Chemical Reactions Follow the matter 1 Chemical Reactions





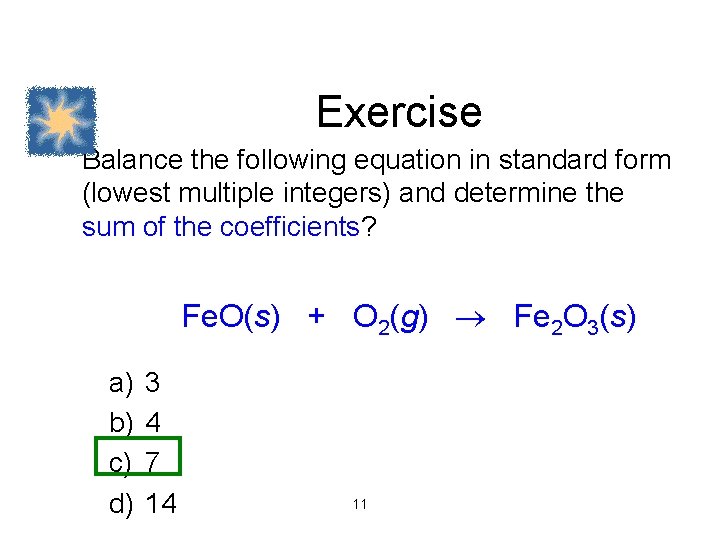

- Slides: 15

Chemical Reactions Follow the matter… 1

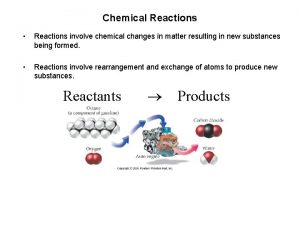
Chemical Reactions • Two chemicals have interacted in some way so that a new substance or substances are formed • Sometimes indicated by a color change, a flash of light, heat given off, etc…. • The point is, the new substances are not the same as the old • But, there is something that doesn’t change…The Law 2

Law of Conservation of Matter • “Matter cannot be created or destroyed, only rearranged into new substances. ” • We credit Antoine de Lavoisier with the best demonstrations of this law. • He performed by reactions, most of them involving blowing things up, but could always account for all the matter before, during and after the reaction. 3

Chemical Equations • We use chemical equations to describe what happens during a chemical reaction • It is similar to a math equation but not exactly the same • Same format is used • Reactants Products • Arrow can be read as “yields” • All atoms on reactants side must also be present on the products side 4

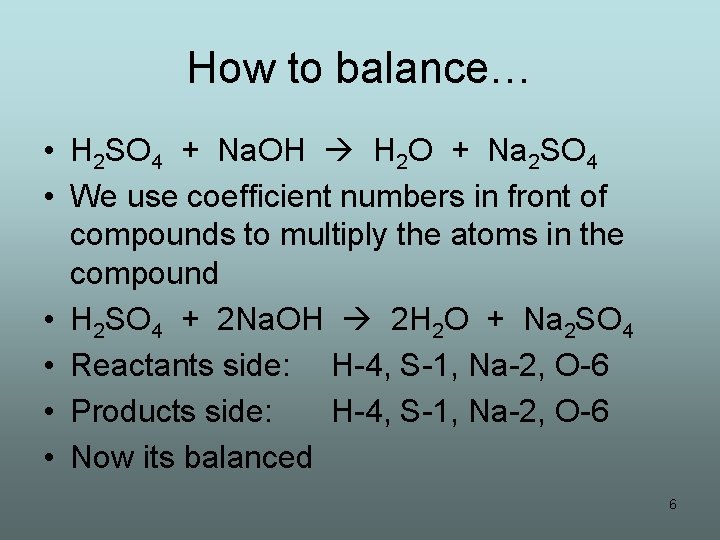
Let’s try one… • H 2 SO 4 + Na. OH H 2 O + Na 2 SO 4 • First we take inventory of all atoms on both sides of the arrow • Reactants side: H-3, S-1, O-5, Na-1 • Products side: H-2, S-1, O-5, Na-2 • Not balanced, we appear to lose a hydrogen and gain a sodium, not allowed • We fix this by balancing the equation 5

How to balance… • H 2 SO 4 + Na. OH H 2 O + Na 2 SO 4 • We use coefficient numbers in front of compounds to multiply the atoms in the compound • H 2 SO 4 + 2 Na. OH 2 H 2 O + Na 2 SO 4 • Reactants side: H-4, S-1, Na-2, O-6 • Products side: H-4, S-1, Na-2, O-6 • Now its balanced 6

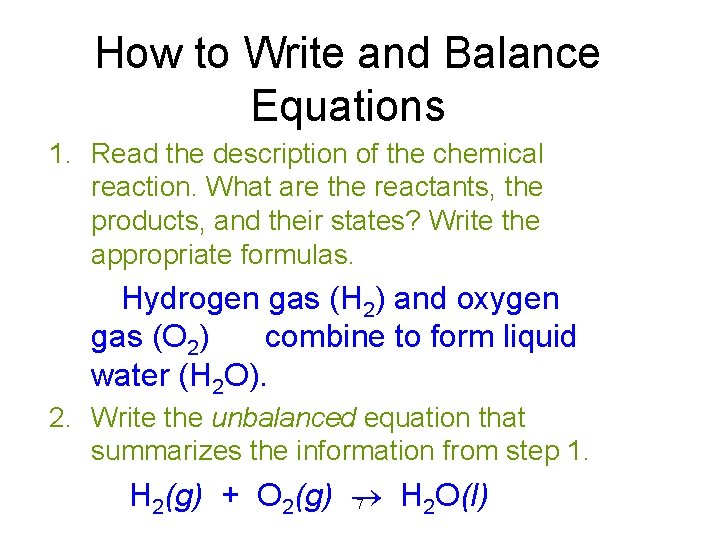
How to Write and Balance Equations 1. Read the description of the chemical reaction. What are the reactants, the products, and their states? Write the appropriate formulas. Hydrogen gas (H 2) and oxygen gas (O 2) combine to form liquid water (H 2 O). 2. Write the unbalanced equation that summarizes the information from step 1. H 2(g) + O 2(g) H 2 O(l) 7

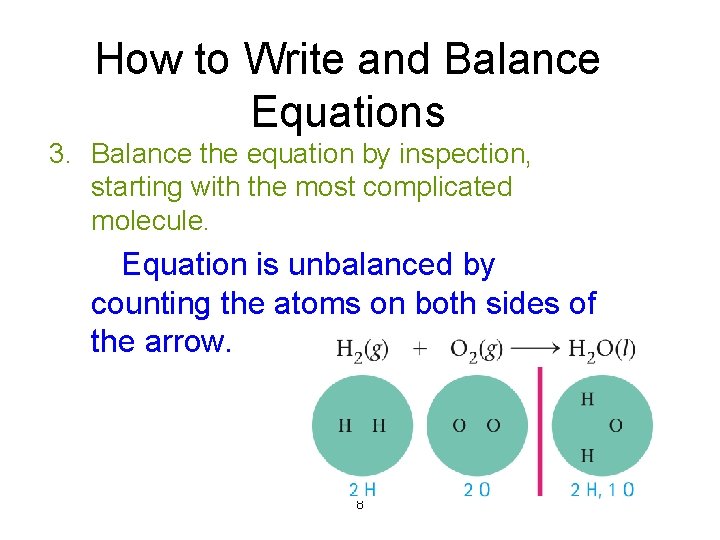
How to Write and Balance Equations 3. Balance the equation by inspection, starting with the most complicated molecule. Equation is unbalanced by counting the atoms on both sides of the arrow. 8

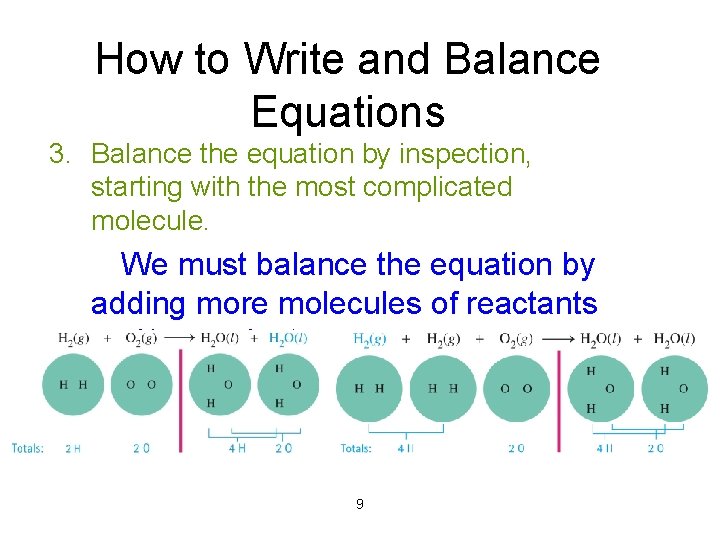
How to Write and Balance Equations 3. Balance the equation by inspection, starting with the most complicated molecule. We must balance the equation by adding more molecules of reactants and/or products. 9

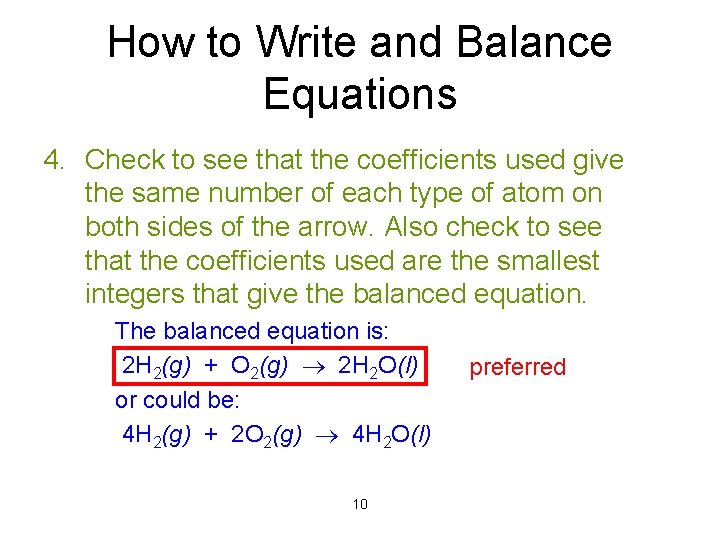
How to Write and Balance Equations 4. Check to see that the coefficients used give the same number of each type of atom on both sides of the arrow. Also check to see that the coefficients used are the smallest integers that give the balanced equation. The balanced equation is: 2 H 2(g) + O 2(g) 2 H 2 O(l) or could be: 4 H 2(g) + 2 O 2(g) 4 H 2 O(l) 10 preferred
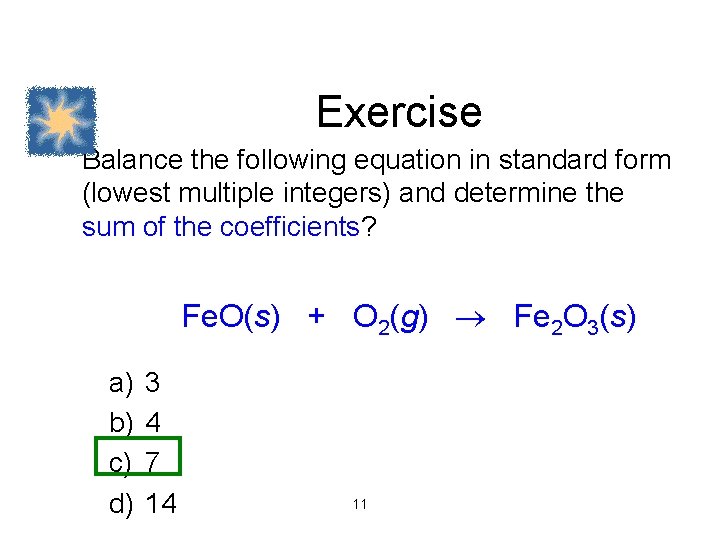
Exercise Balance the following equation in standard form (lowest multiple integers) and determine the sum of the coefficients? Fe. O(s) + O 2(g) Fe 2 O 3(s) a) b) c) d) 3 4 7 14 11

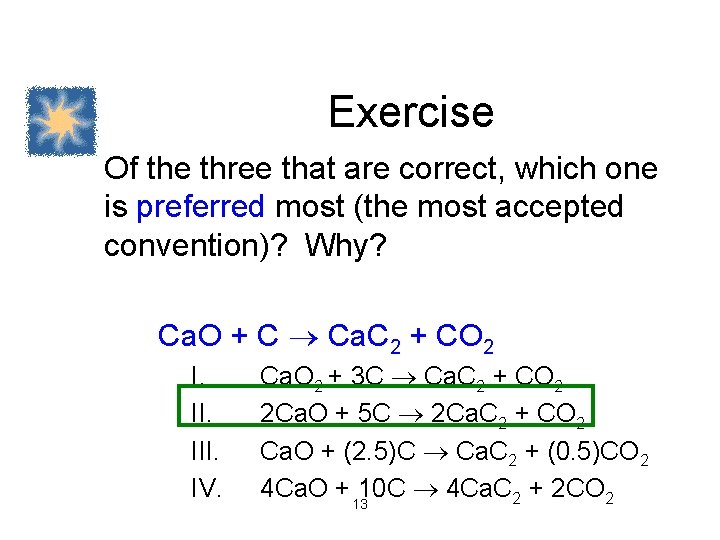
Exercise Which of the following correctly balances the chemical equation given below? There may be more than one correct balanced equation. If a balanced equation is incorrect, explain what is incorrect about it. Ca. O + C Ca. C 2 + CO 2 I. III. IV. Ca. O 2 + 3 C Ca. C 2 + CO 2 2 Ca. O + 5 C 2 Ca. C 2 + CO 2 Ca. O + (2. 5)C Ca. C 2 + (0. 5)CO 2 4 Ca. O + 1210 C 4 Ca. C 2 + 2 CO 2

Exercise Of the three that are correct, which one is preferred most (the most accepted convention)? Why? Ca. O + C Ca. C 2 + CO 2 I. III. IV. Ca. O 2 + 3 C Ca. C 2 + CO 2 2 Ca. O + 5 C 2 Ca. C 2 + CO 2 Ca. O + (2. 5)C Ca. C 2 + (0. 5)CO 2 4 Ca. O + 1310 C 4 Ca. C 2 + 2 CO 2

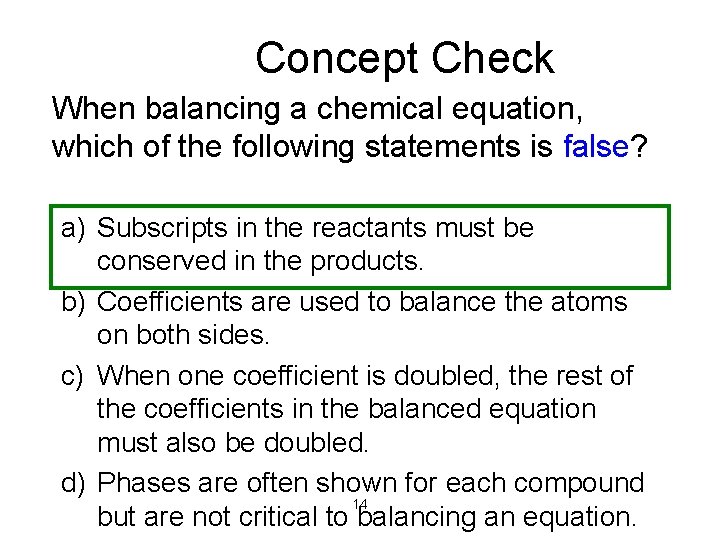
Concept Check When balancing a chemical equation, which of the following statements is false? a) Subscripts in the reactants must be conserved in the products. b) Coefficients are used to balance the atoms on both sides. c) When one coefficient is doubled, the rest of the coefficients in the balanced equation must also be doubled. d) Phases are often shown for each compound 14 but are not critical to balancing an equation.

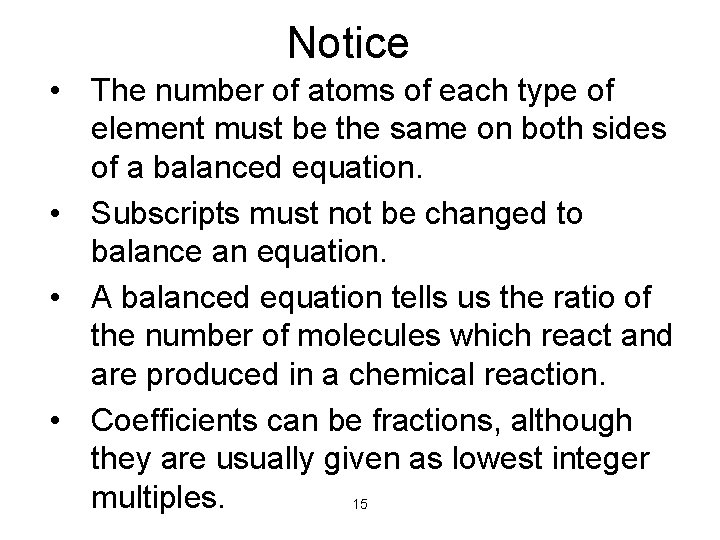
Notice • The number of atoms of each type of element must be the same on both sides of a balanced equation. • Subscripts must not be changed to balance an equation. • A balanced equation tells us the ratio of the number of molecules which react and are produced in a chemical reaction. • Coefficients can be fractions, although they are usually given as lowest integer multiples. 15
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions I will follow you wherever you blank
I will follow you wherever you blank Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Half redox reaction
Half redox reaction Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Ecological succession
Ecological succession Section 1 composition of matter
Section 1 composition of matter Median and lateral apertures
Median and lateral apertures Whats gray matter
Whats gray matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1