromes associated with impaired cholesterol synthesis. ]


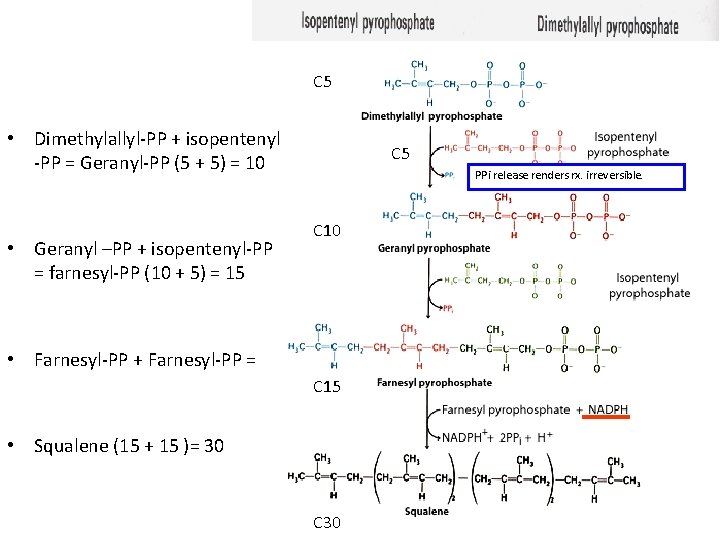
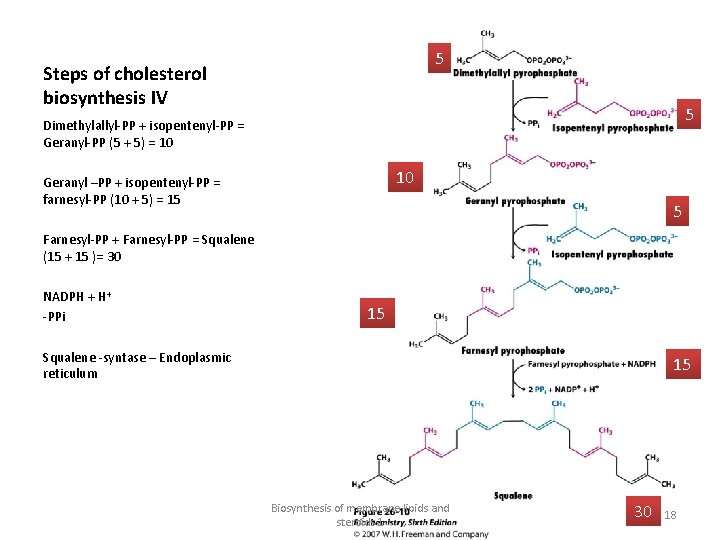
C 5 • Dimethylallyl-PP + isopentenyl -PP = Geranyl-PP (5 + 5) = 10 • Geranyl –PP + isopentenyl-PP = farnesyl-PP (10 + 5) = 15 C 5 PPi release renders rx. irreversible. C 10 • Farnesyl-PP + Farnesyl-PP = C 15 • Squalene (15 + 15 )= 30 C 30

5 Steps of cholesterol biosynthesis IV 5 Dimethylallyl-PP + isopentenyl-PP = Geranyl-PP (5 + 5) = 10 10 Geranyl –PP + isopentenyl-PP = farnesyl-PP (10 + 5) = 15 5 Farnesyl-PP + Farnesyl-PP = Squalene (15 + 15 )= 30 NADPH + H+ -PPi 15 Squalene -syntase – Endoplasmic reticulum 15 Biosynthesis of membrane lipids and steroids 1 30 18

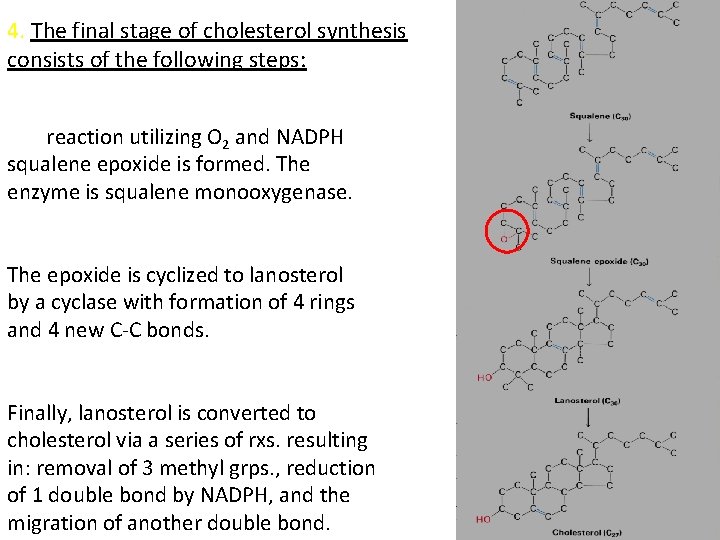
4. The final stage of cholesterol synthesis consists of the following steps: In a reaction utilizing O 2 and NADPH squalene epoxide is formed. The enzyme is squalene monooxygenase. The epoxide is cyclized to lanosterol by a cyclase with formation of 4 rings and 4 new C-C bonds. Finally, lanosterol is converted to cholesterol via a series of rxs. resulting in: removal of 3 methyl grps. , reduction of 1 double bond by NADPH, and the migration of another double bond.

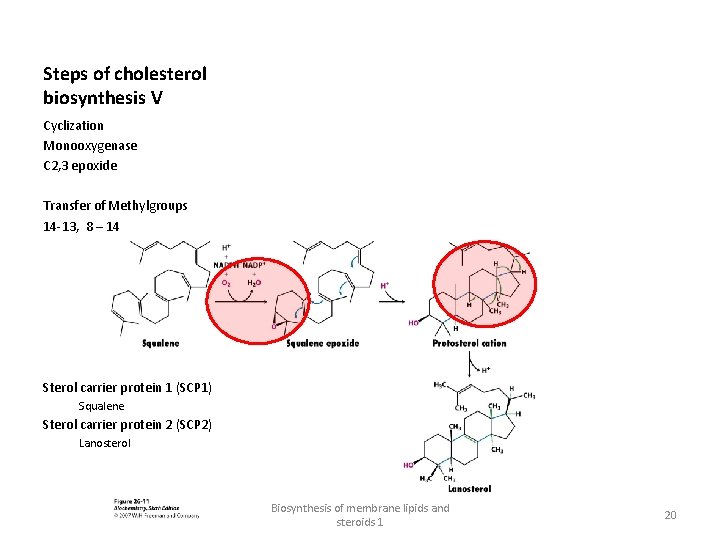
Steps of cholesterol biosynthesis V Cyclization Monooxygenase C 2, 3 epoxide Transfer of Methylgroups 14 -13, 8 – 14 Sterol carrier protein 1 (SCP 1) Squalene Sterol carrier protein 2 (SCP 2) Lanosterol Biosynthesis of membrane lipids and steroids 1 20

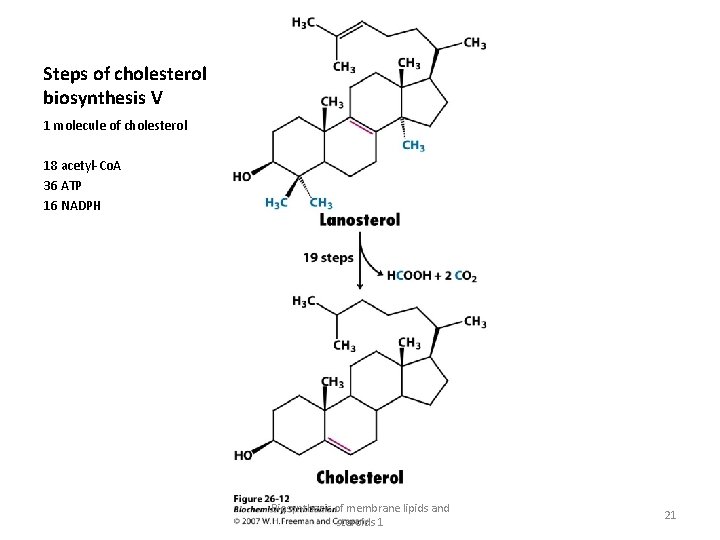
Steps of cholesterol biosynthesis V 1 molecule of cholesterol 18 acetyl-Co. A 36 ATP 16 NADPH Biosynthesis of membrane lipids and steroids 1 21