The Chemical Level of Organization What is matter







- Slides: 28

The Chemical Level of Organization

What is matter? § § It is anything that takes up space and has mass (weight). Types a) Solids – definite shape and volume b) Liquids – definite volume c) Gases – no definite shape and volume § Changes a) Physical changes – do not change the basic nature of a substance (ice melting) b) Chemical changes – change the composition of a substance (food digestion)

What is energy? § It is the ability to do work or to put matter into motion. § Types 1) Kinetic energy – actually doing work (moving objects) 2) Potential energy – inactive or stored energy (batteries)

What are the energy forms? § Chemical energy – stored in chemical substances § Electrical energy – movement of charged particles § Mechanical energy – moving matter § Radiant energy – travels in waves

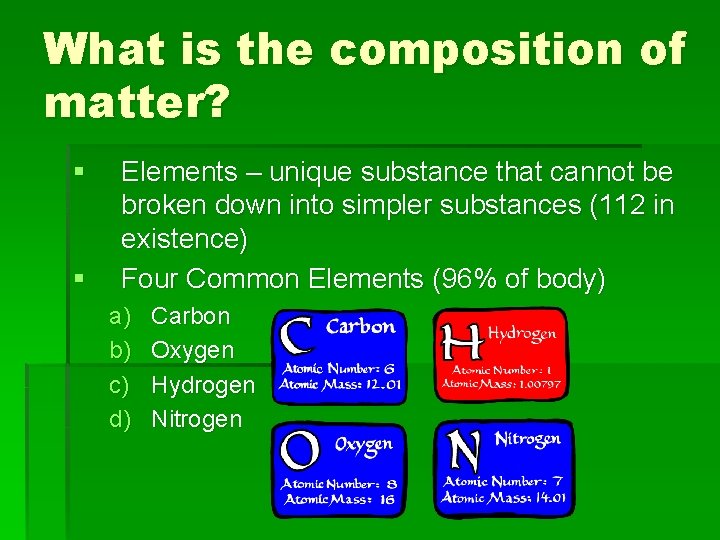
What is the composition of matter? § § Elements – unique substance that cannot be broken down into simpler substances (112 in existence) Four Common Elements (96% of body) a) b) c) d) Carbon Oxygen Hydrogen Nitrogen

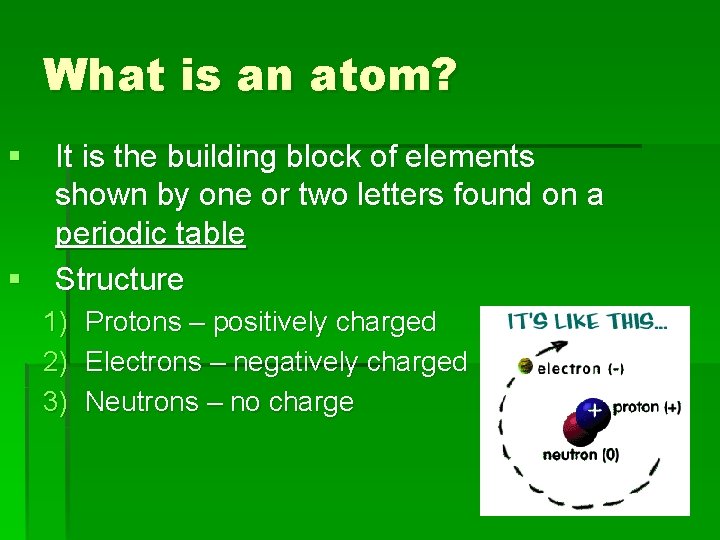
What is an atom? § It is the building block of elements shown by one or two letters found on a periodic table § Structure 1) 2) 3) Protons – positively charged Electrons – negatively charged Neutrons – no charge

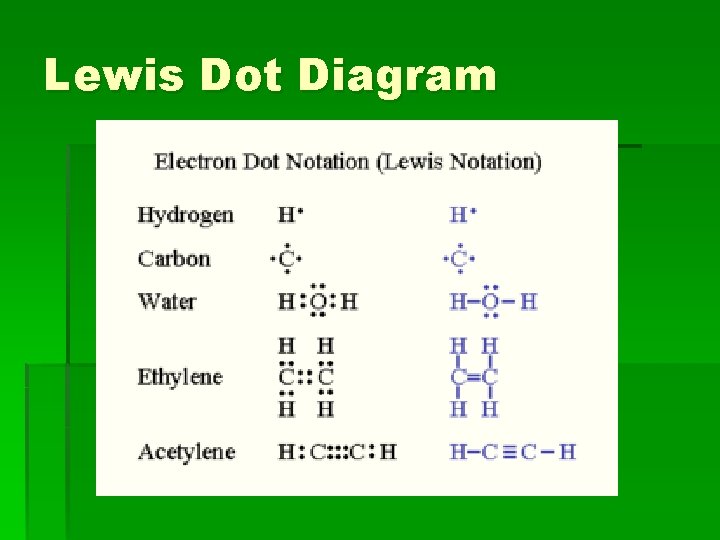
Lewis Dot Diagram

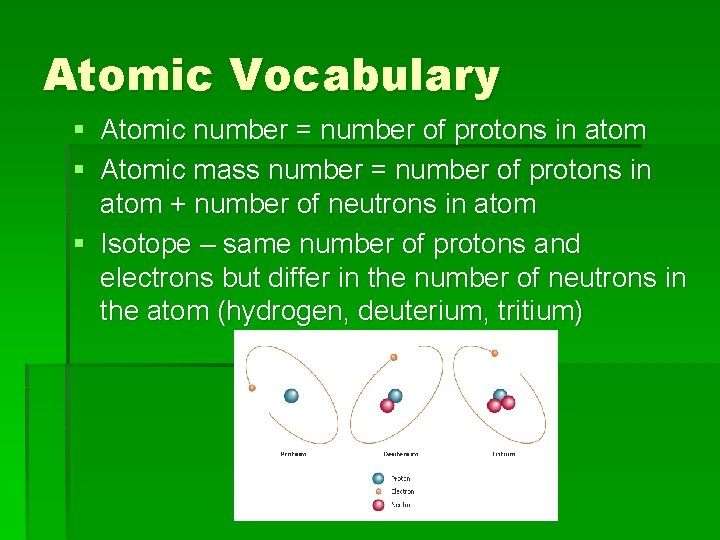
Atomic Vocabulary § Atomic number = number of protons in atom § Atomic mass number = number of protons in atom + number of neutrons in atom § Isotope – same number of protons and electrons but differ in the number of neutrons in the atom (hydrogen, deuterium, tritium)

Atomic Vocabulary § Molecules – two or more atoms that combine chemically (H 2 O) § Compound – two or more different atoms bind together to form a molecule (CH 4) § Chemical reactions – when atoms unite chemically forming chemical bonds

What are chemical bonds? § § It is an energy relationship that involves interactions between the electrons of the reacting atoms. The valence shell or outer energy level is involved in this bonding. 1. 2. 3. First energy shell – holds 2 electrons Second energy shell – holds 8 electrons Third energy shell – holds 8 electrons

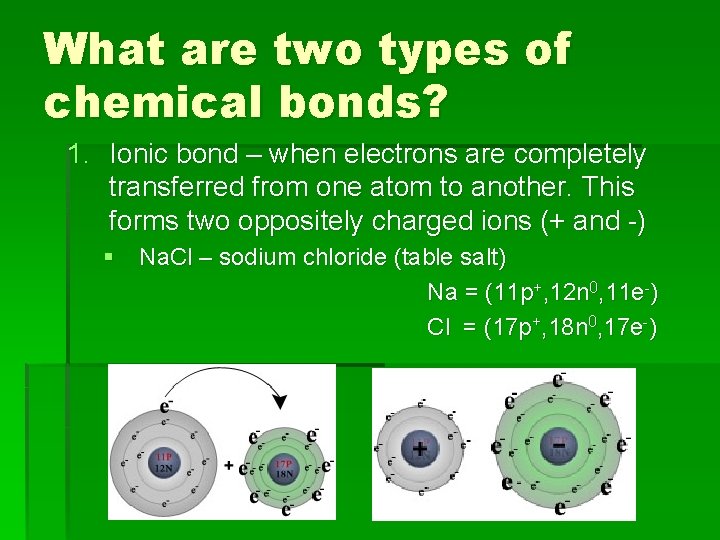
What are two types of chemical bonds? 1. Ionic bond – when electrons are completely transferred from one atom to another. This forms two oppositely charged ions (+ and -) § Na. Cl – sodium chloride (table salt) Na = (11 p+, 12 n 0, 11 e-) Cl = (17 p+, 18 n 0, 17 e-)

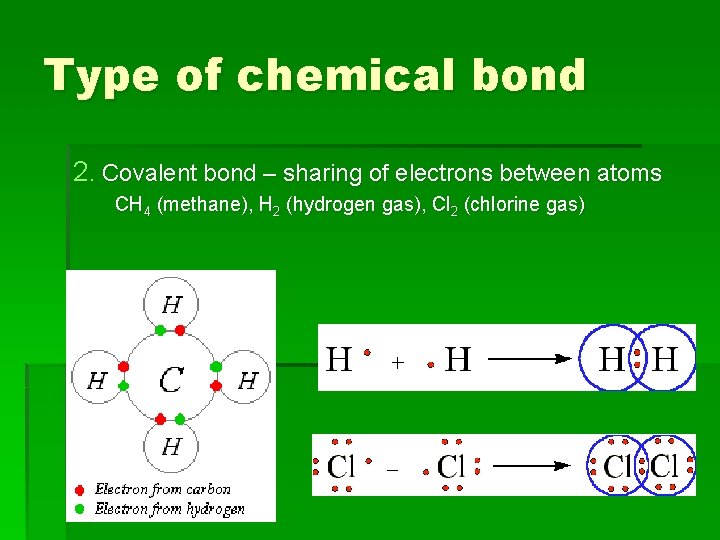
Type of chemical bond 2. Covalent bond – sharing of electrons between atoms CH 4 (methane), H 2 (hydrogen gas), Cl 2 (chlorine gas)

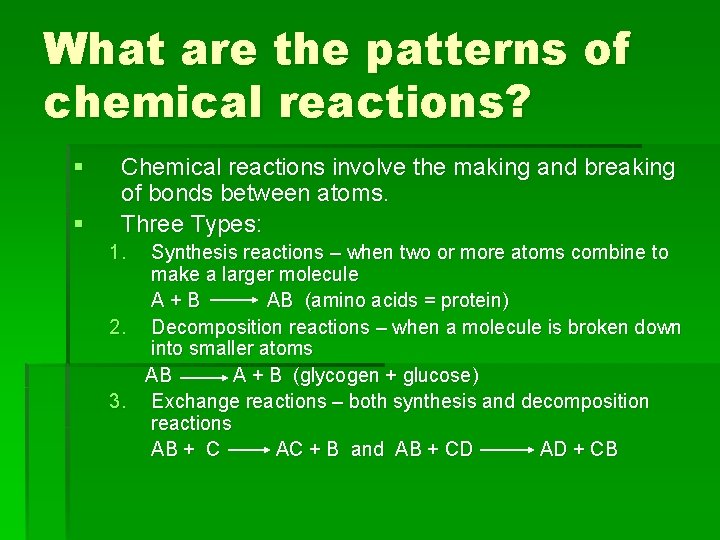
What are the patterns of chemical reactions? § § Chemical reactions involve the making and breaking of bonds between atoms. Three Types: 1. Synthesis reactions – when two or more atoms combine to make a larger molecule A+B AB (amino acids = protein) 2. Decomposition reactions – when a molecule is broken down into smaller atoms AB A + B (glycogen + glucose) 3. Exchange reactions – both synthesis and decomposition reactions AB + C AC + B and AB + CD AD + CB

What are inorganic compounds? § § Compounds that lack carbon Three Types: 1. Water – most abundant compound in body and universal solvent 2. Salts (electrolytes) – nerve transmission, muscle contraction, blood clotting and metabolism 3. Acids – proton donors (H+) 4. Bases – proton acceptors (OH-)

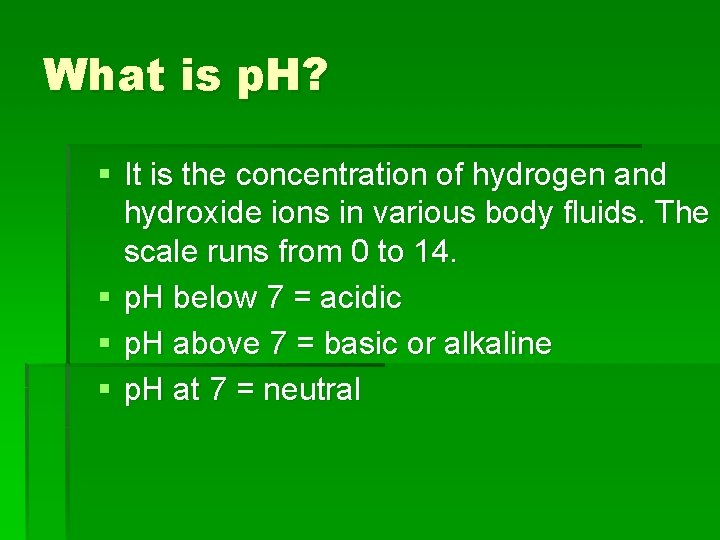
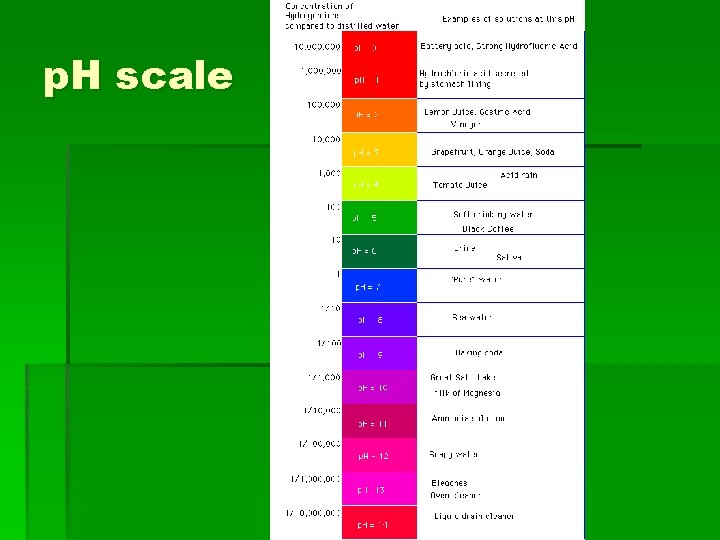
What is p. H? § It is the concentration of hydrogen and hydroxide ions in various body fluids. The scale runs from 0 to 14. § p. H below 7 = acidic § p. H above 7 = basic or alkaline § p. H at 7 = neutral

p. H scale

What are organic compounds? § § § Carbon-containing compounds Contains the four elements – carbon, hydrogen, oxygen, and nitrogen (CHON) Major Types: 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic acids

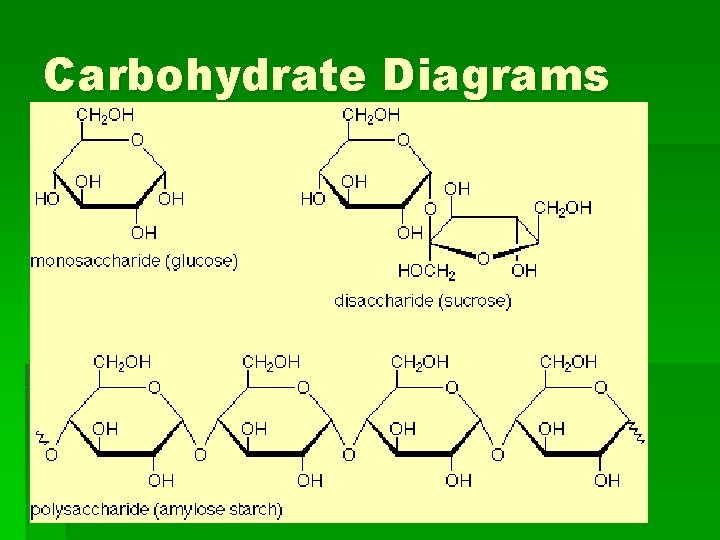
What are carbohydrates? § § § Sugars and starches Contain carbon, hydrogen, and oxygen Classification: a) Monosaccharides – simple or one sugar (glucose) b) Disaccharides – double sugars (sucrose, lactose, maltose) c) Polysaccharides – long branching sugars ( starch and glycogen)

Carbohydrate Diagrams

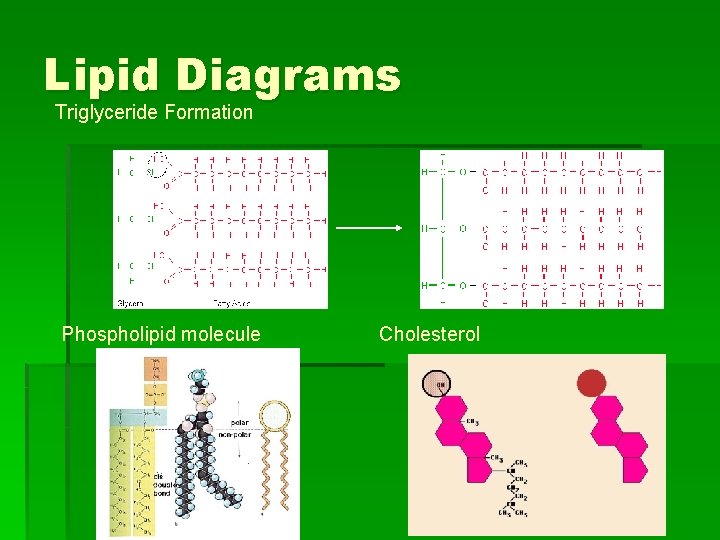
What are lipids? § Neutral fats or triglycerides (glycerol and three fatty acid chains) – most abundant source of energy § Phospholipids – found in cell membrane § Steroids (cholesterol) – found in cell membrane

Lipid Diagrams Triglyceride Formation Phospholipid molecule Cholesterol

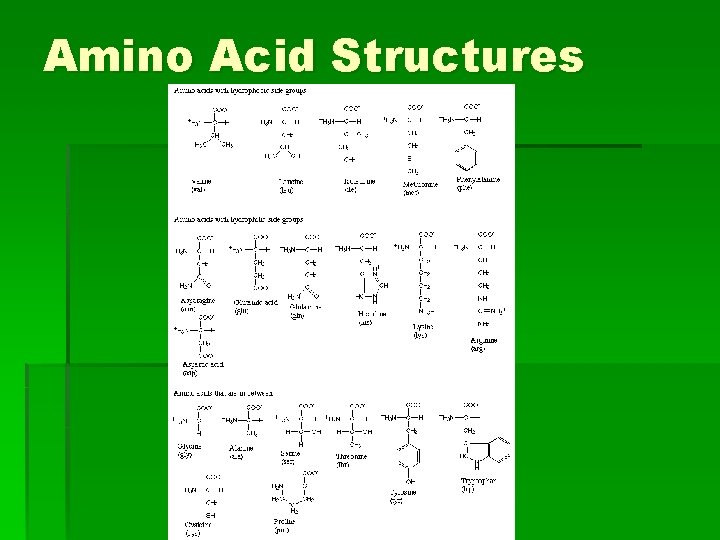
What are proteins? § 50% of organic matter in body § Building blocks are amino acids (20) § Classification: 1. Fibrous proteins or structural proteins (collagen and keratin) 2. Globular proteins or functional proteins (enzymes, hemoglobin, and hormones)

Amino Acid Structures

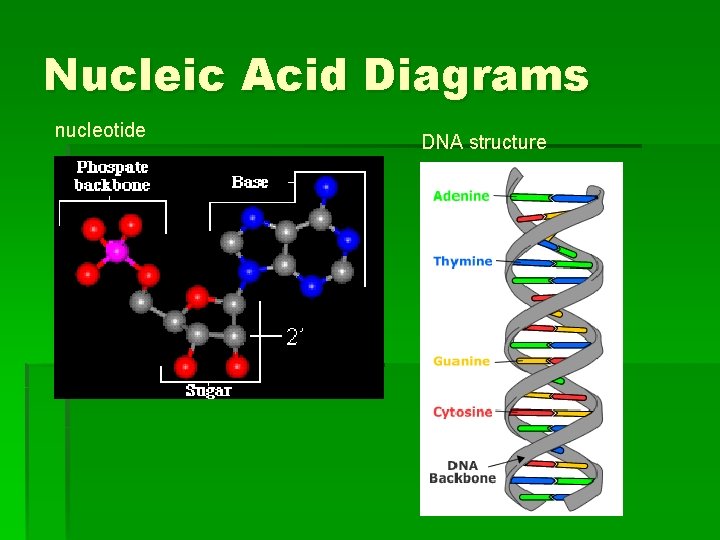
What are nucleic acids § Building block is nucleotide a) b) c) Nitrogenous base Sugar (ribose or deoxyribose) Phosphate group § Deoxyribonucleic acid (DNA) – genetic material of the cell nucleus § Ribonucleic acid (RNA) – directs protein synthesis

Nucleic Acid Diagrams nucleotide DNA structure

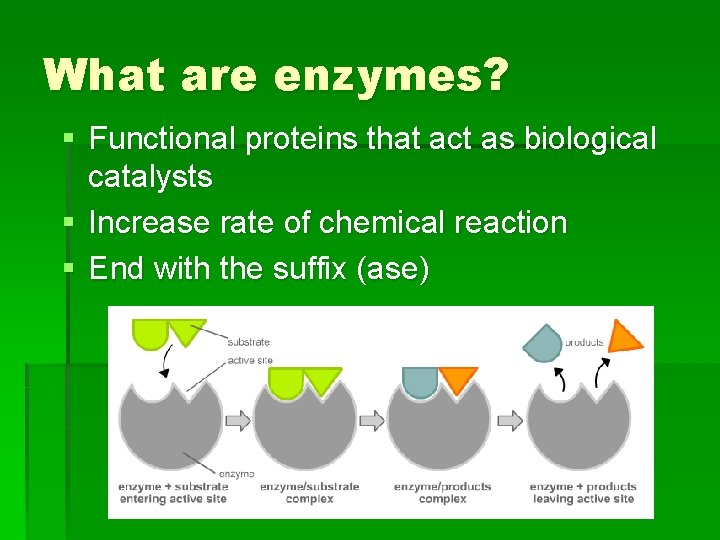
What are enzymes? § Functional proteins that act as biological catalysts § Increase rate of chemical reaction § End with the suffix (ase)

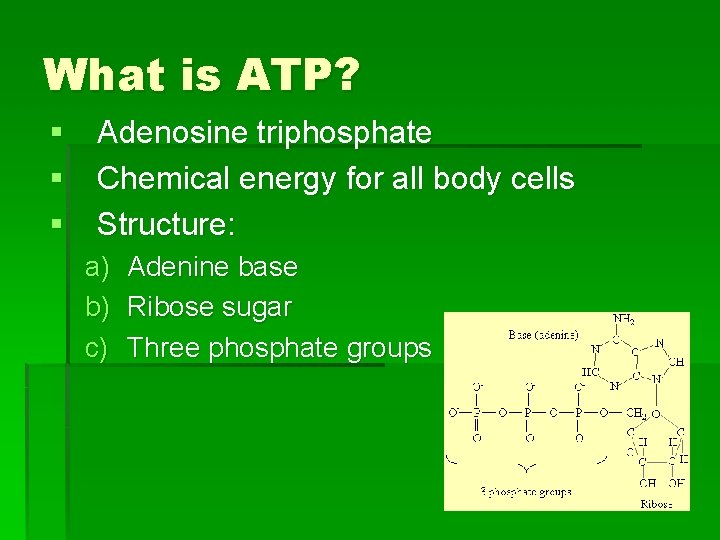
What is ATP? § Adenosine triphosphate § Chemical energy for all body cells § Structure: a) Adenine base b) Ribose sugar c) Three phosphate groups

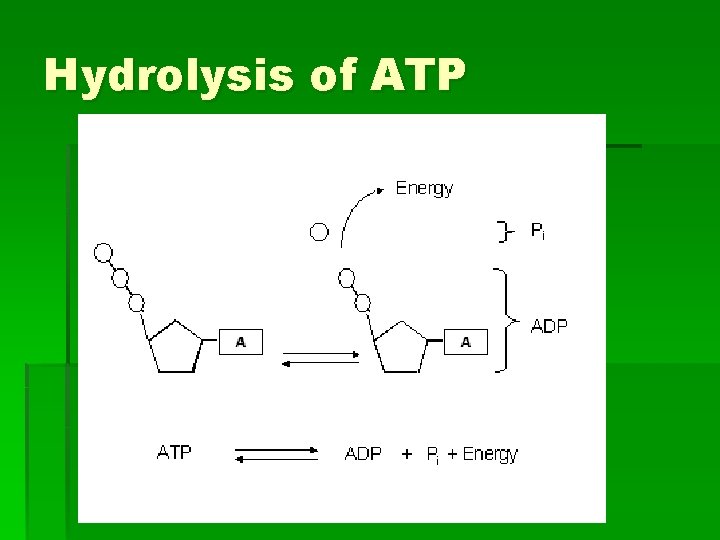
Hydrolysis of ATP
 The chemical level of organization
The chemical level of organization The chemical level of organization chapter 2
The chemical level of organization chapter 2 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Grey matter of nervous system
Grey matter of nervous system Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Primary taste cortex
Primary taste cortex Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter What is gray matter in the brain
What is gray matter in the brain Flow energy review
Flow energy review Physical properties of ice cube
Physical properties of ice cube Example of measurable properties
Example of measurable properties What are chemical properties of matter
What are chemical properties of matter Eating food physical or chemical change
Eating food physical or chemical change Chemical equation with states of matter
Chemical equation with states of matter Process organization in computer organization
Process organization in computer organization Block organization
Block organization Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 7 chemical formulas and chemical compounds
Chapter 7 chemical formulas and chemical compounds Are kc and kp equal
Are kc and kp equal Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào