I Chemical Changes in Matter Chemical Reactions Chemical










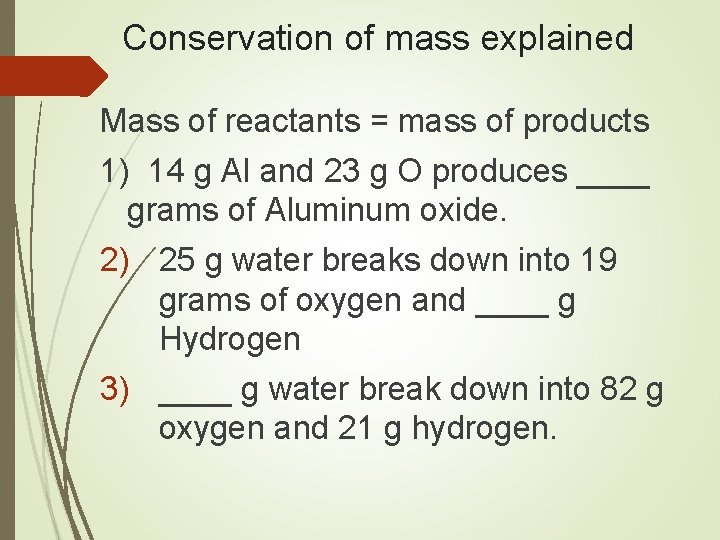
- Slides: 32

I. Chemical Changes in Matter Chemical Reactions Chemical Reaction n Law of Conservation of Mass n Chemical Equations n

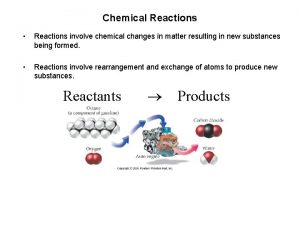
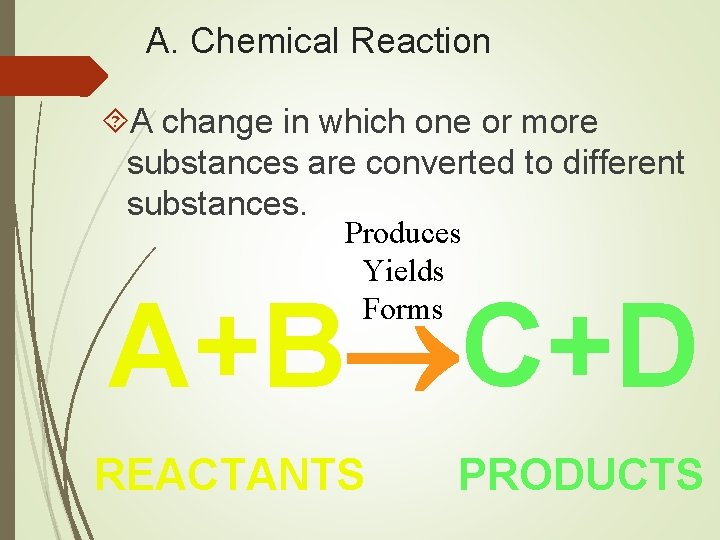
A. Chemical Reaction A change in which one or more substances are converted to different substances. Produces Yields Forms A+B C+D REACTANTS PRODUCTS

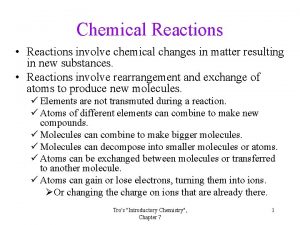
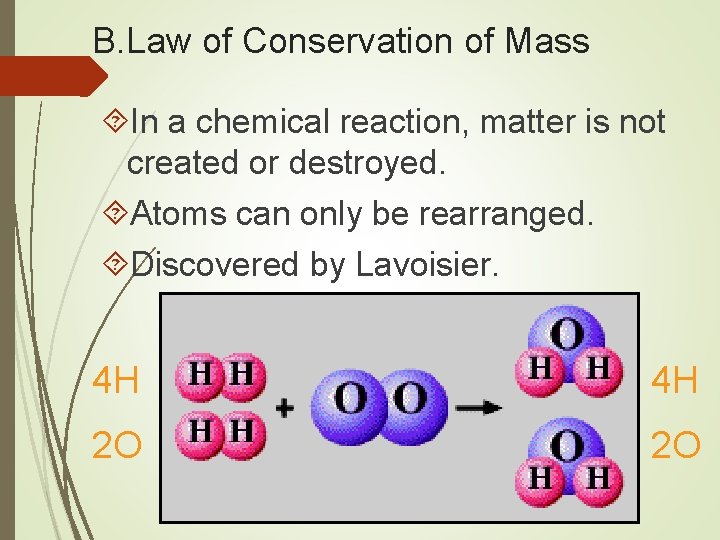
B. Law of Conservation of Mass In a chemical reaction, matter is not created or destroyed. Atoms can only be rearranged. Discovered by Lavoisier. 4 H 4 H 2 O 2 O

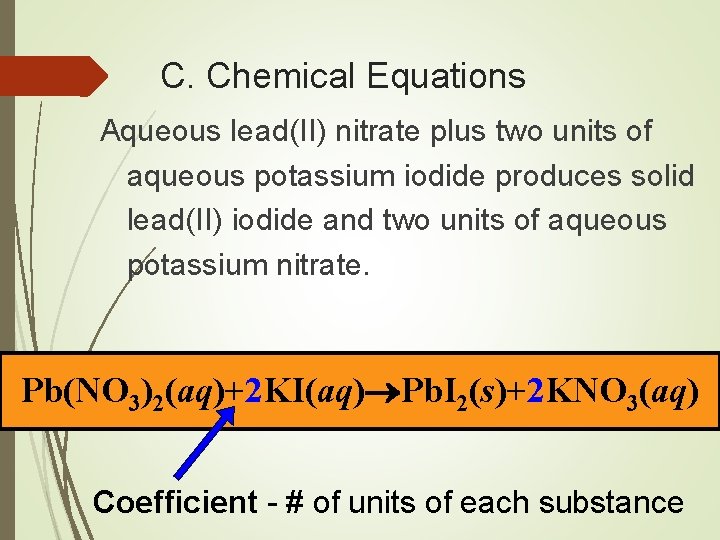
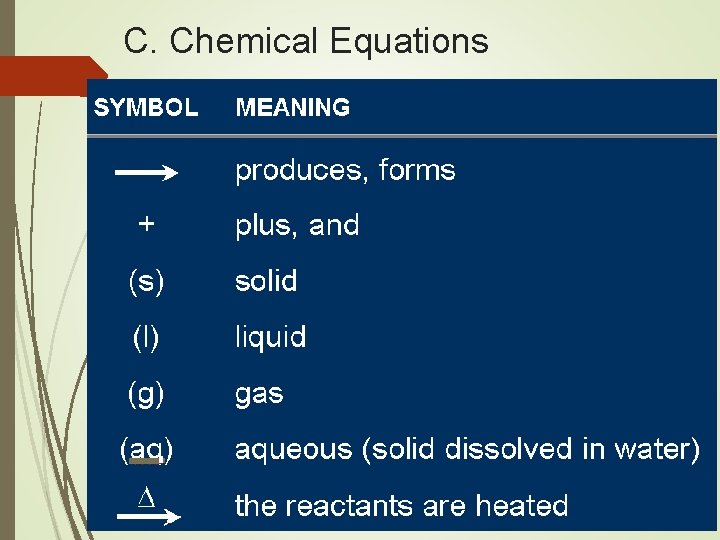
C. Chemical Equations Aqueous lead(II) nitrate plus two units of aqueous potassium iodide produces solid lead(II) iodide and two units of aqueous potassium nitrate. Pb(NO 3)2(aq)+2 KI(aq) Pb. I 2(s)+2 KNO 3(aq) Coefficient - # of units of each substance

C. Chemical Equations Describing Coefficients: individual atom = “atom” 2 Mg 2 atoms of magnesium covalent substance = “molecule” 3 CO 2 3 molecules of carbon dioxide ionic substance = “unit” 4 Mg. O 4 units of magnesium oxide

C. Chemical Equations

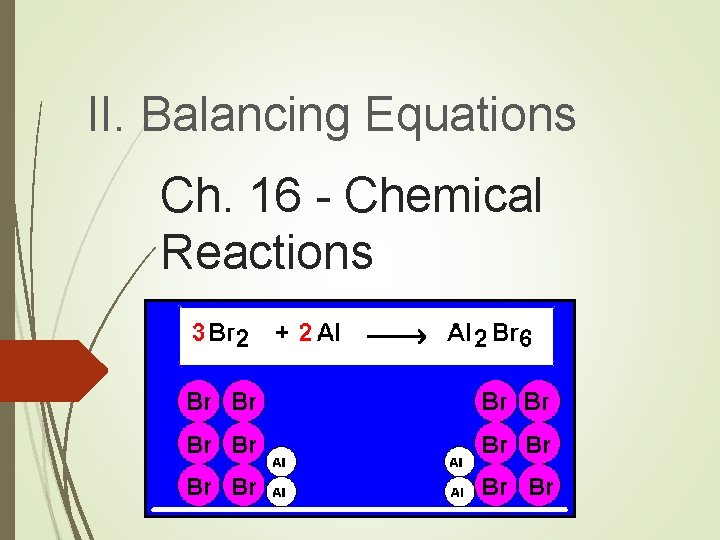
II. Balancing Equations Ch. 16 - Chemical Reactions

A. Steps for Balancing Equations 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make #s equal. Coefficient Subscript = # Atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!

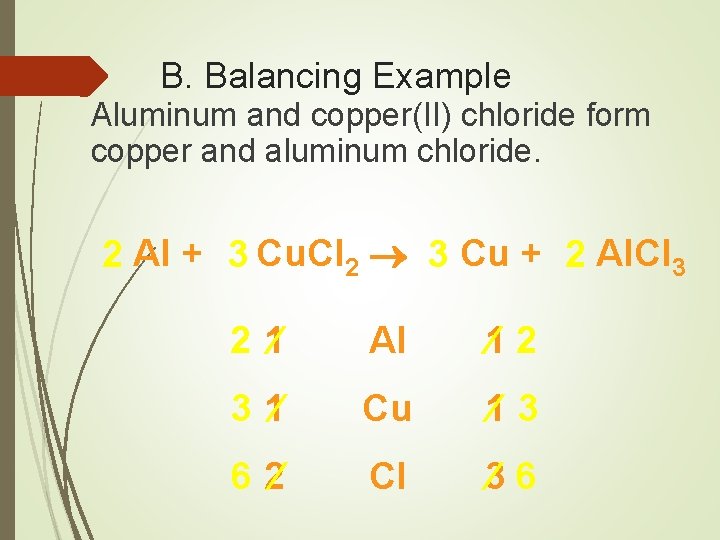
B. Balancing Example Aluminum and copper(II) chloride form copper and aluminum chloride. 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6

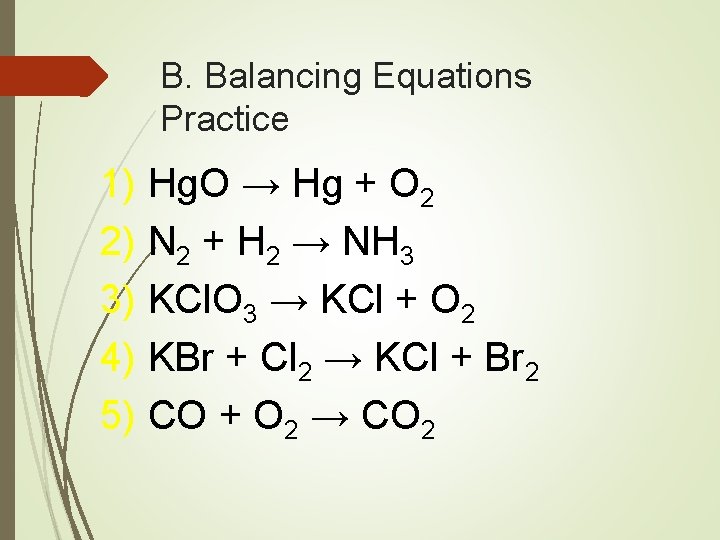
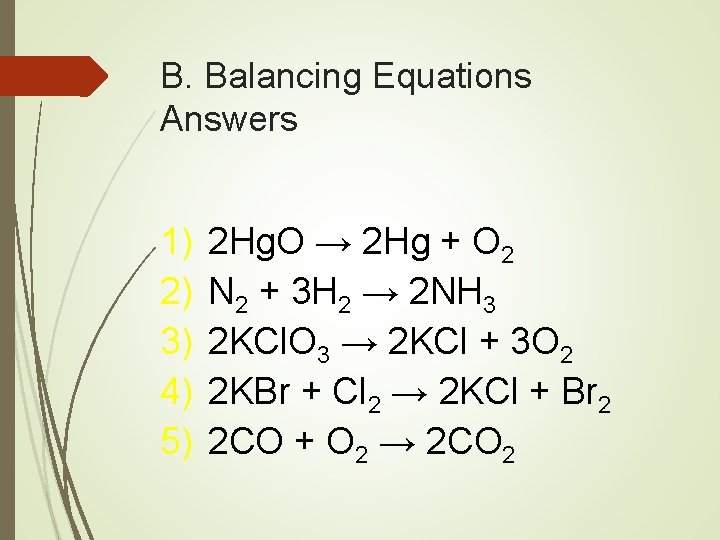
B. Balancing Equations Practice 1) Hg. O → Hg + O 2 2) N 2 + H 2 → NH 3 3) KCl. O 3 → KCl + O 2 4) KBr + Cl 2 → KCl + Br 2 5) CO + O 2 → CO 2

B. Balancing Equations Answers 1) 2) 3) 4) 5) 2 Hg. O → 2 Hg + O 2 N 2 + 3 H 2 → 2 NH 3 2 KCl. O 3 → 2 KCl + 3 O 2 2 KBr + Cl 2 → 2 KCl + Br 2 2 CO + O 2 → 2 CO 2

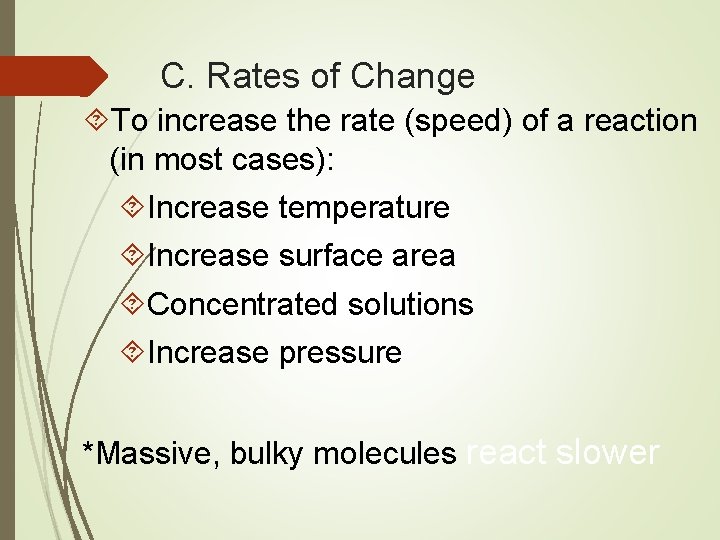
C. Rates of Change To increase the rate (speed) of a reaction (in most cases): Increase temperature Increase surface area Concentrated solutions Increase pressure *Massive, bulky molecules react slower

D. Catalysts A substance that speeds up a chemical reaction without being permanently changed itself. They are not reactants nor products. Enzymes are proteins that are catalysts for chemical reactions in living things.

E. Inhibitors Substances that are used to combine with one of the reactants to prevent certain reactions from occurring. Ex: Food preservatives; lemon juice on cut fruit to keep it from turning brown.

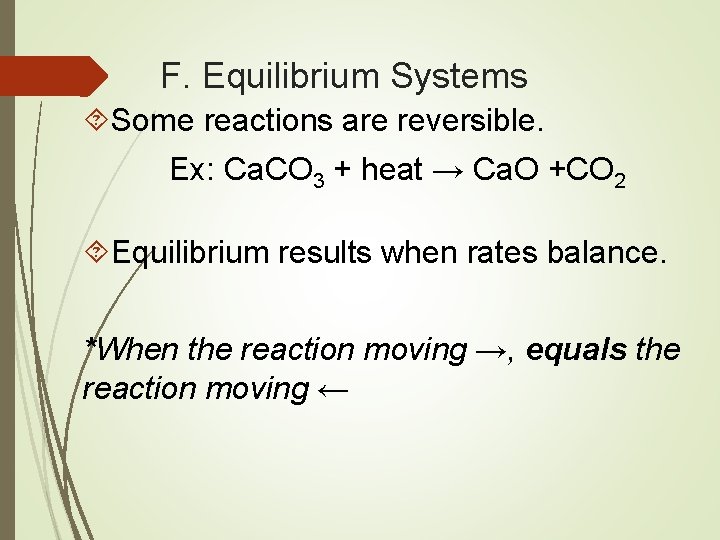
F. Equilibrium Systems Some reactions are reversible. Ex: Ca. CO 3 + heat → Ca. O +CO 2 Equilibrium results when rates balance. *When the reaction moving →, equals the reaction moving ←

III. Types of Reactions Ch. 7 - Chemical Reactions Synthesis n Decomposition n. Single-displacement n. Double-displacement n. Combustion n

Five (5) Main Types of Chemical Reactions: 1) Synthesis 2) Decomposition 3) Single-displacement (replacement) 4) Double-displacement (replacement) 5) Combustion

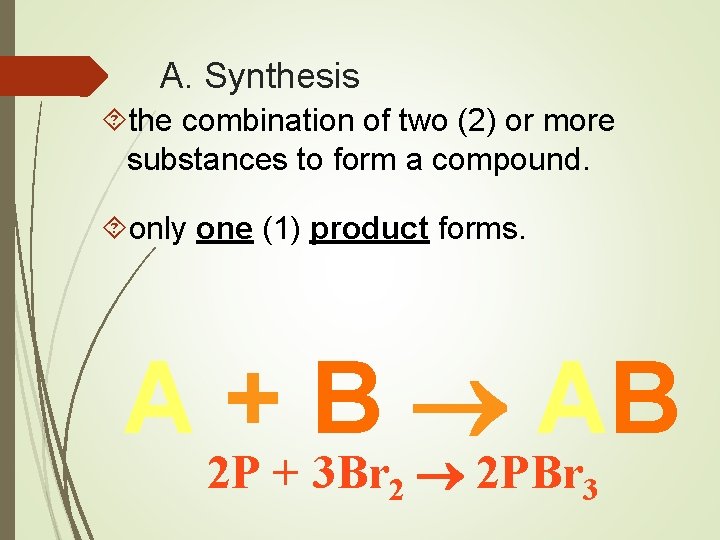
A. Synthesis the combination of two (2) or more substances to form a compound. only one (1) product forms. A + B AB 2 P + 3 Br 2 2 PBr 3

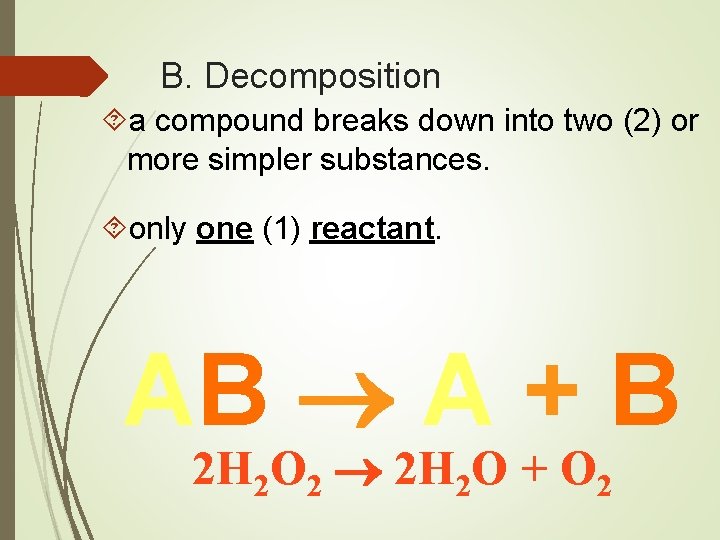
B. Decomposition a compound breaks down into two (2) or more simpler substances. only one (1) reactant. AB A + B 2 H 2 O 2 2 H 2 O + O 2

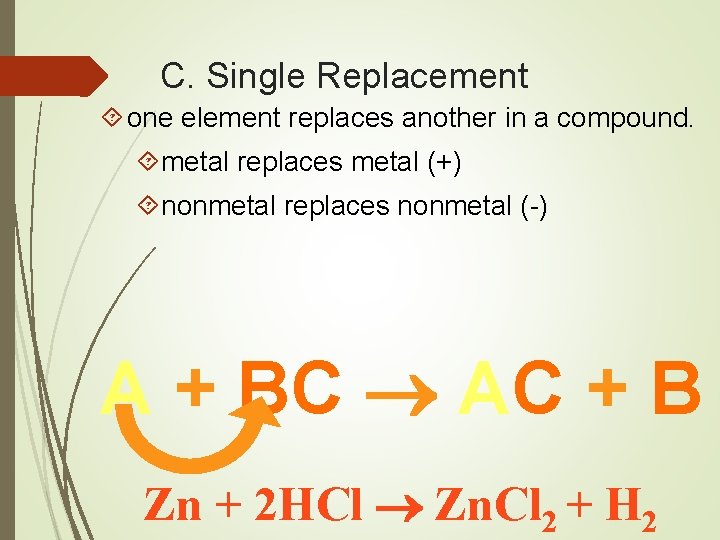
C. Single Replacement one element replaces another in a compound. metal replaces metal (+) nonmetal replaces nonmetal (-) A + BC AC + B Zn + 2 HCl Zn. Cl 2 + H 2

D. Double Replacement ions in two compounds “change partners”. cation(+) of one compound combines with anion(-) of the other AB + CD → AD + CB 2 KOH + Cu. SO 4 K 2 SO 4 + Cu(OH)2

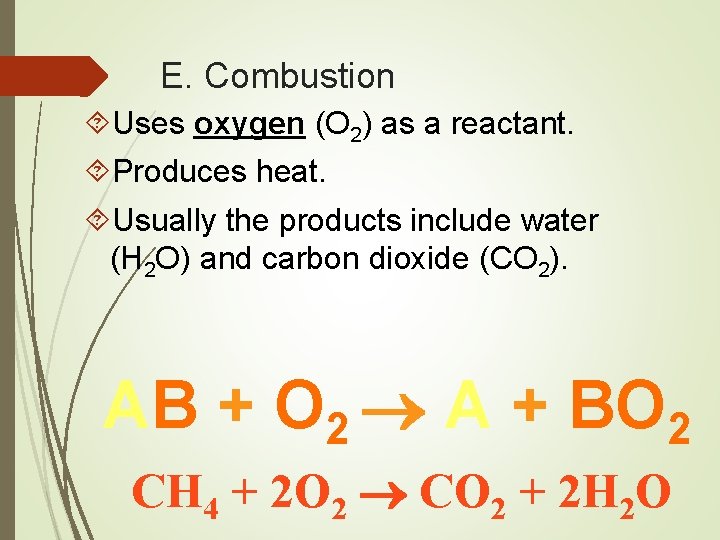
E. Combustion Uses oxygen (O 2) as a reactant. Produces heat. Usually the products include water (H 2 O) and carbon dioxide (CO 2). AB + O 2 A + BO 2 CH 4 + 2 O 2 CO 2 + 2 H 2 O

Types of Chemical Reactions Practice ANSWERS 1. Synthesis 11. Synthesis 2. Decomposition 12. Single replacement 3. Combustion 13. Synthesis 4. Single replacement 14. Double replacement 5. Double replacement 15. Decomposition 6. Double replacement 16. Synthesis 7. Combustion 17. Decomposition 8. Double replacement 18. Single replacement 9. Combustion 19. Single replacement 10. Double replacement 20. Double replacement

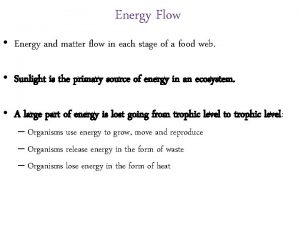
IV. Energy & Chemical Reactions Ch. 7 - Chemical Reactions n. Signs of reactions n. Energy Changes n. Endothermic Reactions n Exothermic Reactions

5 Signs of a Chemical Reaction Production of a gas Production of a precipitant Change in color Change in odor Production of light or heat

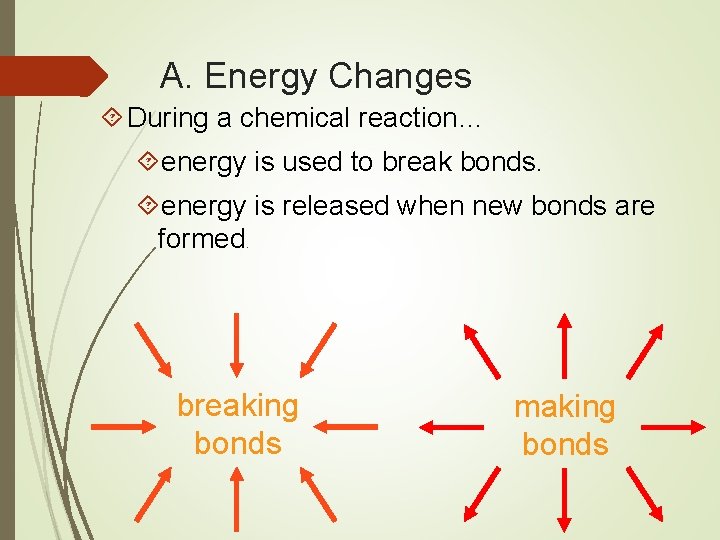
A. Energy Changes During a chemical reaction… energy is used to break bonds. energy is released when new bonds are formed. breaking bonds making bonds

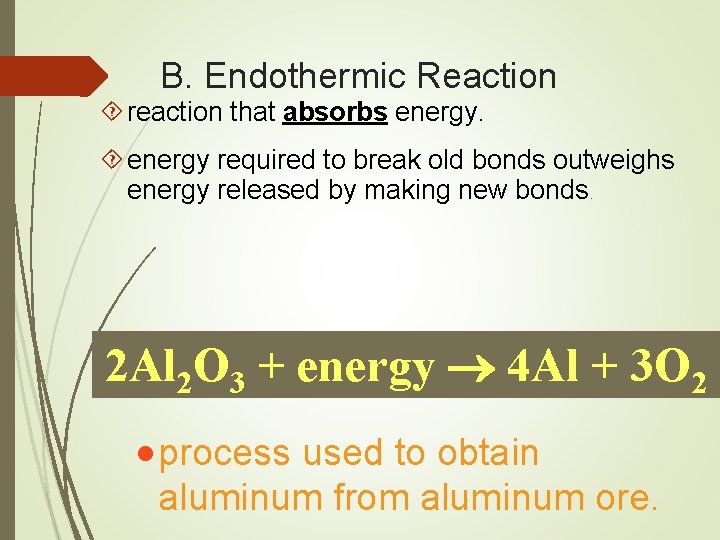
B. Endothermic Reaction reaction that absorbs energy required to break old bonds outweighs energy released by making new bonds. 2 Al 2 O 3 + energy 4 Al + 3 O 2 · process used to obtain aluminum from aluminum ore.

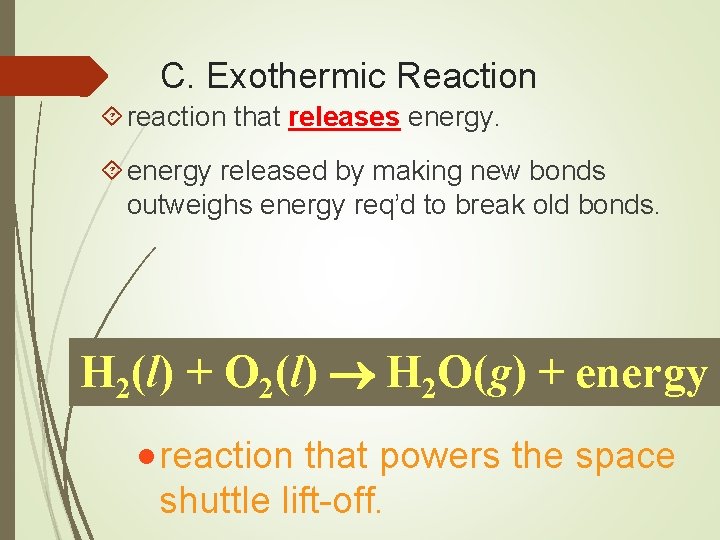
C. Exothermic Reaction reaction that releases energy released by making new bonds outweighs energy req’d to break old bonds. H 2(l) + O 2(l) H 2 O(g) + energy · reaction that powers the space shuttle lift-off.

Identify each as endothermic or exothermic 1. Container gets warm 2. Container gets cold 3. Ice forms 4. Steam is released 5. H 2 + CO 2 -> H 2 O + CO + 394 k. J 6. N 2 O 4+ 57. 2 k. J -> 2 NO 2

V. Law of Conservation of Mass Ch. 7 - Chemical Reactions n application nexamples

Conservation of mass explained In all chemical reactions mass is conserved The mass of reactants MUST equal the mass of products. This fact can be used to determine the amount of a missing reactant or product.
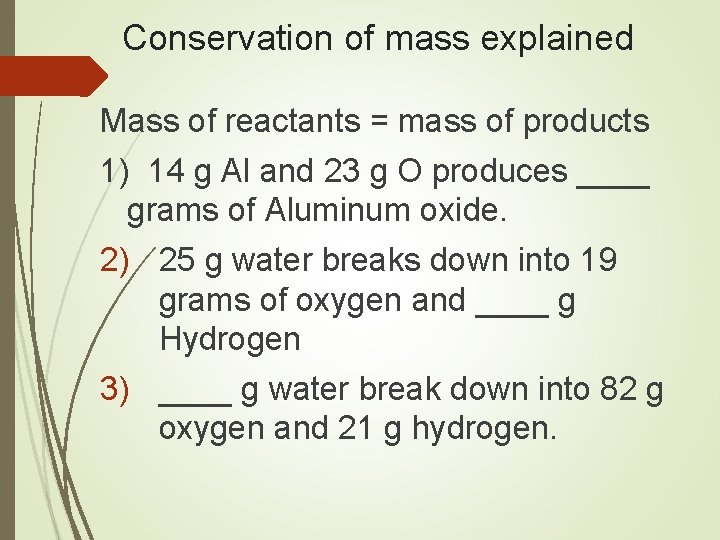
Conservation of mass explained Mass of reactants = mass of products 1) 14 g Al and 23 g O produces ____ grams of Aluminum oxide. 2) 25 g water breaks down into 19 grams of oxygen and ____ g Hydrogen 3) ____ g water break down into 82 g oxygen and 21 g hydrogen.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Types of reactions
Types of reactions Sample of chemical changes
Sample of chemical changes True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. How to write half reactions
How to write half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Elizabeth mulroney
Elizabeth mulroney Chemistry matter and its changes
Chemistry matter and its changes Phase changes of matter
Phase changes of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Changes of state of matter
Changes of state of matter Matter-properties and changes answer key
Matter-properties and changes answer key Melting freezing evaporation condensation sublimation
Melting freezing evaporation condensation sublimation Physical changes of matter
Physical changes of matter Two types of changes
Two types of changes Matter concept map
Matter concept map Phases changes of matter
Phases changes of matter Which is a “big idea” for matter and change?
Which is a “big idea” for matter and change? Cerebral aqueduct
Cerebral aqueduct Flow energy review
Flow energy review Gray matter in the brain
Gray matter in the brain Composition of matter section 1
Composition of matter section 1 Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Frontal and parietal lobes
Frontal and parietal lobes Section 1 composition of matter
Section 1 composition of matter Unit 11 chemical reactions
Unit 11 chemical reactions Classification of chemical reactions worksheet
Classification of chemical reactions worksheet Predicting products of chemical reactions
Predicting products of chemical reactions